Abstract
Endolymphatic sac tumor (ELST) is a rare low-grade locally aggressive neoplasm of the inner ear that may occur sporadically or in the setting of von Hippel-Lindau syndrome. We herein present a case of sporadic ELST in a 39-year-old man, treated using an interdisciplinary approach (surgery + radiotherapy), with a 10-year follow-up. The patient presented with hearing loss of sudden onset. The treatment of choice for ELST is radical tumor resection, which is associated with a good long-term prognosis. Remission may last for years, but there may be local recurrences, probably as a result of incomplete resection. Adjuvant radiotherapy is an option in case of recurrence and could be discussed after incomplete resection. The purpose of this report is to call attention to ELSTs, which are difficult to diagnose due to their rarity and variety of presentations.
Keywords: Lateral skull base, endolymphatic sac, hearing loss, vertigo, tinnitus
Introduction
Endolymphatic sac tumors (ELSTs) are rare low-grade papillary epithelial neoplasms (adenocarcinomas) with a slow growth pattern. The lesion was first described by Hassard et al. in 1984 [1]. The tumors show locally invasive and infiltrative growth, but are not known to metastasize [1]. ELST has been published under different names in the literature (Heffner tumor, aggressive papillary middle ear tumor, and low-grade adenocarcinoma of endolymphatic sac origin). ELST may arise sporadically or in von Hippel-Lindau (VHL) disease [2]. The symptoms (hearing loss with or without tinnitus, vertigo, impairment of cranial nerve function) and the physical and neuro-otologic examination and imaging findings (computed tomography, magnetic resonance imaging) are not specific for tumors of the cerebellopontine angle (CPA) [3]. The final diagnosis can often only be reached through histopathological and immunohistochemical evaluation of the tumor specimen. The differential diagnosis in tumors of the CPA includes paraganglioma, chondroid tumor, atypical schwannoma, meningioma, and also rare entities such as aneurysm, hemangioblastoma, craniopharyngioma, and choroid plexus papilloma. According to a recently published literature review, some 150 cases of ELST have been reported to date [4]. We present here a further case of ELST that was treated using an interdisciplinary approach, with a 10-year follow-up period.
Case decription
A 39-year-old man presented in May 2003 with left-sided hearing loss that had developed suddenly 4 months previously. The patient had been exposed to noise for many years in his job as an assembly worker. The patient’s history was inconclusive for trauma and he had not previously noticed any hearing loss, tinnitus, or vertigo. The audiogram showed combined pantonal hearing loss up to 70 dB (Figure 1). His latmedical history was unremarkable except for allergic rhinitis. The clinical examination, including ear microscopy and testing of cranial nerve function, did not show any pathological findings. The initial treatment consisted of rheological infusion therapy carried out by his otologist. Readmission for further diagnostic work-up was recommended if no improvement followed this. In September 2003, the patient presented again due to persistent hearing loss, with no further neuro-otologic symptoms.
Figure 1.

The audiogram at first presentation, showing severe pantonal combined hearing loss up to 75 dB, with a conductive component of up to 30 dB at 500 Hz.
Magnetic resonance imaging (MRI) was performed and identified a large, lobulated lesion, 3.5 × 1.8 × 2.5 cm in size, with a heterogeneous high signal intensity, on T2-weighted images and avid enhancement of the solid parts on T1-weighted images after injection of contrast medium into the retrolabyrinthine temporal bone. Computed tomography (CT) showed a moth-eaten pattern of bone destruction. There was no peripheral thin rim of expanded bone, suggesting slow tumor growth. The lesion extended as far as the jugular foramen (Figure 2A-D). The differential diagnosis included jugulotympanic paraganglioma, chondrosarcoma, and ELST. A biopsy was taken via a transoccipital approach in October 2003.
A.

T1 SE axial magnetic resonance imaging (MRI), showing the internal carotid artery (ICA) and the cochlea and labyrinth infiltrated by tumor; (B) and T1 SE gadolinium MRI, showing solid and cystic parts of a tumor 3.5 × 1.8 × 2.5 cm in size in the left retrocochlear temporal bone. (C and D) Showing the tumor in T2 SE coronar and sagittal slides.
The histopathological examination revealed a low-grade epithelial neoplasm of the endolymphatic sac, showing papillary and cystic structures lined with bland cuboidal cells with low proliferative activity (Figure 3A, 3B). The tumor cells in the superficial layers covering the papillae stained positively with antibodies against the epithelial marker pancytokeratin KL-1 (Figure 3C). There was a thin layer of CD31-positive endothelial cells beneath the epithelium (Figure 3D).
Figure 3.
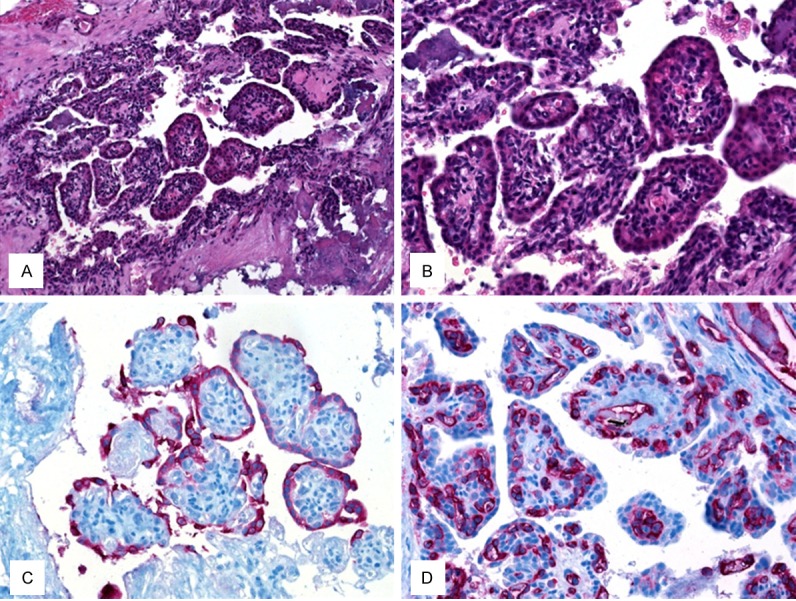
HE staining in (A, × 200) and (B, × 400) showed prominent papillary fronds within a cystic structure. The papillae were lined by bland cuboidal cells. (C) The tumor cells in the superficial layers covering the papillae stained positively with antibodies against pancytokeratin KL-1. (D) A thin layer of CD31-positive endothelial cells was seen beneath surface epithelium.
The ELST was treated on an interdisciplinary basis by head and neck surgeons and neurosurgeons in December 2003. Surgery consisted of resection of the tumor via a transcervical–transmastoid access route with neuronavigation guidance. A macroscopically complete resection was achieved intraoperatively. The final histological examination showed R1 tumor margin status in samples from the lateral petrous bone. The interdisciplinary tumor board discussed and finally recommended image-guided high-conformal stereotactic radiotherapy of the primary tumor region (with Novalis™), and this was carried out from January to April 2004, with a total dosage of 60 Gy. The course was uneventful except for a temporary slight left-sided paresis of the marginal branch of the facial nerve postoperatively. The audiogram remained unchanged. A detailed neuro-otological examination showed sufficient vestibular compensation. Genetic testing showed no evidence of VHL gene mutation, so that a case of sporadic ELST must be assumed. The latest MRI follow-up examination was performed in April 2013, showing no signs of tumor recurrence (Figure 4) or treatment side effects. At the time of writing, the patient had been tumor-free for 10 years, with complete facial nerve recovery.
Figure 4.
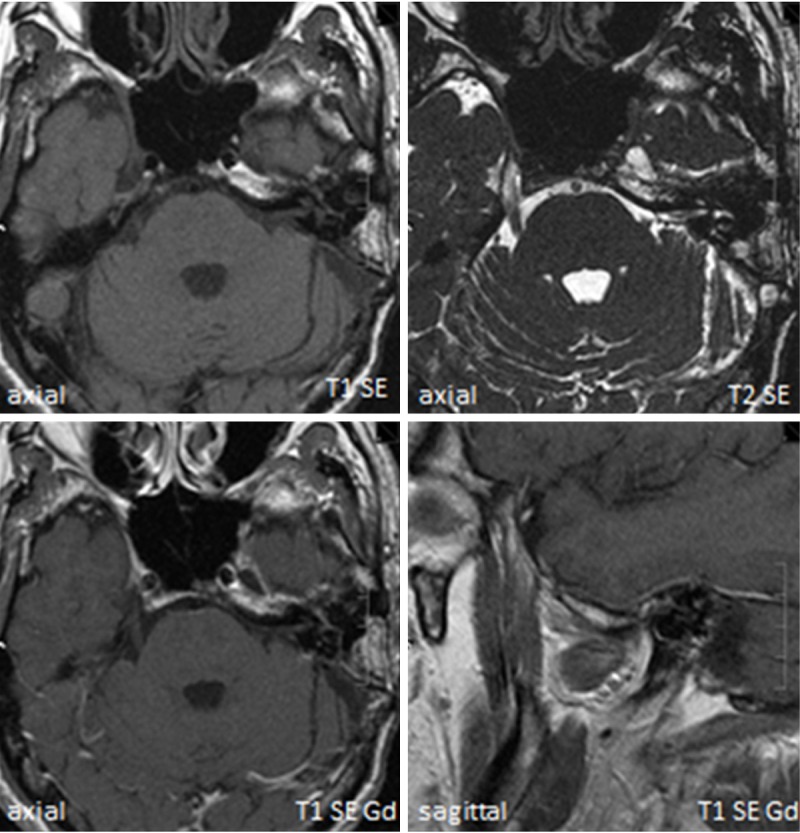
There were no signs of tumor recurrence at the most recent magnetic resonance imaging follow-up examination (in April 2013). Gd, gadolinium.
Discussion
ELSTs are difficult to diagnose, due to their rarity and the wide variety of their presentations. Mean age of patients without von Hippel-Lindau (VHL) disease is reported to be 52 years, whereas in patients with VHL disease the mean age is reported to be 31 years [5]. Since ELST often occurs in the setting of VHL disease, patients with ELST should be screened for VHL gene mutation [2]. Patients characteristically present with hearing loss, tinnitus, and vertigo. This combination of symptoms may mimic Ménière’s disease [6]. Sudden-onset deafness or severe hearing loss, as in this patient, has been the most common presenting symptom in most series, and early imaging therefore needs to be performed if no other cause is identifiable.
The location of the tumor in the present case (the retrocochlear temporal bone) is suggestive for ELST. Osseous erosions with bone spicules, representing bone fragments rather than bone formation, have typically been found in ELSTs [7]. Peripheral bone expansion may be found as a sign of slow progression, differentiating the lesion from more aggressive tumors such as chondrosarcoma and from metastasis, which was absent in this case. Small ELSTs do not involve the jugular foramen, distinguishing this entity from paraganglioma. In contrast to large tumors, which may demonstrate flow voids as a sign of marked hypervascularity, this finding is not typical for small lesions. However, both small and large ELSTs show avid contrast enhancement.
The treatment of choice in ELST is primary complete tumor resection, which is associated with a good long-term prognosis [8]. As stated by a recently published literature review early surgical excision is the best treatment when the tumor is small and remission may last for years in these cases. When the tumor is large, complete excision could be unachievable due to the anatomic complexity of the endolymphatic sac and distinct patterns of extension [5]. Remission after surgery may last for years, but there may also be local recurrences after surgery, probably as a result of incomplete resection. Adjuvant radiotherapy is an option after incomplete resection [9], although there are no larger studies focusing on the role of adjuvant radiotherapy [5]. In advanced cases or in case of recurrence radiotherapy alone may be the sole option [5,10].
In our case, the mass was large, destructed the mastoid process of the left petrous bone and extended to involve both the left medial mastoid as well as the middle ear, so it was difficult to extirpate surgically. The final histological examination showed R1 tumor margin status in samples from the lateral petrous part of the temporal bone. The discussion in the interdisciplinary tumor board focused on several concerns. The young age of the patient and the fact that only limited success of radiotherapy is expectable in a patient with a large or unresectable recurrence with high mortality due to uncontrolled intracranial growth, have led to the indication for postoperative stereotactic radiotherapy [7,11]. Starting daily image guidance during radiotherapy and the use of conformal radiotherapy may have helped prevent long-term side effects in this case.
Conclusion
In patients with hearing loss, vertigo, tinnitus, or facial nerve paresis of unknown origin, ELST should be included in the differential diagnosis, and precise MRI examination plays a paramount role in the diagnostic work-up. In summary, the combined treatment of the sporadic ELST described here has led to local tumor control with low morbidity for 10 years at the time of writing. Due to the lack of evidence in the literature concerning the role of postoperative adjuvant radiotherapy of residual ELST, we like to point out that radiotherapy could also have been spared until progression, as there is no standard treatment recommendation. An interdisciplinary treatment approach including surgeons, radiologists, pathologists, and radiotherapists should be provided for patients with ELST in order to achieve local control of these locally invasive and infiltrative tumors.
Disclosure of conflict of interest
None.
References
- 1.Hassard AD, Boudreau SF, Cron CC. Adenoma of the endolymphatic sac. J Otolaryngol. 1984;13:213–16. [PubMed] [Google Scholar]
- 2.Kim HJ, Hagan M, Butman JA, Baggenstos M, Brewer C, Zalewski C, Linehan WM, Lonser RR. Surgical resection of endolymphatic sac tumors in von Hippel-Lindau disease: findings, results, and indications. Laryngoscope. 2013;123:477–83. doi: 10.1002/lary.23646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mukherji SK, Albernaz VS, Lo WW, Gaffey MJ, Megerian CA, Feghali JG, Brook A, Lewin JS, Lanzieri CF, Talbot JM, Meyer JR, Carmody RF, Weissman JL, Smirniotopoulos JG, Rao VM, Jinkins JR, Castillo M. Papillary endolymphatic sac tumors: CT, MR imaging, and angiographic findings in 20 patients. Radiology. 1997;202:801–8. doi: 10.1148/radiology.202.3.9051037. [DOI] [PubMed] [Google Scholar]
- 4.Virk JS, Randhawa PS, Saeed SR. Endolymphatic sac tumour: case report and literature review. J Laryngol Otol. 2013;127:408–10. doi: 10.1017/S0022215113000327. [DOI] [PubMed] [Google Scholar]
- 5.Sun YH, Wen W, Wu JH, Song JM, Guan H, Wang KX, Xu MQ. Endolymphatic sac tumor: case report and review of the literature. Diagn Pathol. 2012 Apr 2;7:36. doi: 10.1186/1746-1596-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee KJ, Kirsch CF, Lai C, Ishiyama A. Endolymphatic sac tumor presenting with Ménière’s disease. Otolaryngol Head Neck Surg. 2010;142:915–16. doi: 10.1016/j.otohns.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Bell D, Gidley P, Levine N, Fuller GN. Endolymphatic sac tumor (aggressive papillary tumor of middle ear and temporal bone): sine qua non radiology-pathology and the University of Texas MD Anderson Cancer Center experience. Ann Diagn Pathol. 2011;15:117–23. doi: 10.1016/j.anndiagpath.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Hansen MR, Luxford WM. Surgical outcomes in patients with endolymphatic sac tumors. Laryngoscope. 2004;114:147–4. doi: 10.1097/00005537-200408000-00028. [DOI] [PubMed] [Google Scholar]
- 9.Balasubramaniam S, Deshpande RB, Misra BK. Gamma knife radiosurgery in jugular foramen endolymphatic sac adenocarcinoma. J Clin Neurosci. 2009;16:710–11. doi: 10.1016/j.jocn.2008.07.086. [DOI] [PubMed] [Google Scholar]
- 10.Hou ZH, Huang DL, Han DY, Dai P, Young WY, Yang SM. Surgical treatment of endolymphatic sac tumor. Acta Otolaryngol. 2012;132:329–36. doi: 10.3109/00016489.2011.640349. [DOI] [PubMed] [Google Scholar]
- 11.Stanley J, Pickett B. Endolymphatic sac tumors. In: Jackler RK, editor. Tumors of the ear and temporal bone. Philadelphia: Lippincott Williams & Wilkins; 2000. p. 156. [Google Scholar]


