Abstract
Globin family was famous for oxygen supply function of its members such as hemoglobin and myoglobin. With the progress of research, several members of this protein family have been proven to play roles in tumors including glioma. Androglobin (ADGB) is a recently identified member of globin family with very few studies about its function. In the present study, we show that ADGB plays an oncogene role in glioma. Lentiviral vector mediated ADGB knockdown inhibited the proliferation of glioma cell lines determined by MTT assay and colony formation assay. ADGB knockdown also increased the apoptosis of glioma cell line U251 assessed by flow cytometry. In addition, western blot showed that ADGB knockdown altered levels of several proteins related to proliferation, survival or apoptosis in U251 cells. These findings suggest ADGB is involved in the progression of glioma in vitro.
Keywords: Androglobin, glioma, proliferation, apoptosis
Introduction
Globins occur in all three kingdoms of life: bacteria, Archaea and eukaryotes [1]. They can be divided to three types: single-domain globins and two types of chimeric globins, flavohemoglobins and globin-coupled sensors [2]. Except for the well-known intracellular oxygen transport role, globins have diverse functions such as reaction with nitric oxide, peroxidase activity and reaction with free radicals, binding and transport of sulfide and oxygen sensing [3]. Several members of globin superfamily have been identified to play roles in many kinds of cancers [4-6] including glioblastoma [7-10].
Androglobin (ADGB) is a recently identified new member of globin family, which is expressed in heart, brain, lung and testis of mouse, with extremely high level in testes [11]. ADGB mRNA level does not obviously elevated in experimental hypoxia treated human testicular germ cell tumor (NCCIT), embryonal carcinoma (NTERA-2 clone D1) and fibrosarcoma (HT1080) derived cell lines [11]. It is suggested that ADGB harbors other functions rather than oxygen transport. In this study, we propose that ADGB plays a role in the progression of glioma. Lentiviral vector was used to mediate RNA knockdown of ADGB in glioma cell lines. We found ADGB knockdown decreased the growth of glioma cell lines U87, U251, U373 revealed by MTT assay and the colony formation of glioma cell lines U87 and U251. ADGB knockdown also increased apoptosis in U251. In addition, the results from U251 cell line showed that ADGB knockdown altered phosphorylation level of some proteins such as STAT3, Akt, ERK1/2. ADGB knockdown also caused increased level of cleaved caspase-3 and decreased level of cyclin D1 and Bcl-2.
Materials and methods
Cell culture and reagents
Glioma cell lines U87, U251, U373 (Institute of Biochemistry and Cell Biology, Shanghai, China) were cultured in DMEM (Dulbeccos Modified Eagle Medium, Invitrogen, Carlsbad, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Invitrogen) at 37°C with 5% CO2 in a humidified atmosphere. SYBR Premix Ex Taq (Takara, Dalian, China), was used when conducting quantitative real-time PCR. We used antibodies to the following proteins in this study: p-STAT3 (Tyr705), STAT3, p-Akt (Ser473), Akt, p-ERK1/2 (Thr202/Tyr204), ERK1/2, cleaved caspase-3 (Tyr705) (Cell Signaling Technology, Beverly, USA), Cyclin D1 (Boster, Wuhan, China), Bcl-2 (Bioworld Technology, Nanjing, China), GAPDH (Kangchen, Shanghai, China).
Lentivirus-mediated shRNA knockdown
Lentiviral shRNA plasmids with or without targeting ADGB sequence were transfected into 293T cells using Lipofectamine 2000 together with the gag/pol packaging vector and the VSVG encoding plasmid in order to produce the shADGB lentiviruses or the control lentiviruses. The shRNA sequence was: 5’-CCGGGCATTTAGCAAGAGAATATGTGTCGCCTGTAAATGCTTTT-3’. Lentiviruses were packaged with the help of Neuronbiotech Company (Shanghai, China).
Quantitative real-time PCR
The knockdown efficiency of lentiviral shRNA in cells was determined by quantitative PCR analysis. U373 cells in 6-well plate with about 30% confluence were trasnsduced with control or shADGB lentiviruses (MOI = 10). Four days later, RNA was extracted, reverse transcribed and analyzed by quantitative real-time PCR. Total RNA was extracted using Trizol (Invitrogen), according to the manufacturer’s instructions. The primer sets for quantitative real-time PCR were: ADGB: 5’-AGACCCTCATCAGAAGTGCAG-3’; 5’-GCTACCAGAGGACAAGACCTACT-3’. GAPDH: 5’-AAGGTGAAGGTCGGAGTCAAC-3’; 5’-GGGGTCATTGATGGCAACAATA-3’.
Western blot
Cells were collected and lysed in lysis buffer (20 mM Tris-HCl pH 7.5, 2 mM EDTA, 1% NP-40, 150 mM NaCl, 1 mg/ml SDS, 0.25 mg/ml sodium deoxycholate) supplemented with 1 × PMSF, 1 × phosphatase inhibitor and 1 × protease inhibitor cocktail. Western blot was carried out as previously described [12].
MTT assay
To measure the proliferation rate, cells were seeded onto 96-well plates in 150 μl of DMEM with 10% FBS, at a density of 1500 cells/well. Each day for the next five days, 100 μg of MTT was added to the corresponding well. After an additional 4 h in culture, 150 μl of DMSO was added and optical density measured at 490 nm in a multiwell spectrophotometer.
Colony formation assay
Cells were seeded onto 6-well plates at a density of 300 cells/well in triplicate. After 10 days in culture, cells were fixed with methanol and stained with 0.5% crystal violet. The colony formation rate was calculated by dividing the number of colonies by the number of seeded cells.
Flow cytometry assay
The Annexin V Apoptosis Detection Kit APC (eBioscience, San Diego, USA) was used to detect and quantify apoptosis by flow cytometry. Cells in the log phase of growth were harvested according to the manufacturer’s protocol and analyzed using a FACS Calibur Flow Cytometer. Data were analyzed by FlowJo software. Apoptosis Detection was carried out as previously described [12].
Results
Lentivirus mediated ADGB knockdown results in decreased mRNA level in U373
To test the effectiveness of our shRNA, U373 cells in 6-well plate with about 30% confluence were trasnsduced with control or shADGB lentiviruses (MOI = 10). Four days later, RNA was extracted, reverse transcribed and analyzed by quantitative real-time PCR. ADGB mRNA level was changed to less than 50% by the shADGB lentiviruses in U373 (Figure 1). Due to the unavailability of good quality antibody to ADGB, we did not acquire results to show the effectiveness of ADGB knockdown at translational level.
Figure 1.
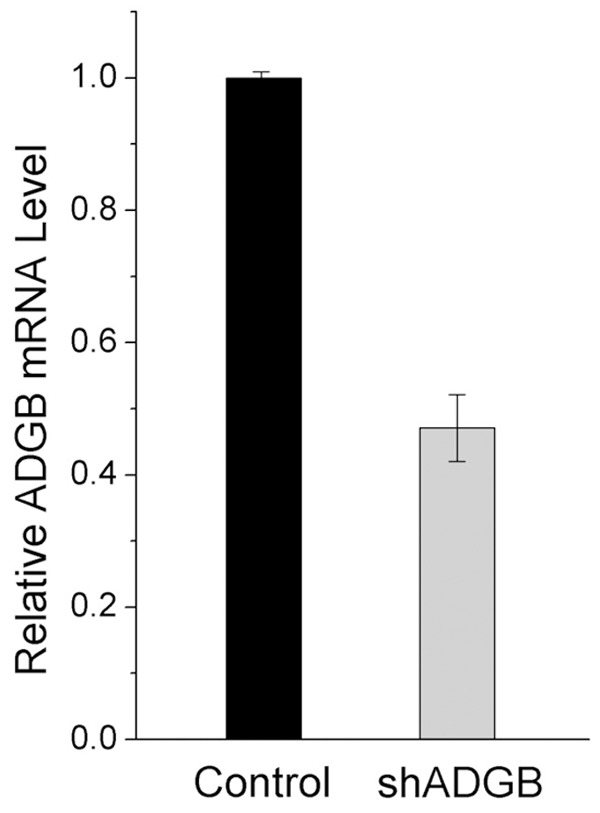
Lentivirus mediated ADGB knockdown results in decreased mRNA level in U373. U373 cells in 6-well plate with about 30% confluence were trasnsfected with control or shADGB lentiviruses (MOI = 10). Four days later, RNA was extracted, reverse transcribed and analyzed by quantitative real-time PCR.
ADGB knockdown inhibited the growth of glioma cell lines U251, U87, and U373
Cells seeded onto 96-well plates at a density of 1500 cells/well were transfected with control or shADGB lentiviruses. Each day for the next five days, 100 μg of MTT was added to the corresponding well. After an additional 4 h in culture, 150 μl of DMSO was added and optical density measured at 490 nm in a multiwell spectrophotometer. The results showed that growth of three glioma cell lines U251, U87, and U373 were all inhibited upon ADGB knockdown (Figure 2).
Figure 2.
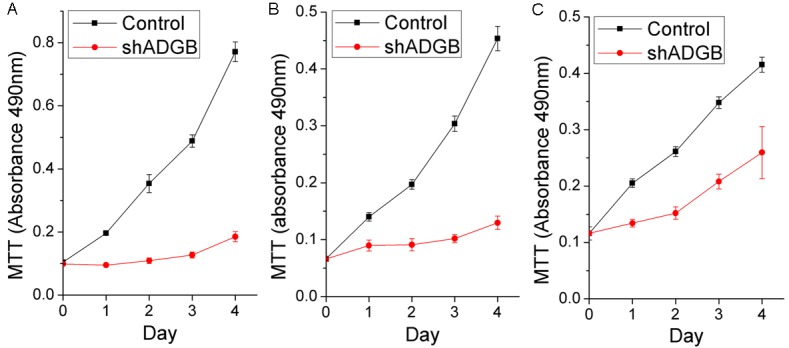
ADGB Knockdown inhibits the growth of glioma cell lines U251, U87, and U373. Proliferation rates of U87, U251 and U373 cells trasnsfected with control or shADGB lentiviruses (MOI = 10) were measured by MTT assay (n = 5). It was showed that the growth of three glioma cell lines U251 (A), U87 (B), and U373 (C) were all inhibited.
ADGB knockdown inhibited the colony formation of glioma cell lines U251 and U87
To test the role of ADGB knockdown on the colony formation of glioma cell lines, glioma cells seeded onto 6-well plates were transfected with control or shADGB lentiviruses. After 10 days in culture, cells were fixed with methanol and stained with 0.5% crystal violet. The image was captured by a normal digital camera. The results showed that ADGB Knockdown reduced the colony numbers of U87 and U251 significantly (Figure 3).
Figure 3.
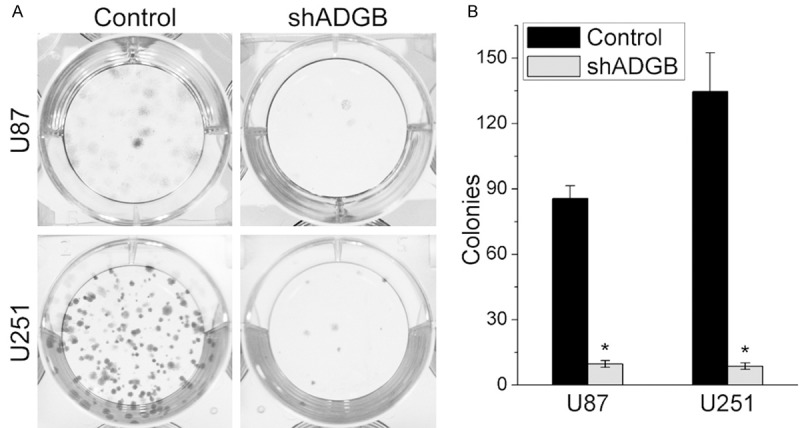
ADGB Knockdown inhibits the colony formation of glioma cell lines U251 and U87. Glioma cells seeded onto 6-well plates were transfected with control or shADGB lentiviruses. After 10 days in culture, cells were fixed with methanol and stained with 0.5% crystal violet. The image was captured by a normal digital camera. A. Reprsentative colony formation results of U251 and U87 cells. B. Quantification of colony numbers, Error bars represent SD. *, P < 0.05.
ADGB knockdown induced apoptosis in glioma cell line U251
To detect whether ADGB knockdown induce apoptosis of glioma cells, the Annexin V Apoptosis Detection Kit APC was used. The proportation of apoptotic cells assessed by flow cytometry assay was increased in U251 cell line (Figure 4). The percentage of Annexin V positive cells in ADGB knockdown group is 11.63%, while it is only 3.37% in control group.
Figure 4.
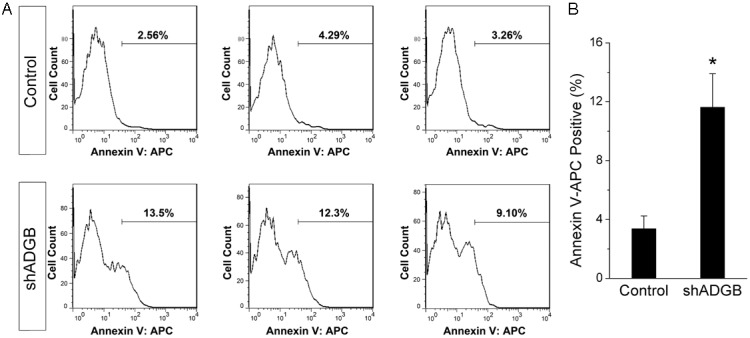
ADGB knockdown induces apoptosis in glioma cell line U251. Apoptosis of U251 cells were analyzed by flow cytometry. The number of Annexin V-APC positive apoptotic cells are shown as percentages of total cells. A. Histogram of Annexin V-APC positive cells. B. Bar chart of the percentage of Annexin V-APC positive cells. Error bars represent SD; n = 3. *, P < 0.05.
ADGB knockdown altered levels of proteins related to proliferation or apoptosis in U251 cells
Western blots showed that ADGB knockdown decreased the phosphorylated level of STAT3 and Akt (Figure 5A, 5B). ADGB knockdown also decreased the expression level of Cyclin D1 and Bcl-2, and increased the level of cleaved caspase-3, which is consistent with our above results. GAPDH was a sample loading control (Figure 5C).
Figure 5.
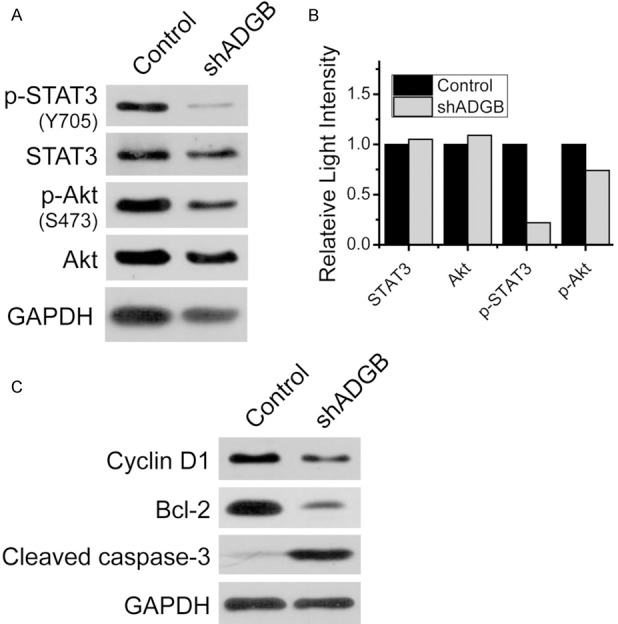
ADGB knockdown altered levels of proteins related to proliferation, survival or apoptosis in U251 cells. A. Western blot showed that ADGB knockdown decreased the phosphorylated levels of STAT3 and Akt. B. Grayscale ratios of STAT3/GAPDH or Akt/GAPDH revealed that the expression levels of STAT3 and Akt were not changed in ADGB knockdown group. Grayscale ratios of P-STAT3/STAT3 and P-Akt/Akt reveald that the phosphorylated levels of STAT3 and Akt were decreased in ADGB knockdown group. C. ADGB knockdown also decreased the expression level of Cyclin D1, Bcl-2 and increased the expression level of cleaved caspase-3. GAPDH was sample loading control.
Discussion
The roles of globins in cancers have drew much attention of scientists in recent years. The globins have been found in many kinds of cancers such as breast cancer [13], hepatocellular carcinoma [6], cervical cancer [14], and glioma [8]. Neuroglobin, cytoglobin, and hemoglobin have been found to be expressed in glioblastoma multiforme cells [4,7,8,10].
Previous studies have revealed that globins have diverse functions in cancers. Marwan Emara and his colleagues concluded that “the expression of different hemoglobins may constitute a part of series of active defense mechanisms supporting these cells to resist various types of treatments including chemotherapy and radiotherapy” [7]. Whether ADGB has similar functions in glioma cells is remain to be explored. Cytoglobin was firstly identified as a tumor suppressor [15,16], but further studies showed that cytoglobin also harbored oncogene functions [17]. Our results suggest that ADGB has the oncogene function in glioma cell lines.
Signal transducers and activators of transcription 3 (STAT3) is a transcription factor essential for cell growth, division, movement and apoptosis [18]. Our results showed that ADGB knockdown reduced the STAT3 activity, but the expression of its downstream target genes still needs further study. PI3K/Akt pathway is one of the major signaling pathways that are involved in the progression of glioma [19] and activation of the Akt pathway is sufficient to allow conversion of human anaplastic astrocytoma to human glioblastoma multiforme [19,20]. Our Preliminary data revealed that ADGB knockdown decreased the phosphorylated level of Akt, which suggested that ADGB might acting through Akt pathway. Blc-2 is a regulator of apoptosis, either inducing (pro-apoptotic) it or inhibiting it [21]. Cleaved caspase-3 is an executioner caspase in the execution-phase of cell apoptosis [22]. Our results about the changed level of Bcl-2 and cleaved caspase-3 suggested that ADGB knockdown triggered programmed cell death, which was consistant with the results of flow cytometry assay. In a word, ADGB knockdown inhibited the proliferation of glioma cells and induced their apoptosis in vitro.
Acknowledgements
This work was supported by the Exploratory Research Foundation of ECUST to Zelan Hu and Jing Zheng, and by the Shanghai Committee of Science and Techonology (Grant 11DZ226 0600).
Disclosure of conflict of interest
None.
References
- 1.Vinogradov SN, Hoogewijs D, Bailly X, Mizuguchi K, Dewilde S, Moens L, Vanfleteren JR. A model of globin evolution. Gene. 2007;398:132–142. doi: 10.1016/j.gene.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 2.Vinogradov SN, Hoogewijs D, Bailly X, Arredondo-Peter R, Gough J, Dewilde S, Moens L, Vanfleteren JR. A phylogenomic profile of globins. BMC Evol Biol. 2006;6:31. doi: 10.1186/1471-2148-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vinogradov SN, Moens L. Diversity of globin function: enzymatic, transport, storage, and sensing. J Biol Chem. 2008;283:8773–8777. doi: 10.1074/jbc.R700029200. [DOI] [PubMed] [Google Scholar]
- 4.Emara M, Turner AR, Allalunis-Turner J. Hypoxic regulation of cytoglobin and neuroglobin expression in human normal and tumor tissues. Cancer Cell Int. 2010;10:33. doi: 10.1186/1475-2867-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oleksiewicz U, Daskoulidou N, Liloglou T, Tasopoulou K, Bryan J, Gosney JR, Field JK, Xinarianos G. Neuroglobin and myoglobin in non-small cell lung cancer: expression, regulation and prognosis. Lung Cancer. 2011;74:411–418. doi: 10.1016/j.lungcan.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Lan SJ, Liu QR, Liu JM, Chen XQ. Neuroglobin, a novel intracellular hexa-coordinated globin, functions as a tumor suppressor in hepatocellular carcinoma via Raf/MAPK/Erk. Mol Pharmacol. 2013;83:1109–1119. doi: 10.1124/mol.112.083634. [DOI] [PubMed] [Google Scholar]
- 7.Emara M, Turner AR, Allalunis-Turner J. Adult, embryonic and fetal hemoglobin are expressed in human glioblastoma cells. Int J Oncol. 2014;44:514–520. doi: 10.3892/ijo.2013.2186. [DOI] [PubMed] [Google Scholar]
- 8.Emara M, Salloum N, Allalunis-Turner J. Expression and hypoxic up-regulation of neuroglobin in human glioblastoma cells. Mol Oncol. 2009;3:45–53. doi: 10.1016/j.molonc.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu HW, Huang YJ, Xie ZY, Lin L, Guo YC, Zhuang ZR, Lin XP, Zhou W, Li M, Huang HH, Wei XL, Man K, Zhang GJ. The expression of cytoglobin as a prognostic factor in gliomas: a retrospective analysis of 88 patients. BMC Cancer. 2013;13:247. doi: 10.1186/1471-2407-13-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang J, Ma I, Allalunis-Turner J. Knockdown of cytoglobin expression sensitizes human glioma cells to radiation and oxidative stress. Radiat Res. 2011;176:198–207. doi: 10.1667/rr2517.1. [DOI] [PubMed] [Google Scholar]
- 11.Hoogewijs D, Ebner B, Germani F, Hoffmann FG, Fabrizius A, Moens L, Burmester T, Dewilde S, Storz JF, Vinogradov SN, Hankeln T. Androglobin: a chimeric globin in metazoans that is preferentially expressed in Mammalian testes. Mol Biol Evol. 2012;29:1105–1114. doi: 10.1093/molbev/msr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu Z, Li K, Xu D, Liu Y, Tang H, Xie Q, Xie L, Liu J, Wang H, Gong Y, Hu Z, Zheng J. ZFX regulates glioma cell proliferation and survival in vitro and in vivo. J Neurooncol. 2013;112:17–25. doi: 10.1007/s11060-012-1032-z. [DOI] [PubMed] [Google Scholar]
- 13.Gorr TA, Wichmann D, Pilarsky C, Theurillat JP, Fabrizius A, Laufs T, Bauer T, Koslowski M, Horn S, Burmester T, Hankeln T, Kristiansen G. Old proteins - new locations: myoglobin, haemoglobin, neuroglobin and cytoglobin in solid tumours and cancer cells. Acta Physiol (Oxf) 2011;202:563–581. doi: 10.1111/j.1748-1716.2010.02205.x. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Wu Z, Wang Y, Mei Q, Fu X, Han W. Characterization of adult alpha- and beta-globin elevated by hydrogen peroxide in cervical cancer cells that play a cytoprotective role against oxidative insults. PLoS One. 2013;8:e54342. doi: 10.1371/journal.pone.0054342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shivapurkar N, Stastny V, Okumura N, Girard L, Xie Y, Prinsen C, Thunnissen FB, Wistuba II, Czerniak B, Frenkel E, Roth JA, Liloglou T, Xinarianos G, Field JK, Minna JD, Gazdar AF. Cytoglobin, the newest member of the globin family, functions as a tumor suppressor gene. Cancer Res. 2008;68:7448–7456. doi: 10.1158/0008-5472.CAN-08-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawada N. [Current situation and future prospect of cytoglobin research] . Nihon Rinsho. 2013;71:927–935. [PubMed] [Google Scholar]
- 17.Oleksiewicz U, Liloglou T, Tasopoulou KM, Daskoulidou N, Bryan J, Gosney JR, Field JK, Xinarianos G. Cytoglobin has bimodal: tumour suppressor and oncogene functions in lung cancer cell lines. Hum Mol Genet. 2013;22:3207–3217. doi: 10.1093/hmg/ddt174. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Crowe PJ, Goldstein D, Yang JL. STAT3 inhibition, a novel approach to enhancing targeted therapy in human cancers (review) Int J Oncol. 2012;41:1181–1191. doi: 10.3892/ijo.2012.1568. [DOI] [PubMed] [Google Scholar]
- 19.Jacques-Silva MC, Bernardi A, Rodnight R, Lenz G. ERK, PKC and PI3K/Akt pathways mediate extracellular ATP and adenosine-induced proliferation of U138-MG human glioma cell line. Oncology. 2004;67:450–459. doi: 10.1159/000082930. [DOI] [PubMed] [Google Scholar]
- 20.Sonoda Y, Ozawa T, Aldape KD, Deen DF, Berger MS, Pieper RO. Akt pathway activation converts anaplastic astrocytoma to glioblastoma multiforme in a human astrocyte model of glioma. Cancer Res. 2001;61:6674–6678. [PubMed] [Google Scholar]
- 21.Garcia-Saez AJ. The secrets of the Bcl-2 family. Cell Death Differ. 2012;19:1733–1740. doi: 10.1038/cdd.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicholson DW, Thornberry NA. Apoptosis. Life and death decisions. Science. 2003;299:214–215. doi: 10.1126/science.1081274. [DOI] [PubMed] [Google Scholar]


