Abstract
Epstein-Barr Virus (EBV) is a herpesvirus usually infecting B-cells but may occasionally infect T- or natural killer (NK)-cells. EBV-associated T- or NK-cell lymphoproliferations represent a continuous spectrum of diseases ranging from asymptomatic infection, infectious mononucleosis (IM), to clonal and malignant lymphoproliferations including systemic EBV-positive T/NK-cell lymphoproliferative disease (EBV-T/NK-LPD) of childhood and hydroa-vacciniforme-like lymphoma of the skin. The clonal diseases are more prevalent in East Asia and exhibit overlapping clinical and pathological features with chronic active EBV infection. Here we report our experience on 10 cases of EBV-associated T-cell lymphoproliferation from Taiwan including five males and five females with a median age of 18 years old (range, 15-28). The most common clinical symptoms were fever, neck mass and hepatosplenomegaly. Eight of these patients showed elevated lactate dehydrogenase level and half of the patients had cytopenia. All patients had either elevated EBV antibody titers or increased serum EBV DNA levels. Five cases were clinically IM-like with polyclonal (3 cases) or clonal (2 cases) T-cell lymphoproliferation. Two patients each had chronic active EBV infection (CAEBV) and hemophagocytic lymphohistiocytosis (HLH). One patient had both CAEBV and HLH. One of the HLH patients with marrow infiltration by intra-sinusoidal large atypical lymphocytes experienced a fulminant course. In a median follow-up time of 21.5 months, seven patients were free of disease, one was alive with disease, and two died of disease in 31 and 3 months, respectively, despite chemotherapy. We confirmed a wide clinicopathological range of EVB-associated T-cell lymphoproliferation in Taiwan. Furthermore, monomorphic LPD and the single case with fulminant course as defined by Ohshima et al (Pathol Int 2018) as categories A3 and B, respectively, died of disease despite chemotherapy. Our report, the largest series in the recent decade from Taiwan, adds to the understanding of these rare diseases with variable clinical and histopathological presentations.
Keywords: Epstein-Barr virus, hemophagocytic lymphohistiocytosis, infectious mononucleosis, systemic T/natural killer-cell lymphoproliferative disease, T/natural killer-cell lymphoproliferation, Taiwan
Introduction
Epstein-Barr virus (EBV), also called human herpesvirus 4, is a ubiquitous DNA virus belonging to the γ subfamily of herpesviruses. Over 90% of humans are infected with EBV and the infection persists for life. Although most people are asymptomatic, EBV infection may cause a continuous range of symptoms from benign to severe diseases depending on the immunological response of the individuals. In most instances, EBV infects B-cells. In rare instances of primary acute or chronic active EBV infection (CAEBV), EBV may infect T- or natural killer (NK)-cells and may cause a wide spectrum of diseases including infectious mononucleosis (IM)-like T-LPD, EBV-associated hemophagocytic lymphohistiocytosis (HLH; or hemophagocytic syndrome), and EBV-associated systemic T/NK-cell lymphoproliferative disorders (EBV-T/NK-LPDs) of childhood [1-8]. Furthermore, EBV is also associated with various hematologic malignancies such as lymphomatoid granulomatosis, extranodal NK/T-cell lymphoma, aggressive NK-cell leukemia, diffuse large B cell lymphoma of the elderly and post-transplant lymphoproliferative disorders [9].
EBV-T/NK-LPD of children and young adults is a systemic illness characterized by clonal proliferation of EBV-infected T- or NK-cells. It is characterized by fever, lymphadenopathy, and splenomegaly developing after primary EBV infection in patients without known immunodeficiency. This rare group of diseases is associated with high morbidity and mortality, and appears to be more prevalent in East Asian countries. According to the 4th edition of World Health Organization (WHO) classification of tumor of hematopoietic and lymphoid tissue in 2008, two major types of EBV-associated T-cell LPDs of childhood have been reported in the pediatric group [10]. Hydroa vacciniforme-like lymphoma is a cutaneous malignancy with an indolent course but usually progresses over time. Systemic EBV-T/NK-LPD of childhood is an aggressive and fulminant form and may be associated with chronic active EBV infection. However, this group of diseases spans a wider spectrum than described in the WHO classification scheme with some borderline cases not fitting into the diagnostic criteria of either disease [8]. In this report, we presented the clinical and histopathological features of 10 patients with EVB-associated T-cell lymphoproliferation, the largest series in recent years from Taiwan, to add to the understanding of the clinicopathological spectrum of these rare diseases.
Material and methods
We retrospectively reviewed the in-house and consultation cases (to S.-S.C) at Chi-Mei Medical Center, Tainan, Taiwan from January 2009 to June 2013. The cases diagnosed as EBV-positive T- or NK-cell lymphoproliferation were selected with the exclusion of aggressive NK-cell leukemia or extranodal NK/T-cell lymphoma, nodal type with leukemic transformation. The specimens analyzed included peripheral blood, bone marrow, liver and lymph node whenever available. We reviewed the medical records and the overall survival was measured from the date of diagnosis to the date of last follow-up or death. Consents from patients were obtained and the study was conducted in accordance with the guidelines of the Declaration of Helsinki.
Flow cytometric immunophenotyping was performed as previously described [11]. In brief, peripheral blood mononuclear cells in ethylenediaminetetraacetic acid (EDTA) were used for three-color flow cytometric immunophenotypic with FACScan (BD Biosciences, San Jose, CA., U.S.A.) after mononuclear cell enrichment by centrifugation with Ficoll-Paque PLUS (Amersham Biosciences, Uppsala, Sweden). Bright CD3 expressors within the lymphocyte gate (bright CD45/low side scatter) were analyzed using the CellQuest software (BD Biosciences). Four-color gating with CD8 expressors were further analyzed when they represented the dominant population. Immunohistochemical studies were performed using the streptavidin-biotin peroxidase method (LSAB kit; Dako Corporation, Carpenteria, CA) and an antigen retrieval technique was applied when needed for each individual antibody as previously described [12]. The antibodies used included CD2, CD3, CD4, CD5, CD7, CD8, CD20, CD56, TIA1 and CD30. In situ hybridization for EBV-encoded mRNA (EBER) was done and the percentage of EBER-positive cells was estimated. Polymerase chain reaction analysis for TCRγ-chain gene rearrangement using paraffin section was also performed as previously described [13]. Double stains for EBER/CD20, EBER/CD3 and EBER/CD56 were performed in selected cases.
Result
A total of 10 cases were identified and their clinical features are summarized in Table 1 There were five males and five females with a median age of 18 years old (range, 15-28). The clinical symptoms and signs included fever (7 cases), hepatomegaly (6), splenomegaly (7), and lymphadenopathy (7). All cases showed either lymphadenopathy and/or hepatosplenomegaly. Peripheral blood cytopenia were noted in five cases, three of them were associated with HLH. Lymphocytosis was noted in four patients and the lymphocytes were comprised mainly of large granular lymphocytes (LGL). Eight cases had elevated lactate dehydrogenase (LDH) levels, and six had abnormal liver function tests. The EBV antibody profile, pathologic category as defined by the criteria of Ohshima et al [5], clonality studies, treatment modality, and follow-up data are listed in Table 2. All cases showed either elevated EBV antibody titers or increased EBV DNA copy numbers in the serum.
Table 1.
Clinical and laboratory findings
| No./Sex/Age | Clinical Dx | Fever | Hepatomegaly | Splenomegaly | LAP | HLH | Hb | WBC | Platelet | LFT | LDH |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/M/15 | IM | + | + | + | + | - | Normal | LGL-lymphocytosis | Normal | Abnormal | Elevated |
| 2/F/19 | IM | - | NA | NA | + | - | Anemia | LGL-lymphocytosis | Normal | Abnormal | NA |
| 3/M/21 | IM | - | + | + | + | - | Normal | LGL-Lymphocytosis | Normal | Abnormal | Elevated |
| 4/F/21 | IM | + | - | + | - | - | Normal | Non-LGL-lymphocytosis | Normal | Abnormal | Elevated |
| 5/M/16 | IM | - | NA | NA | + | - | Normal | Normal | Normal | NA | Elevated |
| 6/M/17 | CAEBV | + | - | - | + | - | Normal | Normal | Normal | Normal | Normal |
| 7/F/16 | HLH | + | + | + | - | + | Anemia | Leukopenia | Thrombocytopenia | Abnormal | Elevated |
| 8/F/16 | CAEBV | + | + | + | + | - | Anemia | Normal | Normal | Normal | Elevated |
| 9/M/28 | CAEBV & HLH | + | + | + | + | + | Anemia | Leukopenia | Thrombocytopenia | Normal | Elevated |
| 10/F/28 | HLH | + | + | + | - | + | Anemia | Leukopenia | Thrombocytopenia | Abnormal | Elevated |
Abbreviations: Dx, diagnosis; Hb, hemoglobin; HLH, histiocytic lymphohistiocytosis or hemophagocytic syndrome; IM, infectious mononucleosis; LAP, lymphadenopathy; LDH, lactate dehydrogenase; LFT, liver function tests; LGL, large granular lymphocyte; NA, not available.
Table 2.
EBV viral profile, pathological, immunophenotypic and clonality study findings
| Case | Category@ | EBV | Phenotype | TCR clonality | Treatment | Outcome (m) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| VCA-IgM | VCA-IgG | EA | EBNA | Viral load# | EBER | ||||||
| 1 | NA | + | + | NA | NA | NA | NA | Cytotoxic T | Clonal | Supportive | NED (10) |
| 2 | NA | NA | NA | NA | NA | +* | NA | Cytotoxic T | Clonal | Supportive & antibiotics | NED (28) |
| 3 | NA | + | + | - | - | NA | + (LN: 10%) | Cytotoxic T | Polyclonal | Supportive & antibiotics | NED (13) |
| 4 | NA | NA | NA | NA | NA | 375/ml | + (BM & liver: 1%) | Cytotoxic T | Polyclonal | Etoposide & prednisolone | NED (14) |
| 5 | NA | NA | + | - | - | NA | + (LN 5%) | Cytotoxic T | Polyclonal | Supportive | NED (4) |
| 6 | A1 | - | + | - | + | 8,497 | + (LN 5%) | Helper T | Polyclonal | Supportive | NED (4) |
| 7 | A1 | - | + | NA | NA | NA | + (LN: 30%) | Cytotoxic T | Polyclonal | IVIG & prednisolone; then immunochemotherapy* | NED (31) |
| 8 | A2 | - | + | - | + | 3,623, 6,160, 13,781 | + (LN: 30%) | Helper T | Clonal | Prednisolone | AWD (51) |
| 9 | A3 | - | + | + | + | 210 | + (BM: 90%; liver: 30%) | Cytotoxic T | Poor DNA | Chemotherapy | DOD (31) |
| 10 | B | - | NA | NA | NA | 56,8324 | + (BM) | CD4/CD8 double negative | Polyclonal (poor DNA for study) | ESHAP | DOD (3) |
The EBV viral load was presented as copies number/ml with sequential testing results listed for Case 8.
Pathological categorization of EBV-associated T/NK LPD according to Ohshima et al. [5].
Immunochemotherapy regimen for Case 7 included prednisolone, cyclosporine and etoposide.
Abbreviations: CAEBV, chronic active EBV infection; Dx, diagnosis; EA, early antigen; EBER, in situ hybridization for EBV-encoded mRNA; ESHAP, etoposide, methylprednisolone, high-dose cytarabine, and cisplatin; IVIG, intravenous immunoglobulin; NA, not available; NED, no evidence of disease; TCR, T-cell receptor gene rearrangement; VCA, viral capsid antigen.
The most common clinical presentation was IM-like pattern (five patients) with monoclonal (Cases 1 & 2) or polyclonal (Cases 3-5) T-cell proliferation. Two cases (Cases 1 and 2) were diagnosed by flow cytometric immunophenotyping and clinical information without nodal biopsy. The peripheral blood of these patients showed LGL lymphocytosis. Flow cytometric analysis revealed cytotoxic T-cell phenotype with clonal TCRγ-chain gene rearrangement (Figure 1). Incorporating the clinical symptoms of fever and lymphadenopathy, these two cases were diagnosed as IM-like T-cell lymphoproliferation. Nodal biopsies of the other IM-like cases (Cases 3-5) showed diffuse architecture effacement by a predominantly T-cell lymphocytic infiltration accompanied by a mild proliferation of post-capillary venules and an admixture of a few histiocytes and plasma cells with a few residual lymphoid follicles. The liver biopsy from Case 4 (Figure 2) showed a dense portal infiltration of small to medium-sized CD8-positive T lymphocytes with extension into the sinusoids resembling hepatosplenic T cell lymphoma (HSTCL), a differential diagnosis excluded by the expression of CD5, CD7, CD8 but not CD56 or TCR-γ in this case. Scanty intrasinusoidal lymphocytes were positive for EBER. Three cases (Cases 7, 9, and 10) showed HLH in the marrow aspirate. The clinical courses of two cases (Cases 8 & 9) were compatible with CAEBV. Case 10 experienced a fulminant course and the marrow biopsy showed intro-sinusoidal infiltration by large atypical lymphocytes (Figure 3).
Figure 1.

Case 2. Infectious mononucleosis-like T cell lymphoproliferation in a 19-year-old female presenting with sore throat, bilateral tonsillar enlargement, and WBC count at 11,800/ul with 74% lymphocytes. Flow cytometry shows that 69% of the gated lymphocytes are CD8-expressing cytotoxic T-cells (not shown). Composite photos of peripheral blood show large granular lymphocytes and atypical lymphoid cells with mild to moderate irregularity in nuclear contours and basophilic cytoplasm with azurophilic granules. Clonality study using BIOMED-2 protocol with tube A reaction for T-cell receptor-γ chain gene shows a clonal result (N, negative control, Po, polyclonal control, M, DNA size marker).
Figure 2.
Liver biopsy of Case 4, IM-like T-cell lymphoproliferation. (A) A dense infiltration by small to medium-sized atypical lymphocytes is noted in the portal area (left-upper corner) with atypical lymphocytes extending into sinusoid (x400). (B) Most of the infiltrating lymphocytes are CD8-positive T lymphocytes (x400). These lymphocytes express βF1 (C: x1,000) and granzyme B (D: x400) but not TCR-γ (not shown). (E) Scanty cells are positive for EBER (x1,000). The 21-year-old female underwent chemotherapy with etoposide and steroid due to the clinical suspicion of HLH although there was no definitive morphological evidence in the marrow aspirate smears. There was no evidence of relapse in a follow-up of 14 months.
Figure 3.
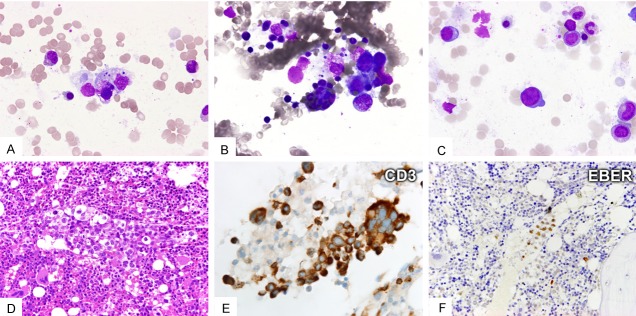
Case 10. Systemic EBV+T-LPD in a 26 year-old female presenting with fever, hepatosplenomegaly and cytopenia. Marrow aspirate shows hemophagocytosis (A and B) and scanty large atypical lymphocytes (C). Bone marrow biopsy shows intravascular infiltration by large atypical lymphocytes (D: HE stain, x400) expressing CD3 (E: x1000) but not CD20, CD4 or CD8 (not shown). Some of these large atypical lymphocytes are positive for EBER (F: x400). The DNA quality is suboptimal for clonality study. She died in three months despite intensive chemotherapy.
Overall, the most common phenotype of the proliferating cells was cytotoxic T lymphocytes (7 cases). Two were of helper T-cell phenotype. The proliferating cells of the last patient was double-negative for both CD4 and CD8, and also negative for both βF1 and TCR-γ. The cellular lineage (T vs. NK) was indeterminate as the DNA quality was unsatisfactory for clonality study. The proportion of EBV-positive cells in the biopsy specimens ranged from 1% to 99% of the infiltrating cells. Combining the presentation and clinical courses with duration of at least 6 months, the pathological states of these cases were categorized into two as category A1 (Cases 6 & 7), one A2 (Case 8), one A3 (Case 9), and one B (Case 10) by the proposed criteria for EBV-T/NK-LPD by Ohshima et al [5].
The treatment modality for these patients was heterogeneous. The first six patients received supportive treatment only as most of them showed IM-like T-cell proliferation with relatively mild symptoms. One patient (Case 8) received prednisolone alone, one (Case 7) received intravenous immunoglobulin (IVIG) plus corticosteroid as immunomodulation therapy, and the remaining two patients (Cases 9 & 10) received chemotherapy. The median follow-up time was 21.5 months (range, 3-51). Seven patients showed no evidence of disease at the last follow-up including five patients receiving supportive treatment only. One patient (Case 8), Ohshima category A2, was alive with disease for 51 months. The two patients with monomorphic proliferation of atypical lymphocytes, Ohshima categories A3 (Case 9) and B (Case 10), died of disease at 31 and 3 months, respectively, despite intensive chemotherapy.
Discussion
In this report, we presented the clinical and histopathological features of 10 patients with EBV-associated T-cell lymphoproliferation. To our knowledge, this is the largest series in the recent decade from Taiwan. Diagnosis of such cases with great heterogeneity in clinical presentation and histologic features is challenge to pathologists when clinical information and EBER studies were lacking or inadequate. The hematological and histopathological features ranged from LGL lymphocytosis, polymorphic or polyclonal T-cell proliferation in tissues to monotonous lymphocytic proliferation, sometimes complicated by HLH [5]. The histologic features may overlap with reactive proliferation and malignant lymphoma. It is of utmost importance to recognize EBV-associated T-cell lymphoproliferation, particularly those with IM-like features, from peripheral T-cell lymphoma to avoid unnecessary treatment [5]. In our series, there were six cases showed IM-like clinical features with polyclonal or clonal T-cell proliferation. One of these cases (Case 6) showed overlapping features with CAEBV because of the long-lasting symptoms for more than one year.
The major diagnostic challenge was the distinction from malignant lymphoma, especially peripheral T-cell lymphoma (PTCL). In Case 4, the intrasinusoidal infiltration in the liver by atypical T-lymphocytes might mimic HSTCL, a neoplasm usually of γδ-T cell origin with frequent CD56 expression but not CD5 or CD8. Furthermore, HSTCL is usually negative for EBER. The distinct phenotype and EBV association of our case argued against the diagnosis of HSTCL. In Case 6, the nodal biopsy showed architecture effacement with dissecting collagen bands mimicking nodular sclerosis Hodgkin lymphoma in low magnifications. The proliferated cells are mainly T-lymphocytes and double-labeling for EBER/CD3, EBER/CD20 and EBER/CD56 revealed that the EBV-infected cells were CD3+CD56- T lymphocytes, ruling out the possibility of Hodgkin lymphoma. While the large Hodgkin-like cells expressed CD20 and CD45, indicating B-cell immunoblasts, not Reed-Sternberg cells. In Case 10, the clinical course was fulminant and marrow biopsy revealed intrasinusoidal infiltration by large atypical lymphocytes, resembling aggressive lymphomas with marrow involvement or intravascular lymphomas. The differential diagnosis between systemic EBV-positive T/NK-LPD of childhood type and other large cell lymphomas was very difficult in this case. The correct diagnosis was reached through the integration of the histopathology, immunohistochemistry, and EBER in the context of clinical history and laboratory data of EBV infection (including antibody profile and EVB DNA copy numbers in the serum).
In our five patients with IM-like lesions, the proliferating cells were all cytotoxic T-cells. In the remaining five cases with CAEBV and/or HLH, the phenotype included two with cytotoxic type, two helpers, and one indeterminate. There was no proven case of NK-cell proliferation in our series. In a study of 108 non-immunocompromised patients with systemic EBV-T/NK-LPD, Kimura et al. [8] found that the clinical categories of the patients were associated with the immunophenotype of EBV-infected cells. In CAEBV and EBV-associated HLH, around 60% of cases the infected cells were αβ T-cells, and in 75% patients with hydroa vacciniforme, the infected cells were γδ T-cells. In contrast, most patients (89%) with severe mosquito bite allergy had EBV-infected NK cells.
Currently there is no standard treatment protocol for patients with systemic EBV-T/NK-LPD. After bone marrow transplantation (BMT), most patients recovered completely from severe CAEBV and systemic EBV-T/NK-LPD [8,14,15]. However, there are still risks of complications for BMT and the criteria for selecting patient for BMT still need further assessment. The two fatality patients in our series were the eldest, both 28 years old, and they presented with fever, hepatosplenomegaly, pancytopenia and HLH. The pathological classification was Ohshima category A3 and B, respectively. The poor prognosis of these patients was in keeping with the report from Ohshima et al [5]. In addition to the pathological classification, Kimura et al. found that age ≥8 years, thrombocytopenia (platelet count <104/μL at diagnosis), and T lineage of the EBV-infected cells were associated with a poor outcome in patients with systemic EBV-T/NK-LPD [16]. BMT may be the treatment of choice for cases presenting with these adverse factors.
In the 4th Asian Hematopathology Workshop held in Seoul, Korea in 2012, the participants recommended the umbrella term “EBV-associated T/NK-LPD” to cover the entire spectrum of EBV-associated lesions in childhood from reactive to neoplastic process. The neoplastic process was subdivided into systemic and cutaneous forms. Systemic T/NK-LPD of childhood is a fulminant disease associated with proliferation of polyclonal, oligoclonal, or monoclonal T- or NK-cells, and aggressive NK-cell leukemia in children can be included in this category. Furthermore, as the disease affects not only children but also young adults and the cell lineages include T- and NK-cells, thus the proposed term was “EBV-T/NK-LPD of childhood type” to replace the WHO nomenclature of “EBV-T-LPD of childhood” [17].
In brief, we presented ten cases of EBV-associated T-cell lymphoproliferative disorder in adolescents and young adults from Taiwan. It is a heterogeneous disease with variable clinical presentation and histopathological features. Patients with IM-like diseases recovered with only supportive treatment. Those with monotonous proliferation and fulminant course and HLH had an adverse outcome despite intensive chemotherapy.
Acknowledgements
This work was supported by the research grant NSC102-2320-B-384-001 (to S.-S.C.) from National Science Council, Taipei, Taiwan and was presented at the 5th Asian Hematopathology Symposium in Nagoya on January 26th, 2014.
Addendum
The 6th patient, a victim of CAEBV, relapsed 6 months after diagnosis with cervical lymphadenopathy and the enlarged nodes partially regressed with supportive treatment. The clinical course was consistent with CAEBV.
Disclosure of conflict of interest
None declared.
References
- 1.Su IJ, Lin DT, Hsieh HC, Lee SH, Chen J, Chen RL, Lee CY, Chen JY. Fatal primary Epstein-Barr virus infection masquerading as histiocytic medullary reticulosis in young children in Taiwan. Hematol Pathol. 1990;4:189–195. [PubMed] [Google Scholar]
- 2.Su IJ, Chen RL, Lin DT, Lin KS, Chen CC. Epstein-Barr virus (EBV) infects T lymphocytes in childhood EBV-associated hemophagocytic syndrome in Taiwan. Am J Pathol. 1994;144:1219–1225. [PMC free article] [PubMed] [Google Scholar]
- 3.Quintanilla-Martinez L, Kumar S, Fend F, Reyes E, Teruya-Feldstein J, Kingma DW, Sorbara L, Raffeld M, Straus SE, Jaffe ES. Fulminant EBV(+) T-cell lymphoproliferative disorder following acute/chronic EBV infection: a distinct clinicopathologic syndrome. Blood. 2000;96:443–451. [PubMed] [Google Scholar]
- 4.Kimura H, Hoshino Y, Kanegane H, Tsuge I, Okamura T, Kawa K, Morishima T. Clinical and virologic characteristics of chronic active Epstein-Barr virus infection. Blood. 2001;98:280–286. doi: 10.1182/blood.v98.2.280. [DOI] [PubMed] [Google Scholar]
- 5.Ohshima K, Kimura H, Yoshino T, Kim CW, Ko YH, Lee SS, Peh SC, Chan JK, Group CS. Proposed categorization of pathological states of EBV-associated T/natural killer-cell lymphoproliferative disorder (LPD) in children and young adults: overlap with chronic active EBV infection and infantile fulminant EBV T-LPD. Pathol Int. 2008;58:209–217. doi: 10.1111/j.1440-1827.2008.02213.x. [DOI] [PubMed] [Google Scholar]
- 6.Isobe Y, Aritaka N, Setoguchi Y, Ito Y, Kimura H, Hamano Y, Sugimoto K, Komatsu N. T/NK cell type chronic active Epstein-Barr virus disease in adults: an underlying condition for Epstein- Barr virus-associated T/NK-cell lymphoma. J Clin Pathol. 2012;65:278–282. doi: 10.1136/jclinpath-2011-200523. [DOI] [PubMed] [Google Scholar]
- 7.He HL, Wang MC, Huang WT. Infectious mononucleosis mimicking malignant T-cell lymphoma in the nasopharynx: a case report and review of the literature. Int J Clin Exp Pathol. 2013;6:105–109. [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura H, Ito Y, Kawabe S, Gotoh K, Takahashi Y, Kojima S, Naoe T, Esaki S, Kikuta A, Sawada A, Kawa K, Ohshima K, Nakamura S. EBV-associated T/NK-cell lymphoproliferative diseases in nonimmunocompromised hosts: prospective analysis of 108 cases. Blood. 2012;119:673–686. doi: 10.1182/blood-2011-10-381921. [DOI] [PubMed] [Google Scholar]
- 9.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO classification of tumours of haematopoietic and lymphoid tissues. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC; 2008. [Google Scholar]
- 10.Quintanilla-Marinez L, Kimura H, Jaffe ES. EBV-positive T-cell lymphoproliferative disorders of childhood. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO classification of tumours of haemtopoietic and lymphoid tissues. Lyon: IARC; 2008. pp. 278–280. [Google Scholar]
- 11.Hsieh YC, Chang ST, Huang WT, Kuo SY, Chiang TA, Chuang SS. A comparative study of flow cytometric T cell receptor Vbeta repertoire and T cell receptor gene rearrangement in the diagnosis of large granular lymphocytic lymphoproliferation. Int J Lab Hematol. 2013;35:501–9. doi: 10.1111/ijlh.12041. [DOI] [PubMed] [Google Scholar]
- 12.Chuang SS, Chang ST, Chuang WY, Huang WT, Hsieh PP, Tsou MH, Liao YL, Lin SH, Hsieh YC, Lu CL, Sheu MJ, Liu H. NK-cell lineage predicts poor survival in primary intestinal NK-cell and T-cell lymphomas. Am J Surg Pathol. 2009;33:1230–1240. doi: 10.1097/PAS.0b013e3181a95c63. [DOI] [PubMed] [Google Scholar]
- 13.Kuo SY, Liu H, Liao YL, Chang ST, Hsieh YC, Bandoh BA, Du MQ, Chuang SS. A parallel comparison of T-cell clonality assessment between an in-house PCR assay and the BIOMED-2 assay leading to an efficient and cost-effective strategy. J Clin Pathol. 2011;64:536–542. doi: 10.1136/jcp.2010.086637. [DOI] [PubMed] [Google Scholar]
- 14.Sato E, Ohga S, Kuroda H, Yoshiba F, Nishimura M, Nagasawa M, Inoue M, Kawa K. Allogeneic hematopoietic stem cell transplantation for Epstein-Barr virus-associated T/natural killer-cell lymphoproliferative disease in Japan. Am J Hematol. 2008;83:721–727. doi: 10.1002/ajh.21247. [DOI] [PubMed] [Google Scholar]
- 15.Kawa K, Sawada A, Sato M, Okamura T, Sakata N, Kondo O, Kimoto T, Yamada K, Tokimasa S, Yasui M, Inoue M. Excellent outcome of allogeneic hematopoietic SCT with reduced-intensity conditioning for the treatment of chronic active EBV infection. Bone Marrow Transplant. 2011;46:77–83. doi: 10.1038/bmt.2010.122. [DOI] [PubMed] [Google Scholar]
- 16.Kimura H, Morishima T, Kanegane H, Ohga S, Hoshino Y, Maeda A, Imai S, Okano M, Morio T, Yokota S, Tsuchiya S, Yachie A, Imashuku S, Kawa K, Wakiguchi H Japanese Association for Research on Epstein-Barr Virus and Related Diseases. Prognostic factors for chronic active Epstein-Barr virus infection. J Infect Dis. 2003;187:527–533. doi: 10.1086/367988. [DOI] [PubMed] [Google Scholar]
- 17.Ko YH, Kim HJ, Oh YH, Park G, Lee SS, Huh J, Kim CW, Kim I, Ng SB, Tan SY, Chuang SS, Nakamura N, Yoshino T, Nakamura S, Kimura H, Ohshim K. EBV-associated T and NK cell lymphoproliferative disorders: consensus report of the 4th Asian Hematopathology Workshop. J Hematopathol. 2012;5:319. [Google Scholar]



