Abstract
The effects of 13 food extracts and juices, including shellfish, fruits, and vegetables, on the binding ability of human norovirus (NoV) were examined, using P particles of human NoV GII.4 as a research surrogate. The enhancements (positive values) or reductions (negative values) of NoV P particle detection (changes in optical density at 450 nm) in the presence of different food extracts and juices as compared with P particles diluted in phosphate-buffered saline were tested by saliva-binding, enzyme-linked immunosorbent assay in triplicate. In the presence of different food extracts and juices at different concentrations, an increase or decrease of the receptor-binding ability of the NoV P particles was observed. Due to a higher specific binding and thus a higher accumulation of the viral particles, oysters may be contaminated with human NoV more often than other shellfish species (mussel, hard clams, and razor clams). Cranberry and pomegranate juices were shown to reduce the specific binding ability of human NoV P particles. No such binding inhibition effects were observed for the other tested extracts of fresh produce (strawberry, blackberry, blueberry, cherry tomato, spinach, romaine lettuce) or, notably, for raspberry, which has been associated with human NoV outbreaks.
Noroviruses (NoVs), members of the Caliciviridae family, are among the most common agents that cause human acute gastroenteritis all over the world (7, 11, 23). Transmitted mainly through the fecal-oral route, human NoV infection was found to be associated with the consumption of contaminated shellfish or fresh produce (1, 2, 4, 5, 8, 12, 14, 24).
Due to the lack of a suitable animal model or cell culture system, it is still impossible to directly determine the infectivity of human NoVs (3). However, it is highly likely that attachment to a receptor on a cell surface, as the first step of a viral life cycle, is essential to initiate the infection process (15). It has been proven that many human NoV strains bind specifically to certain types of human histo-blood group antigens (HBGAs), complex carbohydrates which are present on red blood cell surfaces and the mucosal epithelium of the respiratory, genitourinary, and digestive tracts and as free oligosaccharides in biologic fluids such as saliva, intestinal contents, milk, and blood. The binding pattern has correspondence with the probability of infection (9, 10, 19, 20). The protruding (P) domain of the major structural protein of NoV capsid forms the outermost surface of the capsid and contains the elements required for viral capsid binding to host carbohydrate receptors (18). When expressed in Escherichia coli, this protein forms subviral particles, the P particles, which may be used as a surrogate to study the binding ability of human NoVs (18).
This study used P particles of human NoV GII.4 as a research surrogate to investigate the effect of 13 food extracts and juices (from various types of shellfish, fruits, and vegetables that are common vehicles of human NoV transmission) on the salivary HBGA-binding ability of human NoVs.
MATERIALS AND METHODS
Expression and purification of the P particles in E. coli
P particles of human NoV GII.4 (Hu/NoV/GII.4/VA387/1998/US, GenBank accession no. AY038600), whose HBGA-binding pattern has been demonstrated in previous studies (9, 10, 20), were prepared as described by Tan and Jiang (18). Briefly, the constructs containing P protein–encoding sequences were expressed in E. coli strain BL21 at room temperature overnight with an induction of 0.5 mM isopropyl-β-D-thiogalactopyranoside (IPTG). Purification of the recombinant glutathione S-transferase (GST)–P fusion protein from bacteria was performed with Glutathione Sepharose 4 Fast Flow (Amersham Biosciences, Piscataway, NJ) according to the manufacturer’s instructions. The fusion protein was eluted by glutathione (Amersham Biosciences), and the P proteins were released from GST by thrombin (Amersham Biosciences) cleavage at room temperature for 16 h. Further purification was conducted by gel filtration using a size exclusion column and/or anion exchange. Protein concentration was measured by quantitative sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Protein samples were loaded onto SDS-PAGE gel with a series of standard bovine serum albumin (BSA) of known concentrations. The concentration of the P protein was determined by comparing the density of the specific protein band with the standards. The specificity was confirmed by a Western blot assay. The final concentration of the P protein was estimated to be 1 mg/ml.
Preparation of food extracts
Various types of shellfish (oyster, mussel, hard clam, and razor clam), fruits (raspberry, strawberry, blackberry, and blueberry), and vegetables (lettuce, cherry tomato, and spinach), as well as two juices (Ocean Spray 100% Cranberry juice, Ocean Spray 100% cranberry-pomegranate juice) were purchased from a local grocery store in Cincinnati, OH, and stored in the laboratory at 4°C. For shellfish, the stomachs and digestive diverticula were removed by dissection. For fruits and vegetables, 10 g of each sample was cut into small pieces (less than 1 cm3/1 g per piece). A volume of 10 ml of phosphate-buffered saline (PBS, pH 7.5) was added, and a suspension was obtained manually by the use of a glass homogenizer; the homogenates were centrifuged at 3,000 ×g for 5 min at 4°C. The supernatant or juice (10 to 20 ml) was neutralized by sodium hydroxide (2 M NaOH) to obtain a pH of 7.0 to 7.5 (the volume increase was less than 1 ml) and was 10-fold diluted by PBS to obtain a series of dilutions with 10 ml for each. The concentration of the undiluted supernatant or juice was defined as 100%. The food extracts with concentrations of 100, 10, 1, 0.1, and 0.01% were used to dilute P particles from a concentration of 1 μg/ml (1:1,000 diluted from the expressed P protein by PBS, 1 ml of each) to 0.1 μg/ml (10 ml of each), respectively. The P particle solutions were incubated at 37°C for 1 h and tested immediately.
Saliva-binding ELISA
The saliva-binding enzyme-linked immunosorbent assay (ELISA) was performed as described by Huang et al. (9, 10), with a few modifications. Briefly, saliva samples of type B (5 to 10 ml), the collection and determination of which was defined previously by Huang et al. (9), were boiled at 100°C for 10 min and centrifuged at 10,000 × g for 5 min. The supernatant (100 μl) was diluted by PBS (1:500) and was used to coat 96-well microtiter plates (100 μl per well) at 4°C overnight. The noncoated wells were included as blank controls. After blocking with 5% nonfat dried milk (bovine lacto transfer technique optimizer, 200 μl per well), the incubated P particle solutions with or without food extracts were added (0.1 μg/ml, 100 μl per well). The bound capsid proteins were detected with hyperimmune guinea pig anti-NoV antisera (1:3,300) and by adding horseradish peroxidase–conjugated goat anti–guinea pig immunoglobulin G (1:2,000, ICN Pharmaceuticals, Aurora, OH). The horseradish peroxidase activity was detected with a TMB (3,3′,5,5′-tetramethyl-benzidine) kit (Kirkegaard & Perry Laboratories, Gaithersburg, MD), and the signal intensities (optical density at 450 nm [OD450]) were read with an enzyme immunoassay spectrum reader (Tecan, Durham, NC).
As described by Huang et al. (10), the guinea pig anti-NoV antiserum was a mixture of the hyperimmune sera of guinea pigs cross-immunized with recombinant virus-like particles from human NoV genogroups I and II: Norwalk virus (M87661), C59 (AF435807), VA115 (AY038598), VA387 (AY038600), MxV (U22498), GrV (AJ004864), HV (U07611), VA207 (AY038599), and MOH (AF397156). The antiserum recognized the authentic virions, virus-like particles, and P particles of a wide range of human NoV strains.
Data analysis
The enhancements (positive values) or reductions (negative values) of NoV P particle detection (ΔOD450) in the presence of different food extracts compared with PBS controls (P particles diluted in PBS) were tested by saliva-binding ELISA in triplicate. The comparisons were made to PBS controls (also in triplicate) on the same ELISA plate. The concentration of P particles in each reaction was held constant, and the only variable in this study was the presence of undiluted or diluted food extracts and juices. Negative controls (PBS or dried milk or food extracts plated on saliva-coated plate without P particles) were run on each ELISA plate. Each error bar represents the data range.
RESULTS AND DISCUSSION
The enhancements (positive values) or reductions (negative values) of NoV P particle detection (ΔOD450) in the presence of different food extracts compared with PBS controls (P particles diluted in PBS) by saliva-binding ELISA are shown in Figures 1 through 4. The OD450 values for the negative controls (PBS or dried milk or food extracts plated on saliva-coated plate without P particles) were all determined to be under 0.05. For each food extract, four concentrations of subsequent 10-fold dilutions were tested.
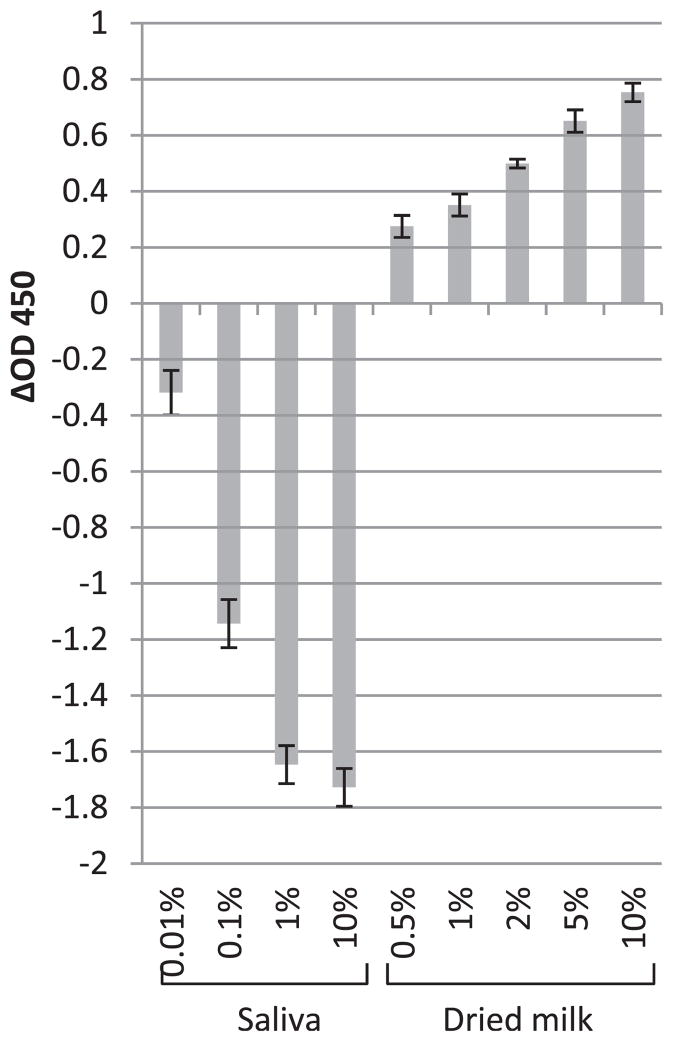
FIGURE 1.
The enhancements (positive values) or reductions (negative values) of NoV P particle detection in the presence of saliva or dried milk compared with PBS controls (P particles diluted in PBS) tested by saliva-binding ELISA. The x axis represents the concentration of saliva or dried milk; the y axis represents the enhancements or reductions of OD450 (ΔOD450). Each data point is an average of triplicates, and each error bar represents the data range.
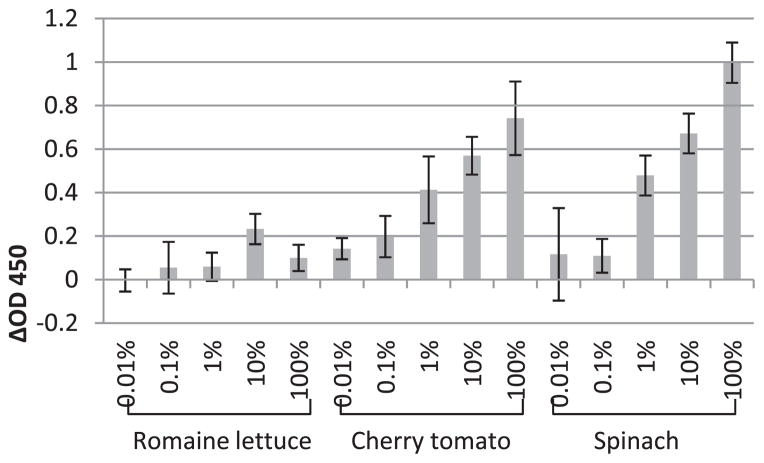
FIGURE 4.
The enhancements (positive values) or reductions (negative values) of NoV P particle detection in the presence of vegetable extract (romaine lettuce, cherry tomato, and spinach) compared with PBS controls (P particles diluted in PBS) tested by saliva-binding ELISA. The x axis represents the concentration of vegetable extract; the y axis represents the enhancements or reductions of OD450 (ΔOD450). Each data point is an average of triplicates, and each error bar represents the data range.
First, the effects of saliva and nonfat dried milk were tested instead of food extract or juice since they are used as the coating and blocking reagent in the ELISA assay. A dose-dependent reduction was obtained for the binding level of P particles after the incubation with saliva (Fig. 1). This shows that the HBGAs in saliva blocked the binding sites on P particles in the solution, keeping them from binding to the salivary HBGA receptors coated on the plate. Interestingly, dried milk, which is recognized as an inert substance in the binding process, could increase the binding level also in a dose-dependent manner (Fig. 1). As the possibility of the dried milk nonspecifically binding to the saliva-coated plate and/or primary or secondary antibody has been ruled out by the negative controls (dried milk plated on saliva-coated plate without P particles), it can be expected that certain inert substances could assist the presenting or binding of P particles to the carbohydrate coated on the plate. One of the tentative explanations could be an aggregation of P particles caused by the nonspecific interactions between the P particles and the food particulates. In addition, from a perspective of biokinetics, it can be assumed that the binding of P particles to the carbohydrates in saliva is a chemical equilibrium that is dynamic and reversible, and a certain amount of nonrelated substances (e.g., milk protein) in the solution may promote the equilibrium to the side forming more protein-carbohydrate complex.
Because foods are complex matrices that contain a wide range of components, it is possible that in these food extracts, compounds that enhance or inhibit the HBGA binding of NoV P particles could both be present.
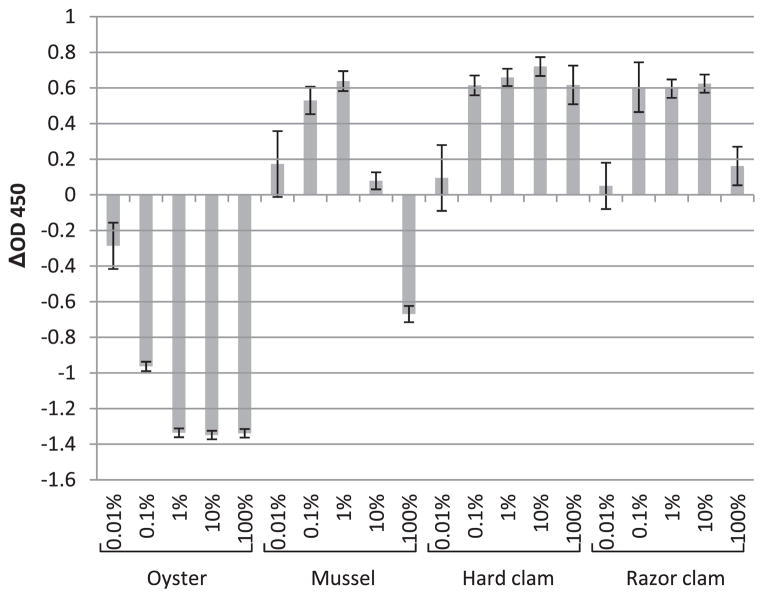
Results shown in Figure 2 indicate that the oyster extract could inhibit the HBGA binding of NoV P particles in a dose-dependent manner from the concentration of 0.01 to 100%, with the optimal decrease in P particle binding obtained with 1% oyster extract. The mussel extract could only inhibit the binding at a higher concentration (100%, Fig. 2). As the concentration of mussel extract decreased (10 and 1%), the blocking effect of the carbohydrates started to be offset by the binding-enhancing effect of the nonrelated substances, which in turn was also decreased as the dilution decreased further (0.1 and 0.01%). In hard clams and razor clams (Fig. 2), the binding-inhibiting effects were barely observed even at high concentrations (100 and 10%). It has been reported that shellfish such as oysters, mussels, and clams express carbohydrates of the HBGA family in their gastrointestinal tissues; this may serve as a possible mechanism of bioaccumulation and thus may explain the important role shellfish play in human NoV transmission (21). As such, it is expected that upon incubation with shellfish extracts, the binding sites of the viruses (or P particles as the surrogate) are blocked, and thus the HBGA-binding levels would be reduced accordingly in vitro. After the contaminated shellfish are consumed, these attached viruses might be released by digestion to become infectious, similar to the release from the blocking of saliva during a typical viral infection process. Since the exact nutritional composition and concentration of the components affecting the binding in different shellfish extracts could be variable, this result may support the observation that in general, human NoV contamination is more often related with oysters (due to the presence of the specific binding compounds) than with the other shellfish species (2, 24).
FIGURE 2.
The enhancements (positive values) or reductions (negative values) of NoV P particle detection in the presence of shellfish extract (oyster, mussel, hard clam, and razor clam) compared with PBS controls (P particles diluted in PBS) tested by saliva-binding ELISA. The x axis represents the concentration of shellfish extract; the y axis represents the enhancements or reductions of OD450 (ΔOD450). Each data point is an average of triplicates, and each error bar represents the data range.
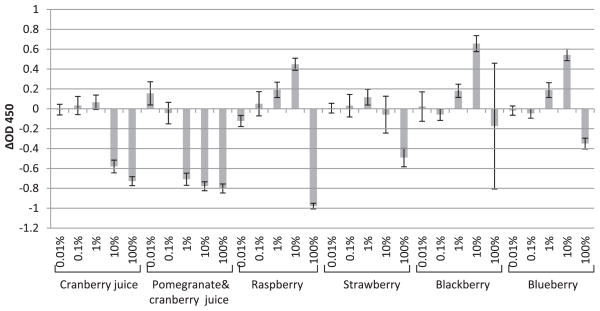
The effects of cranberry juice and cranberry-pomegranate juice, as well as extracts of several common soft fruits (raspberry, strawberry, blackberry, and blueberry), on the HBGA-binding ability of human NoV P particles were investigated. Cranberry juice could reduce the binding level at concentrations of 10 and 100%, while a similar effect could be obtained with cranberry-pomegranate juice with a concentration range of 1% to 100% (Fig. 3). In comparison, the extracts of raspberry, strawberry, blackberry, and blueberry were less effective at inhibiting the binding ability of NoV P particles; a decrease of P particle binding was only obtained by incubation with 100% extracts of these soft fruits (Fig. 3). Cranberry and pomegranate juices have been reported to be able to reduce the infectivity of several human NoV surrogates effectively (16, 17). The polyphenolic compounds, which exist in multiple soft fruits, were determined to be the active components (16, 17). Although the exact mechanism is still not clear, the interaction of plant polyphenolic compounds with the viral capsid protein may cause irreversible damage or reversible blocking on certain spots on the capsid protein. Accordingly, the specific binding ability of P particles treated by plant polyphenols could be reduced. This result demonstrates that cranberry and pomegranate juices interfere with NoV binding. It is expected that these common soft fruit extracts (raspberry, strawberry, blackberry, and blueberry) have a rather limited ability to interfere with binding. In sum, because the soft fruits, in particular raspberry, are susceptible to human NoV contamination due to hand picking, are difficult to wash thoroughly, and have a limited ability to interfere with binding, they are “at risk” produce and have been associated with human NoV outbreaks (4, 8, 12).
FIGURE 3.
The enhancements (positive values) or reductions (negative values) of NoV P particle detection in the presence of fruit juice or extract (cranberry juice, cranberry-pomegranate juice, raspberry, strawberry, blackberry, and blueberry) compared with PBS controls (P particles diluted in PBS) tested by saliva-binding ELISA. The x axis represents the concentration of fruit juice or extract; the y axis represents the enhancements or reductions of OD450 (ΔOD450). Each data point is an average of triplicates, and each error bar represents the data range.
As for vegetables, the extracts of romaine lettuce, cherry tomato, and spinach were tested on the HBGA-binding ability of NoV P particles. With cherry tomato and spinach, enhancement of binding was observed, probably due to the effect of nonrelated substances (as explained for dried milk; Fig. 4). As for romaine lettuce, the presence of lettuce extract did not much alter the binding level of P particles (Fig. 4). In 2010, Gandhi et al. (6) found that the virus-like particles of Norwalk virus could bind to romaine lettuce at a higher level than to other vegetables with certain non–HBGA-binding sites. We assume that this kind of binding may not block the HBGA-binding sites on P particles but could offset the binding enhancing effect that may occur due to nonrelated substances, resulting in a constant binding level with or without the presence of lettuce extract.
Due to the rising number of foodborne outbreaks, human NoV is increasingly being acknowledged as a serious threat to human health that is causing a significant burden of disease worldwide. This study has demonstrated the effects of multiple food extracts on the specific binding ability of NoVs P particles. In summary, the optimal decrease in P particle binding was obtained by incubation with 1% oyster extract; for mussels, only 100% extract led to a decrease in P particle binding, and for hard and razor clams, extracts at concentrations from 0.01 to 100% yielded no decrease. Cranberry juice could reduce the binding level at concentrations of 10 and 100%, while cranberry-pomegranate juice gave similar decreases with a concentration range of 1 to 100%. For raspberry, strawberry, blackberry, and blueberry, a decrease of P particle binding was only obtained by incubation with 100% extracts. None of the vegetables tested in this study gave decrease in P particle binding at any of the tested concentrations.
This study has several limitations. First, there may be differences between situations in vivo and in vitro, between authentic viruses and P particles. Second, the linear relationship between the OD measurements and P particle concentration was imperfect; therefore, in this study the saliva-binding ELISA results could only represent a rough trend for the change of viral-binding abilities. However, the results may support the following conclusions. First, oysters, which have been reported to express carbohydrates of the HBGA family in their gastrointestinal tissues (21), may be associated with human NoV contamination more often than other shellfish species due to a higher specific binding and thus a higher accumulation of the viral particles. Cranberry and pomegranate juices, which have been reported to reduce the infectivity of several human NoV surrogates (16, 17), were demonstrated to have NoV-binding interfering effect by reducing the specific binding ability of human NoV P particles to carbohydrate receptors in saliva samples. For the other tested fresh produce, in particular for raspberry, which has been associated with human NoV outbreaks, the NoV-binding interfering effect could be rather limited because no such binding inhibition effects were observed. Second, since human NoVs are still nonculturable, alternative research approaches that are based upon the viral structural integrity and/or functions have been developed, within which the binding-based assays are considered to be of great value (13, 22). This study reminds researchers that the viral-binding increasing or decreasing effects caused by food matrix in the viral suspensions should be taken into consideration while conducting binding-based assays on NoVs extracted from foods.
Acknowledgments
The research leading to these results has received funding from a Ph.D. grant of the China Scholarship Council (D. Li), a financial support for staying abroad (D. Li) of the Research Foundation-Flanders (Fonds voor Wetenschappelijk Onderzoek [FWO] Vlaanderen), and a U.S. Department of Agriculture grant (X. Jiang).
References
- 1.Baert L, Mattison K, Loisy-Hamon F, Harlow J, Martyres A, Lebeau B, Stals A, Van Coillie E, Herman L, Uyttendaele M. Review: norovirus prevalence in Belgian, Canadian and French fresh produce: a threat to human health? Int J Food Microbiol. 2011;151:261–269. doi: 10.1016/j.ijfoodmicro.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Boxman ILA. Human enteric viruses occurrence in shellfish from European markets. Food Environ Virol. 2010;2:156–166. [Google Scholar]
- 3.Duizer E, Schwab KJ, Neill FH, Atmar RL, Koopmans MPG, Estes MK. Laboratory efforts to cultivate noroviruses. J Gen Virol. 2004;85:79–87. doi: 10.1099/vir.0.19478-0. [DOI] [PubMed] [Google Scholar]
- 4.Falkenhorst G, Krusell L, Lisby M, Madsen SB, Böttiger BE, Mølbak K. Imported frozen raspberries cause a series of Norovirus outbreaks in Denmark. [Accessed 22 September 2005];Euro Surveill. 2005 10(38):pii= 2795. doi: 10.2807/esw.10.38.02795-en. Available at: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=2795. [DOI] [PubMed] [Google Scholar]
- 5.Food and Agriculture Organization of the United Nations/World Health Organization. Microbiological risk assessment series no. 14. FAO/WHO; Rome: 2008. Microbiological hazards in fresh leafy vegetables and herbs: meeting report. [Google Scholar]
- 6.Gandhi KM, Mandrell RE, Tian P. Binding of virus-like particles of Norwalk virus to romaine lettuce veins. Appl Environ Microbiol. 2010;76:7997–8003. doi: 10.1128/AEM.01566-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansman GS, Katayama K, Maneekarn N, Peerakome S, Khamrin P, Tonusin S, Okitsu S, Nishio O, Takeda N, Ushijima H. Genetic diversity of norovirus and sapovirus in hospitalized infants with sporadic cases of acute gastroenteritis in Chiang Mai, Thailand. J Clin Microbiol. 2004;42:1305–1307. doi: 10.1128/JCM.42.3.1305-1307.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hjertqvist M, Johansson A, Svensson N, Abom PE, Magnusson C, Olsson M, Hedlund KO, Andersson Y. Euro Surveill; Four outbreaks of norovirus gastroenteritis after consuming raspberries; Sweden. June–August 2006; 2006. [Accessed 7 September 2006]. p. pii=3038. Available at: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=3038. [DOI] [PubMed] [Google Scholar]
- 9.Huang P, Farkas T, Marionneau S, Zhong W, Ruvoen-Clouet N, Morrow AL, Altaye M, Pickering LK, Newburg DS, LePendu J, Jiang X. Noroviruses bind to human ABO, Lewis, and secretor histoblood group antigens: identification of 4 distinct strain-specific patterns. J Infect Dis. 2003;188:19–31. doi: 10.1086/375742. [DOI] [PubMed] [Google Scholar]
- 10.Huang PW, Farkas T, Zhong WM, Tan M, Thornton S, Morrow AL, Jiang X. Norovirus and histo-blood group antigens: demonstration of a wide spectrum of strain specificity and classification of two major binding groups among multiple binding patterns. J Virol. 2005;79:6714–6722. doi: 10.1128/JVI.79.11.6714-6722.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirkwood CD, Clark R, Bogdanovic-Sakran N, Bishop RF. A 5-year study of the prevalence and genetic diversity of human caliciviruses associated with sporadic cases of acute gastroenteritis in young children admitted to hospital in Melbourne, Australia (1998–2002) J Med Virol. 2005;77:96–101. doi: 10.1002/jmv.20419. [DOI] [PubMed] [Google Scholar]
- 12.Le Guyader FS, Mittelholzer C, Haugarreau L, Hedlund KO, Alsterlund R, Pommepuy M, Svensson L. Detection of noroviruses in raspberries associated with a gastroenteritis outbreak. Int J Food Microbiol. 2004;97:179–186. doi: 10.1016/j.ijfoodmicro.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 13.Li D, Baert L, Van Coillie E, Uyttendaele M. Critical studies on binding-based RT-PCR detection of infectious noro-viruses. J Virol Methods. 2011;177:153–159. doi: 10.1016/j.jviromet.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Nenonen NP, Hannoun C, Horal P, Hernroth B, Bergstrom T. Tracing of norovirus outbreak strains in mussels collected near sewage effluents. Appl Environ Microbiol. 2008;74:2544–2549. doi: 10.1128/AEM.02477-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shors T. Understanding viruses. Jones and Bartlett Publishers; Sudbury, MA: 2009. [Google Scholar]
- 16.Su X, Howell AB, D’Souza DH. The effect of cranberry juice and cranberry proanthocyanidins on the infectivity of human enteric viral surrogates. Food Microbiol. 2010;27:535–540. doi: 10.1016/j.fm.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Su X, Sangster MY, D’Souza DH. In vitro effects of pomegranate juice and pomegranate polyphenols on foodborne viral surrogates. Foodborne Pathog Dis. 2010;7:1473–1479. doi: 10.1089/fpd.2010.0583. [DOI] [PubMed] [Google Scholar]
- 18.Tan M, Jiang X. The P domain of norovirus capsid protein forms a subviral particle that binds to histo-blood group antigen receptors. J Virol. 2005;79:14017–14030. doi: 10.1128/JVI.79.22.14017-14030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan M, Jiang X. Norovirus and its histo-blood group antigen receptors: an answer to a historical puzzle. Trends Microbiol. 2005;13:285–293. doi: 10.1016/j.tim.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Tan M, Jiang X. Norovirus gastroenteritis, carbohydrate receptors, and animal models. PLoS Pathog. 2010;6(8):e1000983. doi: 10.1371/journal.ppat.1000983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian P, Engelbrektson AL, Jiang X, Zhong W, Mandrell RE. Norovirus recognizes histo-blood group antigen on gastrointestinal cells of clams, mussels, and oysters: a possible mechanism of bioaccumulation. J Food Prot. 2007;70:2140–2147. doi: 10.4315/0362-028x-70.9.2140. [DOI] [PubMed] [Google Scholar]
- 22.Tian P, Yang D, Mandrell R. A simple method to recover norovirus from fresh produce with large sample size by using histo-blood group antigen-conjugated to magnetic beads in a recirculating affinity magnetic separation system (RCAMS) Int J Food Microbiol. 2011;147:223–227. doi: 10.1016/j.ijfoodmicro.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 23.Turcios RM, Widdowson MA, Sulka AC, Mead PS, Glass RI. Reevaluation of epidemiological criteria for identifying outbreaks of acute gastroenteritis due to norovirus: United States, 1998–2000. Clin Infect Dis. 2006;42:964–969. doi: 10.1086/500940. [DOI] [PubMed] [Google Scholar]
- 24.Westrell T, Dusch V, Ethelberg S, Harris J, Hjertqvist M, Jourdanda Silva N, Koller A, Lenglet A, Lisby M, Vold L. Euro Surveill; Norovirus outbreaks linked to oyster consumption in the United Kingdom; Norway, France, Sweden and Denmark. 2010; 2010. [Accessed 25 March 2010]. p. pii=19524. Available at: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19524. [PubMed] [Google Scholar]