Abstract
This study measured the antioxidant activity of follicular fluid (FF) in infertile patients and assessed its possible correlation between ovarian stimulation and pregnancy outcomes. Samples from 191 infertile patients undergoing in vitro fertilization-embryo transfer (IVF-ET) were determined by α-diphenyl-β-picrylhydrazyl (DPPH) radical scavenging, reducing power, superoxide radical scavenging, β-Carotene bleaching assay, ferrothiocyanate and thiobarbituric acid assays. The comparison between a positive IVF outcome and FF’s antioxidant activity was also studied. The results showed FF had strong antioxidant activity, which equated to common antioxidants Vc and BHT (100 μg/mL). Patients with endometriosis had less efficient antioxidant activity in FF than that of patients with tubal occlusion or polycystic ovary syndrome. In conclusion, this study detected, for the first time, the antioxidant activity of FF from patients undergoing an IVF and the FF exhibited strong antioxidant activity.
Keywords: Antioxidant activity, follicular fluid, in vitro fertilization, endometriosis
Introduction
Human follicular fluid (FF) forms the microenvironment of the developing oocyte and has an important influence on oocyte quality, sperm-oocyte interaction, sperm-mediated oocyte activation, embryo development and implantation [1-4]. FF is rich in low-molecular weight metabolites, which are direct or indirect regulators of oxidative stress and antioxidant activity [5]. Oxidative stress is defined as disequilibrium between the production and neutralization of reactive oxygen species (ROS), which may occur as a result of excess ROS production [6]. The ROS might further promote oxidative stress, generating peroxidation of lipids and of their degradation products and of the products formed by their interaction with low-density lipoproteins and other proteins. Peroxidized lipids, when undergoing decomposition, may generate products such as malondialdehyde (MDA) and may be recognized as foreign bodies, triggering an antigenic response with the consequent production of antibodies [7]. Thus, increased oxidative stress levels in the FF impair fertilization capabilities and embryonic development [8]. At the same time, FF has its antioxidant system, which include superoxide dismutase (SOD), selenium-dependent glutathione peroxidase (SeGPx), catalase (CAT), thioredoxin and glutathione peroxidase (GPx) [9-11], vitamins A, C, and E [12,13]. The antioxidant activity of the FF has a detrimental effect on oocyte development, embryo development and pregnancy outcome [14]. The free radical activity is also associated with parameters of ovarian responsiveness and in vitro fertilization (IVF) outcome [15]. This suggests clear associations between oxidative stress, antioxidant status and some aspects of ovarian stimulation and outcome, including pregnancy rate [16].
To the best of our knowledge, although there were some researches detected the antioxidant status of FF, the antioxidant capacity of FF from patients undergoing IVF, as yet, had not been reported. Current study was performed with the aim of evaluating the systemic antioxidant activity in the occyte maturation environment and correlating the results with the outcome of controlled ovarian hyperstimulation (COH) and clinical pregnancy rate. For this purpose, FF samples from 191 infertile patients undergoing IVF were determined by α,α-diphenyl-β-picrylhydrazyl (DPPH) radical scavenging, reducing power, superoxide radical scavenging, β-Carotene bleaching assay, ferrothiocyanate and thiobarbituric acid assays.
Materials and methods
Chemicals
α,α-diphenyl-β-picrylhydrazyl (DPPH) was purchased from Sigma-Aldrich (St. Louis, MO). Linoleic acid was purchased from Alfa Aesar (Ward Hill, MA). β-carotene was purchased from Fluka (Menlo Park, CA). Ascorbic acid (Vitamin C), gallic acid and butylated hydroxytoluene (BHT) were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). All other chemicals used for analysis were AnalaR grade and obtained from China Medicine (Group) Shanghai Chemical Reagent Corporation (Shanghai, China).
Study protocol
Ethics approval for this project was obtained from the ethics committee of Tongji Hospital (reference number 130302, approved 10 September 2012). Enrolment occurred between November 2012 and February 2013. All patients who signed written informed consent were undergoing IVF treatment at the Reproductive Medicine Centre, Tongji Hospital.
A total of 191 infertile patients from 22 to 41 years of age (mean, 30.3 ± 4.0) were divided into three groups by different reasons for infertility: tubal occlusion (TO, n = 113), polycystic ovary syndrome (PCOS, n = 39), endometriosis (EMT, n = 39). All women were non-smokers and had been unable to be pregnant naturally for at least one year. A total of 2784 oocytes were retrieved from all patients and 2484 of them were matured oocytes (matured oocyte rate, 89.2%). The protocol for COH according to the procedure was described by our previously published method [17]. Briefly, it consisted of pituitary suppression with the gonadotropin-releasing hormone analogue triptorelin acetate (Decapeptyl, Ferring) administered starting in the midluteal phase of the preceding cycle. When complete pituitary desensitization was confirmed by a low plasma E2 level of ~30 pg/mL and an LH level of ~2 mIU/mL, ovarian stimulation was started with administration of recombinant FSH (Gonal F, Serono; or Puregon, Schering-Plough). Recombinant hCG (250 mg; Ovidrel; Serono) was given to trigger ovulation when two leading follicles reached a mean diameter of 18 mm. Oocytes were retrieved transvaginally 34-36 hours after hCG administration. Usually fewer than two best-quality embryos were transfered on day 2 or 3 after oocyte retrieval, and excess good-quality embryos were cryopreserved for subsequent frozen-embryo transfer (FET) cycles.
Collection and processing of FF
FF were collected during oocyte retrieval by follicle aspiration under ultrasonographic control and centrifuged at 1000×g for 10 min. The clear supernatant was divided into aliquots and frozen immediately at -80°C. Only material from the first aspirated follicle (diameter 20.7 ± 2.3 mm) was used in order to avoid contamination with blood. No contaminated samples were used for further analysis.
IVF procedure
The methods for sperm preparation, IVF and embryo culture have been described previously [18]. Briefly, oocytes and embryos were cultured in sequential embryo culture media. Fertilization check was performed 16-18 h after intracytoplasmic sperm injection. The presence of two pronuclei was defined as normal fertilization and normally fertilized oocytes were continuously cultured in G1 medium (Vitrolife, Sweden) for two more days. Morphological evaluation of the embryos was performed on day 2 (48 h) and day 3 (72 h) based on number of blastomeres, rate of fragmentation, multinucleation of the blastomeres and early compaction [19]. Day 2 or 3 embryos were scored on a scale of 1 (high grade) to 4 (low grade) [20].
DPPH radical scavenging assay
The free-radical scavenging capacity of FF was determined using DPPH according to the procedure described by published method with slight modifications [21]. 0.4 mL FF sample was mixed with 3.6 mL 0.2 mM DPPH in methanol. The mixture was centrifuged at 1000×g for 10 min. The absorbance of clear supernatant was measured at 517 nm. Radical scavenging activity was calculated as the following percentage: [(ADPPH - AS)/ADPPH] ×100 (ADPPH = absorbance of DPPH alone and AS= absorbance of DPPH in the presence of different FF sample). A concentration of Vc, gallic acid and BHT (100 μg/mL) that were identical to the experimental samples were used as reference.
Reducing power assay
The reducing power of FF sample was measured by the method of Oyaizu [22]. Briefly, FF sample was mixed with 2.5 mL potassiumferricyanide (1%), 2.5 mL sodium phosphate buffer (0.2 M, pH 6.6) and 2.5 mL trichloroacetic acid (10%). Mixture was centrifuged at 1000×g for 10 min. 2.5 mL Distilled water and 0.5 mL ferric chloride (0.1%) were mixed with 2.5 mL upper layer, and absorbance was determined at 700 nm. Increased absorbance of the sample indicated increased reducing power. A concentration of Vc, gallic acid and BHT (100 μg/mL) were used as positive comparison.
Superoxide radical scavenging assay
The activity of FF to scavenge superoxide radicals was determined by a pyrogallol auto-oxidation system [23] with slight modifications. Reaction mixtures containing 0.5 mL FF sample in 4.50 mL Tris-HCl buffer, and then 150 μL of pyrogallic acid (3 mM) was added. The absorbance of the sample at 325 nm was determined immediately, and then at 30 s intervals thereafter. The auto-oxidation rate constant (Kb) was calculated from the curve of A325nm vs time. The control did not contain FF sample and a concentration of Vc, gallic acid and BHT (200 μg/mL) identical to the samples was used as a standard.
β-Carotene bleaching assay
Antioxidant capacity of FF was evaluated according to the β-carotene bleaching method [24] with slight modifications. Four mililitres of β-carotene solution were pipetted into a round-bottom flask. After chloroform was removed under vacuum, 80 mg of purified linoleic acid, 800 mg of Tween 80 emulsifier, and 200 mL of aerated distilled water were added to the flask with vigorous shaking. 3.0 mL of this emulsion were added into different tubes containing 0.2 mL FF. Vc, gallic acid and BHT (200 μg/mL) were used for comparative purposes. The zero time absorbance was measured at 470 nm when the emulsion was added into tubes and then recorded absorbance at 30-min intervals in 2 h. Lipid peroxidation (LPO) inhibition was calculated by the equation: LPO inhibition = [(As - Ai)/ As] ×100 (As = absorbance of contorl of assay; Ai = absorbance of 2 h later of assay).
Antioxidant activity in a linoleic acid system using ferrothiocyanate (FTC) and thiobarbituric acid (TBA)
The FTC method was adapted from Osawa and Namiki [25]. 1 mL FF was mixed with 1 mL 2.5% linoleic acid, 2 mL phosphate buffer (50 mM), and 1 mL distilled water. 0.1 mL Aliquots were mixed with 9.7 mL ethanol and 0.1 mL ammonium thiocyanate. 0.1 mL ferrous chloride (20 mM) was added to each sample, and absorbance was measured at 500 nm. Aliquots were withdrawn and assayed in an identical fashion at 24 h intervals until a constant maximum value was reached. Controls without sample and standard containing Vc, gallic acid and BHT (100 μg/mL) in place of FF sample were subjected to the same procedure.
The method of Kikuzaki and Nakatani [26] using TBA was also employed to determine the antioxidant capacity of FF. 1 mL TBA and 1 mL trichloroacetic acid were added to 0.5 mL of reaction solution that was prepared as described in the FTC method. The mixture was placed in a boiling water bath for 15 min and centrifuged at 1500×g for 20 min. Absorbance of the supernatant was determined at 532 nm. The inhibition rate was calculated by the following equation: [(Ac - As)/Ac]c ×100 (Ac = absorbance of control; As = absorbance of sample).
Antioxidant scores of FF samples
The score of each antioxidant assay was calculated using the following equation: Vs/Vmin (Vs = sample value of single antioxidant assay, Vmin = minimum value of single antioxidant assay). The antioxidant scores of FF samples were the total scores of five antioxidant assays.
Pregnancy
Serum human chorionic gonadotrophin was measured for diagnosis of pregnancy 2 weeks after embryo transfer and then was tested serially to monitor the rise in its titre. Clinical pregnancy was defined as the presence of a gestational sac with fetal heart activity on ultrasound examination 5 weeks after oocyte retrieval.
Statistics
All data analysis was performed using Statistical Package for Social Sciences (SPSS) version 13.0. Between-groups data was analysed using either the nonparametric Mann-Whitney U-test (for two groups) or one-way ANOVA (for more than two groups). The clinical pregnancy rates of different groups were analysed by Chi-squared test. Statistical significance was determined at P < 0.05.
Results
In the DPPH radical scavenging assay (Figure 1A), the FF from 191 patients had a wide range in DPPH radical scavenging percentage from 22.08% to 47.82% (mean, 33.82 ± 6.56%). The DPPH radical scavenging ability of patients with TO, PCOS and EMT were 34.11 ± 6.45%, 33.88 ± 7.14% and 32.93 ± 6.39%, respectively. Although EMT group had lower DPPH radical scavenging percentage, there were no significant differences in three groups and standard antioxidants (BHT and Vc). In addition, the DPPH radical scavenging ability of GA (81.51 ± 1.38%) was significant higher than others.
Figure 1.
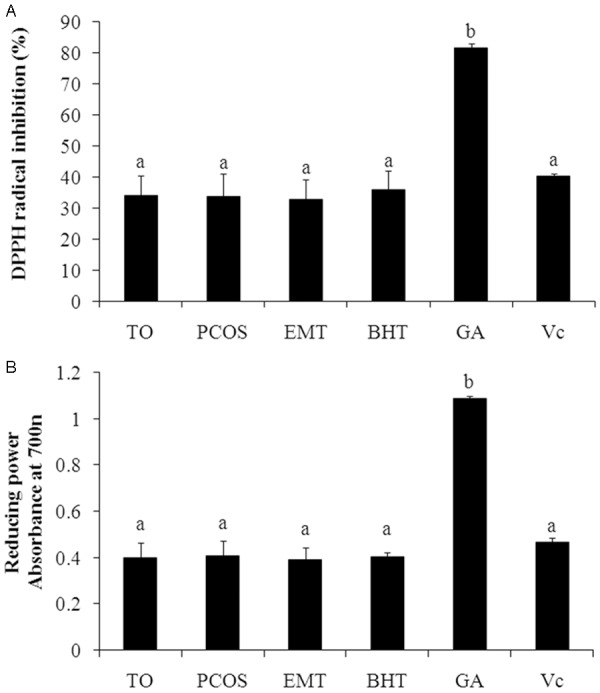
Antioxidant activities of follicular fluid (FF) and reference standards. A. DPPH radical scavenging capacity of FF; B. Reducing power of FF. TO, tubal occlusion; PCOS, polycystic ovary syndrome; EMT, endometriosis; DPPH, α,α-diphenyl-β-picrylhydrazyl; Vc, ascorbic acid; GA, gallic acid; BHT, butylated hydroxytoluene.
As indicated in Figure 1B, all the FF samples showed different reducing capability with the absorbance from 0.308 to 0.532 (mean, 0.399 ± 0.070). The reducing power of patients with TO, PCOS and EMT were 0.400 ± 0.064, 0.406 ± 0.067% and 0.389 ± 0.054%, respectively. And no significant difference was obtained from these three groups. The results of comparison between FF and standard antioxidants (BHT, Vc and GA) had similar patterns to DPPH radical scavenging assay.
In superoxide radical scavenging assay, the scavenging effect of all FF sample on the superoxide anion radical was as effective as BHT as an antioxidant, but less efficient than Vc and GA (Table 1). The PCOS group had stronger scavenging effect than TO and EMT groups, but no significant difference between them (P > 0.05).
Table 1.
Inhibition of pyrogallic acid auto-oxidation by the follicular fluid samples
| Sample | Control | TO | PCOS | EMT | BHT | GA | Vc |
|---|---|---|---|---|---|---|---|
| Kb value (×10-4) | 14.97 ± 0.22a | 13.84 ± 0.29b | 13.77 ± 0.31b | 14.05 ± 0.33b | 13.82 ± 0.18b | 4.26 ± 0.20c | 1.46 ± 0.10d |
TO, tubal occlusion; PCOS, polycystic ovary syndrome; EMT, endometriosis; BHT, butylated hydroxytoluene; GA, gallic acid; Vc, ascorbic acid. Different letters differed significantly (P < 0.05).
The results of β-Carotene bleaching assay showed that FF was more efficient than Vc and GA, but less efficient than BHT (Figure 2A). The results of LPO also proved the antioxidant activity of FF samples (Figure 2B).
Figure 2.
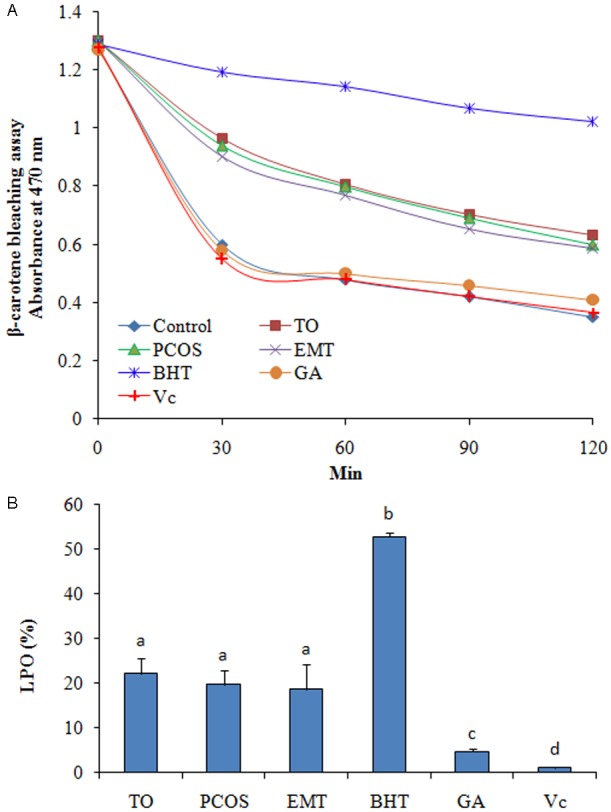
Antioxidant activity of follicular fluid (FF) determined using β-Carotene bleaching assay. TO, tubal occlusion; PCOS, polycystic ovary syndrome; EMT, endometriosis; DPPH, α,α-diphenyl-β-picrylhydrazyl; Vc, ascorbic acid; GA, gallic acid; BHT, butylated hydroxytoluene.
The antioxidant activity exhibited by FF according to the FTC methods was shown in Figure 3A. The results showed that all FF sample had strong antioxidant activity, but less efficient than BHT. The results in Figure 3B showed that the antioxidant activity of TO group had significantly stronger than EMT group (P < 0.05).
Figure 3.
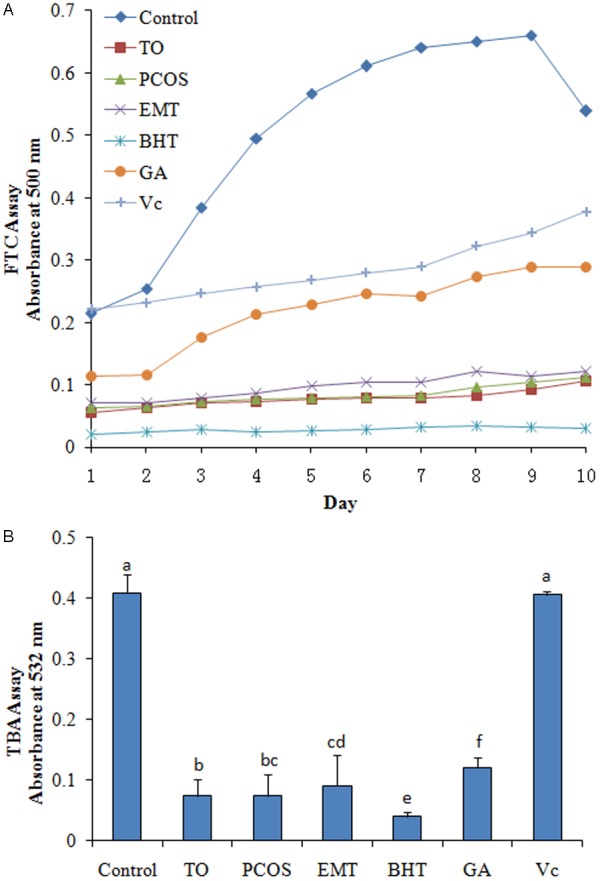
Antioxidant activity monitored daily using the FTC and TBA method. A. FTC assay; B. TBA assay. TO, tubal occlusion; PCOS, polycystic ovary syndrome; EMT, endometriosis; DPPH, α,α-diphenyl-β-picrylhydrazyl; Vc, ascorbic acid; GA, gallic acid; BHT, butylated hydroxytoluene.
The comparison between a positive IVF outcome and FF’s antioxidant activity was studied. Of the enrolled patients, 144 had embryos transferred, resulting in a clinical pregnancy rate of 47.9% (n = 69) per embryo transfer. No statistically significant differences were shown for all of the parameters (Table 2). Although the antioxidant score of pregnant group was higher than that of not pregnant group, there was no significant difference between them (P > 0.05). In different enrolled groups, the antioxidant scores of TO and PCOS group were significant higher than EMT group (P < 0.05) (Figure 4).
Table 2.
Nonparametric Mann-Whitney U-test was used to compare physiological parametersbetween the all patients with different pregnancy outcome
| Pregnant (n = 69) | Not pregnant (n = 75) | |
|---|---|---|
|
|
||
| Mean ± SD | Mean ± SD | |
| Age | 30.5 ± 4.1 | 30.0 ± 4.0 |
| No. of oocytes | 11.2 ± 5.3 | 12.3 ± 5.1 |
| Total Gn (IU) | 2186 ± 462 | 2199 ± 405 |
| Mature oocyte (%) | 91.7 ± 7.2 | 90.9 ± 8.1 |
| Fertilization (%) | 69.5 ± 15.6 | 66.67 ± 14.8 |
| Day 2 grade 1 embryo (%) | 37.8 ± 19.2 | 33.33 ± 18.9 |
| Day 2 grade 1+2 embryo (%) | 66.2 ± 20.2 | 65.8 ± 18.6 |
| Day 3 grade 1 embryo (%) | 30.1 ± 15.7 | 25.00 ± 16.2 |
| Day 3 grade 1+2 embryo (%) | 64.1 ± 20.3 | 57.9 ± 18.5 |
| No. of transferred embryos | 2.01 ± 0.21 | 1.97 ± 0.19 |
| DPPH radical scavenging assaya | 36.3 ± 6.43 | 34.8 ± 6.2 |
| Reducing power assayb | 0.43 ± 0.06 | 0.41 ± 0.06 |
| Superoxide radical scavenging assayc | 13.8 ± 0.33 | 13.7 ± 0.31 |
| β-Carotene bleaching assayd | 21.2 ± 2.72 | 20.3 ± 2.31 |
| TBA assaye | 0.08 ± 0.03 | 0.08 ± 0.04 |
| Antioxidant scores | 8.16 ± 1.10 | 8.01 ± 1.17 |
DPPH radical scavenging percentage (%);
Absorbance at 700 nm;
Auto-oxidation rate constant of pyrogallic acid;
Lipid peroxidation inhibition rate (%);
Absorbance at 532 nm.
Gn, gonadotropin; DPPH, α,α-diphenyl-β-picrylhydrazyl; TBA, thiobarbituric acid.
Figure 4.
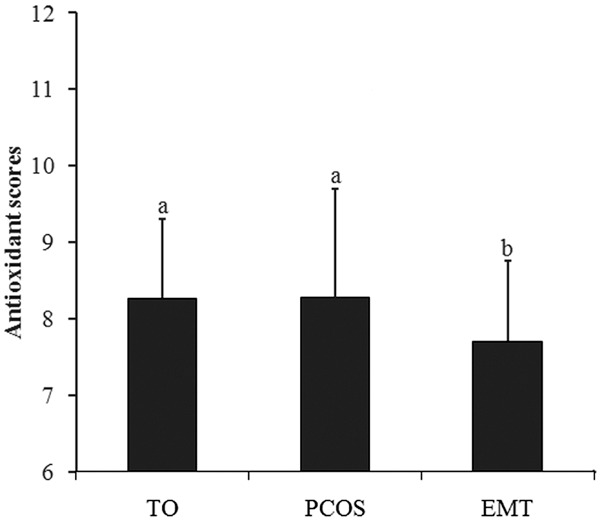
Antioxidant scores of different enrolled groups. TO, tubal occlusion; PCOS, polycystic ovary syndrome; EMT, endometriosis. Different letters differed significantly (P < 0.05).
Discussion
In this work we determined, for the first time, the systemic antioxidant activity of FF from patients undergoing an IVF by five different methods: DPPH radical scavenging, reducing power, superoxide radical scavenging, β-Carotene bleaching assay, ferrothiocyanate and thiobarbituric acid assays. We found the FF from patients undergoing an IVF had strong antioxidant activity and patients with endometriosis had less efficient antioxidant activity in FF than that of patients with tubal occlusion or polycystic ovary syndrome.
DPPH was characterized as a stable free radical by virtue of the delocalisation of the spare electron over the molecule as a whole, so that the molecule does not dimerize, as would be the case with most other free radicals. The delocalization of electron also gives rise to the deep violet color, characterized by an absorption band in ethanol solution centered at about 517 nm. When a solution of DPPH was mixed with that of a substrate that can donate a hydrogen atom, then this gives rise to the reduced form with the loss of this violet color [27]. Moreover, DPPH was the most easy, simple and reasonably costly method and hence it might have been used mostly for the antioxidant activity evaluation of a sample [28]. The reducing power assay was also an important determination of the antioxidant activities of samples. The reducing power of FF was determined by measuring the increase in absorbance at 700 nm upon conversion of ferric ions (Fe3+) to ferrous ions (Fe2+) in the presence of potassium ferricyanide [29]. Increase in absorbance of the reaction mixture indicates an increase in the reducing power of the FF samples. In our study, the FF showed a certain activity of scavenging DPPH radical and reducing power, which equated to common antioxidants Vc and BHT (100 μg/mL).
The superoxide anion radical (·O2 -) was the most common free radical generated by endogenous metabolism. Pyrogallic acid, as an ·O2 - generator, has been often used to investigate the role of ROS in the biological system [30]. Therefore, the free radical scavengers in the FF may slow down the rate of ·O2 - generation. Our result showed the scavenging effect of FF on the superoxide anion radical was as efficient as BHT. It suggested that the FF was capable of scavenging superoxide radicals, and could help prevent or ameliorate oxidative damage.
The antioxidant activity of carotenoids was based on the radical adducts of carotenoid with free radicals from linoleic acid [24]. The linoleic acid free radical attacks the highly unsaturated β-carotene models, causes the β-carotene to lose its chromophore and orange color [31]. The antioxidants from FF can prevent this process and decrease the extent of discoloration, which was measured at 434 nm.
The FTC assay was used to measure the lipid peroxidation. In this study, results showed the absorbance of the control showed a steady increase and reached peak value in day 9. BHT showed a high capacity to inhibit linoleic acid peroxidation with almost constant absorbance. The absorbance of FF increased slowly, which indicates a strong potential for antioxidant ability. Lipid peroxidation with Vc and GA were inferior to the FF and BHT, but stronger than that of the control. Hence, FF had a stronger antioxidant activity than Vc and GA but were less efficient than BHT. During the boiling water treatment, the lower molecular weight malondialdehyde may be measured by the TBA assay, providing a means to evaluate the extent of lipid peroxidation [32]. The TBA results were in agreement with those obtained by the FTC method. In addition, the data obtained demonstrated that the endometriosis group was lower than others in inhibitory ability on lipid oxidation, in agreement with data reported by Petean et al [13]. Overall, these lipid peroxidation results from both the FTC and TBA assays indicated FF had stronge inhibitory ability on lipid oxidation.
All of these proved that FF from patients undergoing an IVF had strong antioxidant activity. We believed the antioxidant compounds in FF play an important role in antioxidant activity. The results from many researches showed the FF had various antioxidant compositions, which including SOD, SeGPx, CAT, GPx [9-11], vitamins A, C, E [12,13] and amino acids [33]. Our results indicated the difference between TO and PCOS group was not significant in antioxidant activity. Similar to our results, Rodrigues, et al [34] found there were no significant differences in oxidative stress markers levels in FF between the PCOS group and tubal factor group. However, to the best of our knowledge, few studies had tested the antioxidant activity of FF by these assays. Therefore, we believed that DPPH radical scavenging, reducing power, superoxide radical scavenging, β-Carotene bleaching assay, ferrothiocyanate and thiobarbituric acid assays were another options to measure the antioxidant activity of human FF.
The physiological parameters between the all patients with different pregnancy outcome were compared in present study. In spite of no significant differences between two groups, there were some information claimed our attention. Previously, with conflicting information, Pasqualotto et al [35] found lipid peroxidation and antioxidant capacity were positively correlated with the pregnancy rate. Oral et al [36] showed that pregnancy rates were found to be decreasing in higher lipid peroxidation levels. In current study, the results of five antioxidant activity assays and antioxidant scores of pregnant groups had slightly better than not pregnant group, but no significant difference was obtained between them. Similar results was reported in Velthut et al.’s research [16]. Therefore, the antioxidant activity of FF needs to be better elucidated in studies on larger series. The mechanism of how systemic antioxidant activity influences folliculogenesis could not be concluded.
For the issue of lower antioxidant scores of endometriosis patients, just like Petean et al. [13] said, the possible occurrence of more serious lipid peroxidation in infertile women with endometriosis, which may contributed, at least in part, to the worse oocyte quality that was related to that disease. This also has been supported by Garrido et al [37]. In contrast, Attaran et al [38] showed that patients with endometriosis and male factor infertility who became pregnant after COH for embryo transfer after IVF presented higher levels of lipid peroxidation in FF than did those who did not get pregnant. Therefore, further studies conducted on larger series will be important for the elucidation of the role of FF’s antioxidant activity in endometriosis.
In conclusion, this primary study detected, as far as was known for the first time, the systemic antioxidant activity of FF from patients undergoing an IVF. The FF showed strong antioxidant activity. Although we did not find a significant difference in antioxidant activity of FF from patients with different pregnancy outcome, we observed a tendency to higher antioxidant activity in the FF of patients with positive pregnancy outcome than in negative group. The FF’s antioxidant score of EMT patients was significant lower than that of TO and PCOS patients. The researches of antioxidant activity of FF in patients undergoing IVF was still few, hence the antioxidant compound of FF and mechanism of how antioxidant activity influences folliculogenesis needs to be better evaluated in our future studies on larger series.
Disclosure of conflict of interest
None.
References
- 1.Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H, Taketani T, Matsuoka A, Yamagata Y, Shimamura K, Morioka H, Ishikawa H, Reiter RJ, Sugino N. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res. 2008;44:280–287. doi: 10.1111/j.1600-079X.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- 2.Taketani T, Tamura H, Taniguchi K, Maekawa R, Sugino N. Influence of oxidative stress on oocyte quality and protective role of melatonin as an antioxidant. Biol Reprod. 2007;77:218. [Google Scholar]
- 3.Tarin JJ, Vendrell FJ, Ten J, Blanes R, van Blerkom J, Cano A. The oxidizing agent tertiary butyl hydroperoxide induces disturbances in spindle organization, c-meiosis, and aneuploidy in mouse oocytes. Mol Hum Reprod. 1996;2:895–901. doi: 10.1093/molehr/2.12.895. [DOI] [PubMed] [Google Scholar]
- 4.Saleh RA, Agarwal A, Nada EA, El-Tonsy MH, Sharma RK, Meyer A, Nelson DR, Thomas AJ. Negative effects of increased sperm DNA damage in relation to seminal oxidative stress in men with idiopathic and male factor infertility. Fertil Steril. 2003;79(Suppl 3):1597–1605. doi: 10.1016/s0015-0282(03)00337-6. [DOI] [PubMed] [Google Scholar]
- 5.Singh R, Sinclair KD. Metabolomics: approaches to assessing oocyte and embryo quality. Theriogenology. 2007;68(Suppl 1):56–62. doi: 10.1016/j.theriogenology.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Diplock AT. Antioxidants and free radicals scavengers. In: Rice-Evans CA, Burdon RH, editors. Free radical damage and its control. Amsterdam: Elsevier; 1994. p. 113. [Google Scholar]
- 7.Murphy AA, Santanam N, Parthasarathy S. Endometriosis: a disease of oxidative stress? Semin Reprod Endocrinol. 1998;16:263–273. doi: 10.1055/s-2007-1016286. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003;79:829–843. doi: 10.1016/s0015-0282(02)04948-8. [DOI] [PubMed] [Google Scholar]
- 9.Carbone MC, Tatone C, Delle Monache S, Marci R, Caserta D, Colonna R, Amicarelli F. Antioxidant enzymatic defences in human follicular fluid: characterization and age-dependent changes. Mol Hum Reprod. 2003;9:639–643. doi: 10.1093/molehr/gag090. [DOI] [PubMed] [Google Scholar]
- 10.Knapen MF, Zusterzeel PL, Peters WH, Steegers EA. Glutathione and glutathione-related enzymes in reproduction. A review. Eur J Obstet Gynecol Reprod Biol. 1999;82:171–184. doi: 10.1016/s0301-2115(98)00242-5. [DOI] [PubMed] [Google Scholar]
- 11.Lambrinoudaki IV, Augoulea A, Christodoulakos GE, Economou EV, Kaparos G, Kontoravdis A, Papadias C, Creatsas G. Measurable serum markers of oxidative stress response in women with endometriosis. Fertil Steril. 2009;91:46–50. doi: 10.1016/j.fertnstert.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 12.Ozkaya MO, Naziroglu M. Multivitamin and mineral supplementation modulates oxidative stress and antioxidant vitamin levels in serum and follicular fluid of women undergoing in vitro fertilization. Fertil Steril. 2010;94:2465–2466. doi: 10.1016/j.fertnstert.2010.01.066. [DOI] [PubMed] [Google Scholar]
- 13.Campos Petean C, Ferriani RA, dos Reis RM, de Moura MD, Jordão AA Jr, Navarro PA. Lipid peroxidation and vitamin E in serum and follicular fluid of infertile women with peritoneal endometriosis submitted to controlled ovarian hyperstimulation: a pilot study. Fertil Steril. 2008;90:2080–2085. doi: 10.1016/j.fertnstert.2007.10.072. [DOI] [PubMed] [Google Scholar]
- 14.Palhares MB Jr, Romão GS, Da Broi MG, Martins WP, Jordão AA Jr. Serum and follicular oxidative stress biomarkers levels and response to controlled ovarian hyperstimulation (COH) in IVF cycles. Fertil Steril. 2012;98:241. [Google Scholar]
- 15.Wiener-Megnazi Z, Vardi L, Lissak A, Shnizer S, Reznick AZ, Ishai D, Lahav-Baratz S, Shiloh H, Koifman M, Dirnfeld M. Oxidative stress indices in follicular fluid as measured by the thermochemiluminescence assay correlate with outcome parameters in in vitro fertilization. Fertil Steril. 2004;82(Suppl 3):1171–1176. doi: 10.1016/j.fertnstert.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Velthut A, Zilmer M, Zilmer K, Kaart T, Karro H, Salumets A. Elevated blood plasma antioxidant status is favourable for achieving IVF/ICSI pregnancy. Reprod Biomed Online. 2013;26:345–352. doi: 10.1016/j.rbmo.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Xu B, Li Z, Zhang H, Jin L, Li Y, Ai J, Zhu G. Serum progesterone level effects on the outcome of in vitro fertilization in patients with different ovarian response: an analysis of more than 10,000 cycles. Fertil Steril. 2012;97:1321–7. doi: 10.1016/j.fertnstert.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 18.Zhu L, Xi Q, Zhang H, Li Y, Ai J, Jin L. Blastocyst culture and cryopreservation to optimize clinical outcomes of warming cycles. Reprod Biomed Online. 2013;27:154–160. doi: 10.1016/j.rbmo.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Papanikolaou EG, D’haeseleer E, Verheyen G, Van de Velde H, Camus M, Van Steirteghem A, Devroey P, Tounaye H. Live birth rate is significantly higher after blastocyst transfer than after cleavage-stage embryo transfer when at least four embryos are available on day 3 of embryo culture. A randomized prospective study. Hum Reprod. 2005;20:3198–3203. doi: 10.1093/humrep/dei217. [DOI] [PubMed] [Google Scholar]
- 20.Veeck LL. An atlas of human gametes and conceptuses: an illustrated reference for assisted reproductive technology. New York: Parthenon Publishing; 1999. [Google Scholar]
- 21.Huang B, Ban X, He J, Tong J, Tian J, Wang Y. Comparative analysis of essential oil components and antioxidant activity of extracts of Nelumbo nucifera from various areas of China. J Agric Food Chem. 2010;58:441–448. doi: 10.1021/jf902643e. [DOI] [PubMed] [Google Scholar]
- 22.Oyaizu M. Studies on products of browning reactions: antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–315. [Google Scholar]
- 23.Xiang Z, Ning Z. Scavenging and antioxidant properties of compound derived from chlorogenic acid in South-China honeysuckle. LWT - Food Science and Technology. 2008;41:1189–1203. [Google Scholar]
- 24.Shon MY, Kim TH, Sung NJ. Antioxidants and free radical scavenging activity of Phellinus baumii (Phellinus of Hymenochaetaceae) extracts. Food Chem Toxicol. 2003;82:593–597. [Google Scholar]
- 25.Osawa T, Namiki M. A novel type of antioxidant isolated from leaf wax of Eucalyptus leaves. Agricultural and Biological Chemistry. 1981;45:735–739. [Google Scholar]
- 26.Kikuzaki H, Nakatani N. Antioxidant effects of some ginger constituents. Journal of Food Science. 1993;58:1407–1410. [Google Scholar]
- 27.Friaa O, Chaleix V, Lecouvey M, Brault D. Reaction between the anesthetic agent propofol and the free radical DPPH in semiaqueous media: kinetics and characterization of the products. Free Radic Biol Med. 2008;45:1011–1018. doi: 10.1016/j.freeradbiomed.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Alam MN, Bristi NJ, Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm J. 2013;21:143–152. doi: 10.1016/j.jsps.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meir S, Kanner J, Akiri B, Hadas SP. Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves. J Agric Food Chem. 1995;43:1813–1815. [Google Scholar]
- 30.Wang J, Zhu J, Liu S, Liu B, Gao Y, Wu Z. Generation of reactive oxygen species in cyanobacteria and green algae induced by allelochemicals of submerged macrophytes. Chemosphere. 2011;85:977–982. doi: 10.1016/j.chemosphere.2011.06.076. [DOI] [PubMed] [Google Scholar]
- 31.Jayaprakasha GK, Singh RP, Sakariah KK. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem. 2001;73:285–290. [Google Scholar]
- 32.Guillen-Sans R, Guzman-Chozas M. The thiobarbituric acid (TBA) reaction in foods: a review. Food Chem. 1998;38:315–330. doi: 10.1080/10408699891274228. [DOI] [PubMed] [Google Scholar]
- 33.Jozwik M, Teng C, Battaglia FC. Amino acid, ammonia and urea concentrations in human pre-ovulatory ovarian follicular fluid. Hum Reprod. 2006;21:2776–2782. doi: 10.1093/humrep/del038. [DOI] [PubMed] [Google Scholar]
- 34.Rodrigues JK, Dib LA, Donabella FC, Ferriani RA, Jordão AA Jr, Navarro PAAS. Oxidative stress markers in follicular fluid (FF) and assisted reproduction outcomes in patients with infertility due to polycystic ovary syndrome (PCOS) or to tubal and/or male factor. Fertil Steril. 2010;94:S144. [Google Scholar]
- 35.Pasqualotto EB, Agarwal A, Sharma RK, Izzo VM, Pinotti JA, Joshi NJ, Rose BI. Effect of oxidative stress in follicular fluid on the outcome of assisted reproductive procedures. Fertil Steril. 2004;81:973–976. doi: 10.1016/j.fertnstert.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 36.Oral O, Kutlu T, Aksoy E, Ficicioglu C, Uslu H, Tugrul S. The effects of oxidative stress on outcomes of assisted reproductive techniques. J Assist Reprod Genet. 2006;23:81–85. doi: 10.1007/s10815-005-9010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garrido N, Navarro J, Garcia-Velasco J, Remoh J, Pellice A, Simon C. The endometrium versus embryonic quality in endometriosis-related infertility. Hum Reprod Update. 2002;8:95–103. doi: 10.1093/humupd/8.1.95. [DOI] [PubMed] [Google Scholar]
- 38.Attaran M, Pasqualotto E, Falcone T, Goldberg JM, Miller KF, Agarwal A, Sharma RK. The effect of follicular fluid reactive oxygen species on the outcome of in vitro fertilization. Int J Fertil Womens Med. 2000;45:314–320. [PubMed] [Google Scholar]


