Abstract
TrkB is the cognate receptor for brain-derived neurotrophic factor (BDNF), a member of the neurotrophin family involved in neuronal survival, neurogenesis and synaptic plasticity. BDNF has been shown to protect photoreceptors from light-induced retinal degeneration (LIRD) and to improve ganglion cell survival following optic nerve damage. However, the utility of BDNF as a retinal neuroprotectant is limited by its short half-life, inability to cross the blood-brain and blood-retinal barriers, and activation of the proapoptotic p75 neurotrophin receptor. N-Acetylserotonin (NAS) is a naturally occurring chemical intermediate in the melatonin biosynthetic pathway in the pineal gland and retina. Its synthesis occurs in a circadian fashion with high levels at night and is suppressed by light exposure. Until recently, NAS was thought to function primarily as a melatonin precursor with little or no biological function of its own. We have now shown that TrkB activation in the retina and hippocampus is circadian in C3H/f+/+ mice, which synthesize NAS, but not in C57BL/6 mice, which have a mutation in the gene encoding the enzyme that converts serotonin to NAS. In addition, treatment of mice exogenous NAS, but not with serotonin or melatonin, activates TrkB during the daytime in a BDNF-independent manner. NAS appears to have neuroprotective properties and its administration reduces caspase 3 activation in the brain in response to kainic acid, a neurotoxic glutamate analog. We have developed structural analogs of NAS that activate TrkB. One of these derivatives, N-[2- (5-hydroxy-1H-indol-3-yl)ethyl]-2-oxopiperideine- 3-carboximide (HIOC), selectively activates TrkB with greater potency than NAS and has a significantly longer biological half-life than NAS after systemic administration. HIOC administration results in long-lasting activation of TrkB and downstream signaling kinases. The compound can pass the blood-brain and blood-retinal barriers when administered systemically and reduces kainic acid-induced neuronal cell death in a TrkB-dependent manner. Systemic administration of HIOC mitigates LIRD, assessed electrophysiologically and morphometrically. Hence, NAS may function as an endogenous circadian neurotrophin-like compound and HIOC is a good lead compound for further drug development for treatment of retinal degenerative diseases.
Keywords: neuroprotection, TrkB receptor, BDNF, light-induced retinal degeneration, circadian rhythm, N-acetylserotonin
96.1. Introduction
Melatonin is a neurohormone synthesized in the retina and pineal gland [1;2]. It transmits circadian signals of darkness and nighttime. Melatonin is synthesized from serotonin and its robust circadian rhythm is attributable primarily to the circadian regulation of arylalkylamine N-acetyltransferase (AANAT) [3], which converts serotonin to N-acetylserotonin. Melatonin acts primarily through two subtypes of G protein-coupled receptors, MT1 and MT2 [4;5], but the neurohormone may also have actions as a free radical scavenger and antioxidant [5]. Until recently, N-acetylserotonin was thought to be merely a metabolic intermediate in melatonin synthesis, although some data suggest that it may have cytoprotective effects due to its antioxidant properties [6].
Neurotrophins are a family of small proteins with neuroprotective effects that play important roles in development and maintenance of neurons, in neuroprogenitor cell proliferation and neurogenesis, and in a variety of other important neuronal functions [7]. Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family that acts primarily on high-affinity tropomyosin-related kinase B (TrkB) receptors, which are transmembrane proteins with an extracellular BDNF-binding domain and an intracellular tyrosine kinase domain [7;8]. Binding of BDNF elicits homodimerization of TrkB and its autophosphorylation. Phospho-TrkB (pTrkB) couples to multiple signaling pathways, including ERK1/2 and AKT, through which it is thought to promote cell survival. BDNF also acts on the low affinity p75NTR receptor, which is also a tyrosine kinase receptor [7].
BDNF has been shown to protect against LIRD and to improve retinal ganglion survival following optic nerve injury through TrkB-dependent mechanisms [9–12]. However, the therapeutic potential of BDNF is limited because (1) it does not cross the blood-retinal barrier or blood-brain barrier and, therefore, must be administered centrally or intravitreally; (2) it has a relatively short half-life; (3) at higher concentrations, it acts on the p75NTR receptor, which has a pro-apoptotic effect. These limitations have led to the search for small molecule activators of TrkB. In a screen of small molecule libraries, Keqiang Ye and colleagues noted that compounds with an indole alkyl moiety, similar in structure to melatonin, N-acetylserotonin, and serotonin, had efficacy in promoting TrkB activation [13].
96.2. Circadian Activation of TrkB in the Retina and Brain
Melatonin synthesis and AANAT activity occur as circadian rhythms with peaks at night in darkness [1;2]. The levels of pTrkB and total TrkB were examined during the day and night in two strains of mice [14]: C3H/f+/+ mice, which have an intact melatonin synthetic pathway [15], and C57BL/6J mice, which have a mutation in AANAT that limits the production of melatonin [16]. Total TrkB protein did not evidently fluctuate as function of time of day in either strain of mouse. However, a circadian rhythm in the levels of pTrkB was observed in the retina and hippocampus of C3H/f+/+ mice, with higher levels at night compared to day. In contrast, pTrkB levels were similar in the day and night in the retina and hippocampus of C57BL/6J mice.
96.3. N-Acetylserotonin Activates TrkB and Its Signaling Pathways
To determine if endogenous indole alkyl compounds can activate TrkB, the effects of serotonin, N-acetylserotonin and melatonin on pTrkB were examined in cultured cortical and hippocampal neurons [14]. Treatment of cultures with BDNF and N-acetylserotonin, but not with serotonin, 5-hydroxyindoleacetic acid, or melatonin, increased the level of pTrkB assessed by immunofluorescence and immunoblot analyses [14]. The effect of N-acetylserotonin appeared to be specific for TrkB, as it had no effect on TrkA or TrkC. It was dose dependent and was observed in the low nM range. Systemic administration of N-acetylserotonin increased pTrkB in the retina and hippocampus, indicating that it crosses the blood-retinal and blood-brain barriers. This conclusion was verified by direct measurement of N-acetylserotonin in the retina and brain following systemic administration [17]. In vitro, N-acetylserotonin was shown to promote TrkB dimerization and activation of downstream signaling kinases, including ERK1/2 and AKT. The effects of N-acetylserotonin were BDNF-independent, as shown in neurons from forebrain-specific BDNF conditional knockout mice. They were blocked by 1NMPP1, a specific inhibitor of Trk tyrosine kinase in TrkB F616A knock-in mouse neurons [18], indicating that they are specifically mediated by TrkB activation.
96.4. Neuroprotective Effect of N-Acetylserotonin
Kainic acid administration promotes neuronal apotoptosis in brains of TrkB F616A mice. The effect of kainic acid was antagonized by systemic administration of N-acetylserotonin, but not of melatonin[14]. Moreover, the neuroprotective effect of N-acetylserotonin was blocked by 1NMPP1, indicating that it was mediated by activation of TrkB. Similarly, BDNF and N-acetylserotonin effectively blocked glutamate-induced apoptosis in cultured cortical neurons [14].
As mentioned, treatment with BDNF protects rats from LIRD [9]. In contrast, N-acetylserotonin had no protective effect against LIRD in mice, even at doses exceeding those required for maximal TrkB activation (K. Ghai and P.M. Iuvone, unpublished observations). The lack of neuroprotection could be due to species differences, protocol differences (constant light vs. short-term high-intensity light), or to the short half-life of N-acetylserotonin.
96.5. Neuroprotective Effect of HIOC, an N-Acetylserotonin Derivative
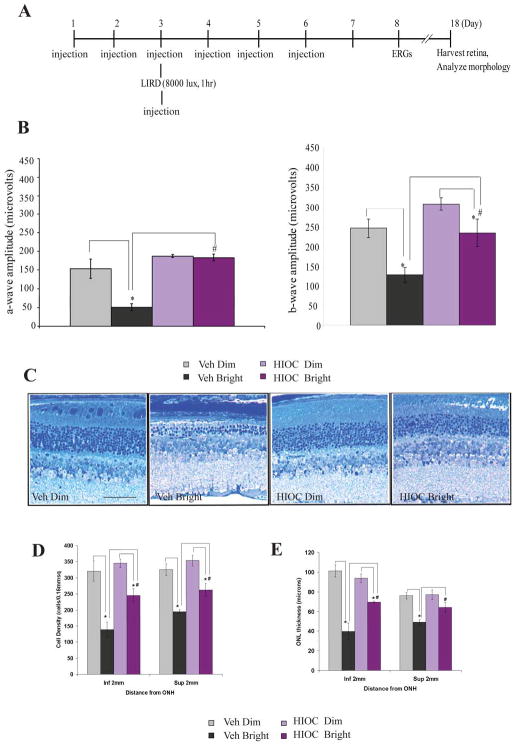
Several N-acetylserotonin derivatives were evaluated for efficacy in activating TrkB [17]. One of these, N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]-2-oxopiperidine-3-carboxamide (HIOC) was potent and effective in activating TrkB and downstream signaling kinases. HIOC passed the blood-brain and blood-retina barriers following systemic administration, as assessed by pharmacokinetic measurements of the drug in blood, retina and brain [17]. It had a significantly longer biological half-life than N-acetylserotonin, and promoted persistent activation of TrkB following systemic administration [17]. HIOC protected cultured neurons from glutamate-induced neuronal cell death. It also protected against kainic acid-induced apoptosis in the brain following systemic administration; the neuroprotective effect of HIOC was TrkB dependent. In contrast to N-acetylserotonin, HIOC potently protected against LIRD, as assessed functionally and histologically [17]. Bright-light exposure (8000 lux for 1 h) markedly reduced a-wave and b-wave amplitudes of the dark-adapted electroretinogram (ERG), measured one week post exposure, and caused reduced thickness of the outer nuclear layer (ONL) and photoreceptor outer segment disruption, measured 16 days post exposure (Fig. 96.1). These deleterious effects of bright light were significantly attenuated with HIOC treatment (Fig. 96.1) [17]. The neuroprotective effect of HIOC was tightly associated with TrkB activation. When assessed 90 min after bright light exposure in vehicle-treated mice, a small increase in pTrkB immunoreactivity was observed just distal to the ONL in the vicinity of photoreceptor inner segments [17]. This may reflect TrkB activation in the microvilli of Müller cells, as photoreceptors are not thought to express TrkB [19]. There was also some increase in label in cells of the ganglion cell layer. In the retinas of mice exposed to bright light and treated with HIOC, there was a massive increase of pTrkB labeling in the retina. The labeling at the level of the inner segments was greatly enhanced, as was that in ganglion cell and inner nuclear layers [17]. TrkB activation in the Müller cell microvilli may be particularly relevant to the photoreceptor-survival effect of HIOC considering its location and a previous study showing that BDNF expression directed to Müller cells is protective in LIRD [10].
Figure 96.1.
HIOC mitigates retinal damage caused by bright light exposure. (A). Experimental design. BALB/c mice (n=4–5/group) were injected with HIOC (40 mg/kg ip) or vehicle, subjected to bright light, ERG recordings, and histological analysis on the schedule depicted in the diagram. (B) ERG measured at a flash intensity of 6.28 cd-s/m2. (C) Representative toluidine blue stained retinal sections from each treatment group. (D, E) Quantitative analysis of ONL cell density and thickness. *,# p<0.05. Reproduced from Shen et al., Proc Natl Acad Sci USA 2012;109: 3540–5. [17] © The Authors.
96.6. Conclusion
These recent studies suggest that N-acetylserotonin, previously thought to be just a precursor of melatonin, is an endogenous circadian neuroprotectant and activator of TrkB receptors. Exogenous N-acetylserotonin may have therapeutic benefit in short-term neurotoxic insults, but probably not for slowly developing neurodegenerative disease due to its short half-life. Synthetic, small molecule activators of TrkB based on the structure of N-acetylserotonin have potential for the treatment of retinal degenerative diseases and HIOC is a good lead compound for this purpose.
Reference List
- 1.Iuvone PM, Tosini G, Pozdeyev N, Haque R, Klein DC, Chaurasia SS. Circadian clocks, clock networks, arylalkylamine N-acetyltransferase, and melatonin in the retina. Prog Retinal Eye Res. 2005;24:433–56. doi: 10.1016/j.preteyeres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Klein DC, Coon SL, Roseboom PH, Weller JL, Bernard M, Gastel JA, Zatz M, Iuvone PM, Rodriguez IR, Begay V, et al. The melatonin rhythm-generating enzyme: Molecular regulation of serotonin N-acetyltransferase in the pineal gland. Recent Prog Hormone Res. 1997;52:307–58. [PubMed] [Google Scholar]
- 3.Bernard M, Iuvone PM, Cassone VM, Roseboom PH, Coon SL, Klein DC. Avian melatonin synthesis: photic and circadian regulation of serotonin N-acetyltransferase mRNA in the chicken pineal gland and retina. J Neurochem. 1997;68:213–24. doi: 10.1046/j.1471-4159.1997.68010213.x. [DOI] [PubMed] [Google Scholar]
- 4.Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005;27:101–10. doi: 10.1385/ENDO:27:2:101. [DOI] [PubMed] [Google Scholar]
- 5.Hardeland R. Antioxidative protection by melatonin: multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine. 2005;27:119–30. doi: 10.1385/endo:27:2:119. [DOI] [PubMed] [Google Scholar]
- 6.Oxenkrug G. Antioxidant effects of N-acetylserotonin: possible mechanisms and clinical implications. Ann NY Acad Sci. 2005;1053:334–47. doi: 10.1196/annals.1344.029. [DOI] [PubMed] [Google Scholar]
- 7.Hennigan A, O’Callaghan RM, Kelly AM. Neurotrophins and their receptors: roles in plasticity, neurodegeneration and neuroprotection. Biochem Soc Trans. 2007;35:424–7. doi: 10.1042/BST0350424. [DOI] [PubMed] [Google Scholar]
- 8.Ohira K, Hayashi M. A new aspect of the TrkB signaling pathway in neural plasticity. Curr Neuropharmacol. 2009;7:276–85. doi: 10.2174/157015909790031210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LaVail MM, Unoki K, Yasumura D, Matthes MT, Yancopoulos GD, Steinberg RH. Multiple growth factors, cytokines, and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc Natl Acad Sci USA. 1992;89:11249–53. doi: 10.1073/pnas.89.23.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gauthier R, Joly S, Pernet V, Lachapelle P, Di Polo A. Brain-Derived Neurotrophic Factor Gene Delivery to Muller Glia Preserves Structure and Function of Light-Damaged Photoreceptors. Invest Ophthalmol Vis Sci. 2005 Sep 1;46:3383–92. doi: 10.1167/iovs.05-0362. [DOI] [PubMed] [Google Scholar]
- 11.Weber AJ, Harman CD, Viswanathan S. Effects of optic nerve injury, glaucoma, and neuroprotection on the survival, structure, and function of ganglion cells in the mammalian retina. J Physiol. 2008;586:4393–400. doi: 10.1113/jphysiol.2008.156729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mansour-Robaey S, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci USA. 1994;91:1632–6. doi: 10.1073/pnas.91.5.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE, et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci USA. 2010;107:2687–92. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jang SW, Liu X, Pradoldej S, Tosini G, Chang Q, Iuvone PM, Ye K. N-acetylserotonin activates TrkB receptor in a circadian rhythm. Proc Natl Acad Sci U S A. 2010;107:3876–81. doi: 10.1073/pnas.0912531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tosini G, Menaker M. The clock in the mouse retina: melatonin synthesis and photoreceptor degeneration. Brain Res. 1998;789:221–8. doi: 10.1016/s0006-8993(97)01446-7. [DOI] [PubMed] [Google Scholar]
- 16.Roseboom PH, Namboodiri MAA, Zimonjic DB, Popescu NC, Rodriguez IR, Gastel JA, Klein DC. Natural melatonin ‘knockdown’ in C57BL/6J mice: rare mechanism truncates serotonin N-acetyltransferase. Mol Brain Res. 1998;63:189–97. doi: 10.1016/s0169-328x(98)00273-3. [DOI] [PubMed] [Google Scholar]
- 17.Shen J, Ghai K, Sompol P, Liu X, Cao X, Iuvone PM, Ye K. N-acetyl serotonin derivatives as potent neuroprotectants for retinas. Proc Natl Acad Sci USA. 2012;109:3540–5. doi: 10.1073/pnas.1119201109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen X, Ye H, Kuruvilla R, Ramanan N, Scangos KW, Zhang C, Johnson NM, England PM, Shokat KM, Ginty DD. A Chemical-Genetic Approach to Studying Neurotrophin Signaling. Neuron. 2005;46:13–21. doi: 10.1016/j.neuron.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Rohrer B, Korenbrot JI, LaVail MM, Reichardt LF, Xu B. Role of neurotrophin receptor TrkB in the maturation of rod photoreceptors and establishment of synaptic transmission to the inner retina. J Neurosci. 1999;19:8919–30. doi: 10.1523/JNEUROSCI.19-20-08919.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]