Abstract
Purpose: The aim of this meta-analysis was to assess the safety of denosumab in postmenopausal women with osteoporosis or low bone mineral density (BMD). Methods: Safety of denosumab was compared with placebo or bisphosphonates. A systematic literature search without language restriction was conducted up to January, 2014. The RevMan 5.1 software was used for statistical analysis. Results: A total of 11 English literatures were eventually identified. The pooled data in the overall analysis revealed that there was no significant difference when compared denosumab with placebo or bisphosphonates in any adverse events (AAE) (RR=0.99, 95% CI=0.98-1.01, p=0.29), serious adverse event (SAE) (RR=1.05, 95% CI=0.98-1.13, p=0.18), neoplasm/cancer (RR=1.14, 95% CI=0.95-1.37, p=0.16) and deaths (RR=0.77, 95% CI=0.57-1.04, p=0.09). However, significant differences were found when compared denosumab with placebo or bisphosphonates in SAE related to infection (RR=1.23, 95% CI=1.00-1.52, p=0.05) and non-vertebral fracture (RR=0.86, 95% CI=0.74-1.00, p=0.05). Subgroup analysis was performed by the type of drugs which was used in the control group. The results of subgroup analysis did not demonstrate the differences between denosumab and bisphosphonates in SAE related to infection (RR=1.13, 95% CI=0.63-2.03) and non-vertebral fracture (RR=1.31, 95% CI=0.87-1.98). Conclusions: Compared to placebo, denosumab treatment significantly decreased the risk of non-vertebral fracture but increased the risk of SAE related to infection in the postmenopausal women with osteoporosis or low BMD. However, no difference between the safety of denosumab and bisphosphonates was found.
Keywords: Denosumab, osteoporosis, postmenopausal women, meta-analysis
Introduction
Osteoporosis is a common disease characterized by a systemic impairment of bone mass, strength, and microarchitecture which increases the propensity of fragility fractures [1]. There is higher prevalence of osteoporosis among postmenopausal women and the elderly [2]. Approximately 30% of all postmenopausal women in the United States and Europe have osteoporosis [3]. In Korea, the prevalence of osteoporosis has been reported to be 31% in postmenopausal women aged 45-64 years, 53% in those aged 65-74 years [4]. Shao et al. [5] reported that the prevalence of osteoporosis was as much as 60% in postmenopausal Chinese women.
The treatment of osteoporosis and prevention of osteoporotic fractures consist of non-drug and drug therapy [6]. Drug therapy of osteoporosis is based on the knowledge of mechanisms of bone turnover and the manipulation of the cellular components of bone turnover in recruitment, activation and apoptosis [7]. Bisphosphonates is one of the drugs that are currently available for postmenopausal osteoporosis by inhibiting bone turnover [8].
Denosumab is a fully human monoclonal antibody to receptor activator of nuclear factor-κB ligand (RANKL) [9]. RANKL is a cytokine member of the tumour necrosis factor family that is the principal final mediator of osteoclastic bone resorption [10]. It is the key molecule responsible for the bone loss observed in osteoporosis [11]. By binding to RANKL and preventing its binding to the RANK receptors on the surface of osteoclasts and osteoclast precursors, denosumab inhibits the development, activation and survival of osteoclasts [12]. Although denosumab had been recommended as one of the clinical medicines by American Association of Clinical Endocrinologists (AACE) [13], it is lack of large sample size studies to perfectly evaluate the effectiveness and safety of denosumab. Therefore, we performed a meta-analysis to assess the safety of denosumab in postmenopausal women with osteoporosis or low bone mineral density (BMD).
Materials and methods
Search strategy
A systematic literature search without language restriction was conducted up to January, 2014 by using the electronic databases such as PubMed, Embase, Springer link, Cochrane library. The key words included “denosumab”, “osteoporosis”, “postmenopausal women”, and “low bone mineral density”. Furthermore, paper literatures were retrieved by manual search. Review articles and reference lists of retrieved Figure 1. Literature search and study selection.articles were also inspected to find additional eligible studies.
Figure 1.
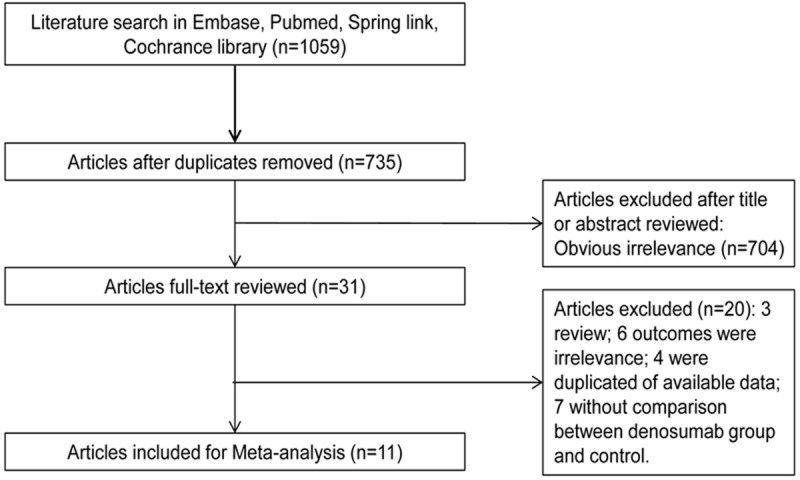
Literature search and study selection.
Study selection
Studies that met the following criteria were included in the meta-analysis: (1) the studies were randomized controlled trials; (2) the subjects were the postmenopausal women with osteoporosis or low BMD; (3) the studies were designed to compare the safety of denosumab with placebo or bisphosphonates; (4) one of the following risk indicators must be included: any adverse events (AAE), serious adverse event (SAE), SAE related to infection, non-vertebral fracture, neoplasm/cancer and deaths. Studies were excluded if they were (1) animal studies; (2) studies that had unavailable data or lack of enough data; (3) reviews, letters and comments; (4) repeated publication articles.
Data extraction and quality assessment
Two evaluators independently selected studies and extracted data. Discrepancies were resolved by discussion with a third investigator. For each study, the following information was extracted: the first author name, year of publication, region, age of subjects, number of subjects, dosage of denosumab and outcomes.
Study quality was assessed using the 6-item instrument developed by Jadad et al [13]. The studies with the score from 3 to 5 are high quality studies. According to Cochrane Library Handbook [14], risk assessment tool in RevMan 5.1 was used to evaluate the risk of bias in the following factors: random sequence generation, allocation concealment, blinding of participants and staff, blinding of outcome assessment, incomplete data of outcome, selective reporting and so on.
Statistical analysis
The risk indicators of AAE, SAE, SAE related to infection, fractures, neoplasm/cancer and deaths were used to assess the safety of denosumab in the postmenopausal women with osteoporosis or low BMD. Placebo or bisphosphonates were regarded as control. The RevMan 5.1 software was used for statistical analysis and risk ratio (OR) and 95% confidence intervals (CIs) as summary statistics were calculated. Heterogeneity of effect size across studies was assessed by using Cochran’s Q and the I2 statistic [15]. A Random-effects model was applied if there was significant heterogeneity (p<0.05, I2>50%). Otherwise, a fixed-effects model was used. Subgroup analysis was performed by the type of drugs which was used in the control group. Publication bias was observed with the funnel plot. Sensitivity analysis was performed by omitting special studies to test the stability of pooled results.
Result
Search results
A total of 1059 potential relevant articles were identified by initial literature research. After removing duplicated articles, 735 literatures were remained. Then, 704 obviously irrelevant literatures were excluded. Finally, 20 literatures were omitted from the remaining 31 literatures. The 20 literatures included three reviews, one animal study, four studies with the same population, six studies without related risk indicators, and seven studies lack of data about the comparison between the denosumab and control group. Finally, based on the included criteria and excluded criteria, 11 literatures [16-26] were included in this meta-analysis. The flow diagram of the search process is shown in Figure 1.
Characteristic of included studies
The characteristics of the included studies are summarized in Table 1. The included literatures were published from 2007 to 2014 in this meta-analysis. The 11 literatures contained 13 studies. The safety of denosumab was compared to placebo in seven [16,17,19-21,24,25] out of the thirteen studies with 4390 patients in placebo group and comparison between bisphosphonates and denosumab was shown in the other six studies [18,20,22-24,26] with 4390 patients in bisphosphonates group. Among the patients in all studies, 6483 were assigned to the denosumab group. Length of the follow-up time was from 9 months to 3 years. All the included literatures were high quality studies with the Jadad scores from 3 to 5. Assessment of risk of bias is shown in Figure 2. There was no high risk of bias in the studies except the study of Kumagai et al. [19], Recknor et al. [22] and Roux et al. [23].
Table 1.
Characteristics of 11 studies included in meta-analysis
| Author | Year | length of study (years) | Location | n, age trial mean (SD) | Treatment of trial | n, age control | Treatment of control | lumbar spine BMD T-scores | Outcomes | Jadad Score |
|---|---|---|---|---|---|---|---|---|---|---|
| Bone | 2008 | 2 | North America | 166 | 60 mg every 6 months | 166 | placebo | (-2.5, -1.0)+ | SAE; AAE; SAE related to infection; Neoplasm; Deaths; Non-vertebral fracture. | 4 |
| 59.8 (7.4) | 58.9 (7.5) | |||||||||
| Cummings | 2009 | 3 | multicenter, international | 3902 | 60 mg every 6 months | 3906 | placebo | (-4.0, -2.5) | SAE; SAE related to infection; Neoplasm; Deaths; Non-vertebral fracture. | 4 |
| 72.3 (5.2) | 72.3 (5.2) | |||||||||
| Ellis | 2008 | 2 | North America | 127 | 60 mg every 6 months | 125 | placebo | (-2.5, -1.0) | SAE; SAE related to infection; Neoplasm; Deaths. | 4 |
| 59.7 (9.7) | 89.2 (8.9) | |||||||||
| Kumagai | 2011 | 9 months | Japan | 30 | 0.03, 0.1, 0.3, 1.0, 3.0 mg/kg | 10 | placebo | NP | AAE | 3 |
| 40-66* | 59.6 (3.1) | |||||||||
| Lewiecki | 2007 | 2 | United States | 319 | 6, 14, 30 mg/ 3 months; 14, 60, 100, 210 mg/6 months | 46 | placebo | (-4.0, -1.5) | SAE; SAE related to infection; Neoplasm; Deaths; AAE | 4 |
| 63.7 (9.1) | ||||||||||
| 62.3 (8.0) | 47 | Alendronate 70 mg/week | ||||||||
| 62.8 (8.2) | ||||||||||
| Nakamura | 2012 | 1 | Japan | 157 | 60 mg every 6 months | 55 | placebo | (-4.0, -2.5) | SAE; SAE related to infection; Neoplasm; AAE | 4 |
| 65.2 (6.8) | 64.6 (7.0) | |||||||||
| Seeman | 2010 | 1 | multicenter, international | 83 | 60 mg every 6 months | 82 | placebo | (-3.0, -2.0) | AAE; SAE | 5 |
| 60.8 (5.2) | ||||||||||
| 60.3 (5.9) | 82 | Alendronate 70 mg/week | ||||||||
| 60.7 (5.2) | ||||||||||
| Brown | 2009 | 1 | multicenter, international | 594 | 60 mg every 6 months | 595 | Alendronate 70 mg/week | ≤2.0 | SAE; AAE; SAE related to infection; Neoplasm; Non-vertebral fracture. | 3 |
| 64.1 (8.6) | ||||||||||
| Kendler | 2010 | 1 | multicenter, international | 253 | 60 mg every 6 months | 251 | Alendronate 70 mg/week | (-4.0, -2.0) | SAE; AAE; SAE related to infection; Neoplasm; Deaths; Non-vertebral fracture. | 5 |
| 66.9 (7.8) | 68.2 (7.7) | |||||||||
| Recknor | 2013 | 1 | United States and Europe | 417 | 60 mg every 6 months | 416 | ibandronate 150 mg/month | (-4.0, -2.0) | AAE; SAE; SAE related to infection; Neoplasm; Non-vertebral fracture; Deaths. | 3 |
| 67.2 (8.1) | 66.2 (7.8) | |||||||||
| Roux | 2014 | 1 | multicenter, international | 435 | 60 mg every 6 months | 435 | risedronate 150 mg/month | -2.3 (1.1)&, | SAE; AAE; SAE related to infection; Deaths; Non-vertebral fracture. | 3 |
| 67.8 (7.0) | 67.7 (6.8) | -2.2 (1.2) |
range of age;
range of lumbar spine BMD T-scores;
mean (SD) of lumbar spine BMD T-scores;
AE: adverse events; SAE: Serious adverse events; AAE: any adverse events; NP: not provided.
Figure 2.
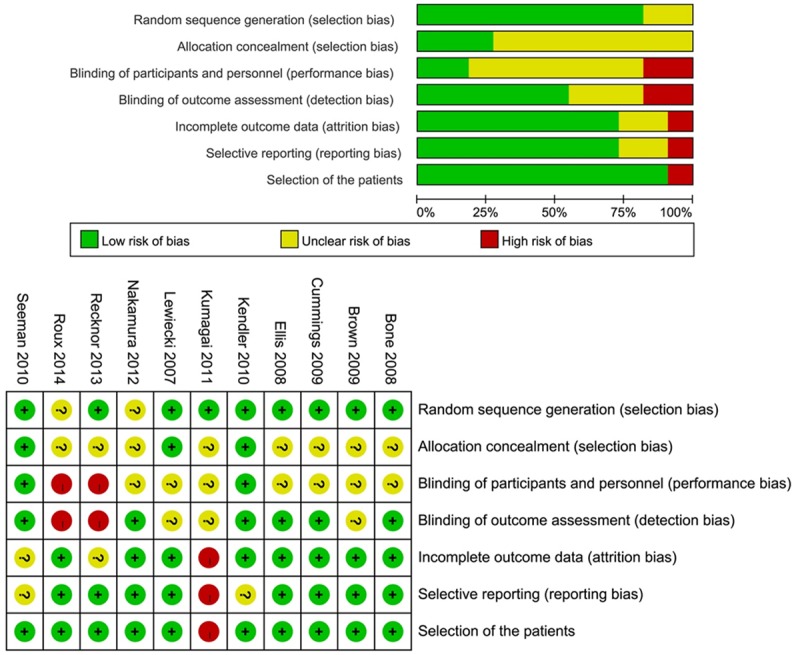
Risk of bias of 11 studies included in meta-analysis.
Comparison of safety
For the six risk indicators, no significant heterogeneity (p>0.05, I2<50%) was detected among the included studies. A fixed-effects model was used to calculate RR and 95% CI. Relevant forest plots are presented in Figures 3, 4. No significant difference between denosumab and control group was demonstrated in AAE (RR=0.99, 95% CI=0.98-1.01, p=0.29), SAE (RR=1.05, 95% CI=0.98-1.13, p=0.18), neoplasm/ cancer (RR=1.14, 95% CI=0.95-1.37, p=0.16) and deaths (RR=0.77, 95% CI=0.57-1.04, p=0.09) (Figure 3). Subjects assigned to denosumab demonstrated evidence of significant risk of SAE related to infection (RR=1.23, 95% CI=1.00-1.52, p=0.05) when compared to controls. Denosumab treatment significantly decreased the risk of non-vertebral fracture (RR=0.86, 95% CI=0.74-1.00, p=0.05) in the postmenopausal women with osteoporosis or low BMD (Figure 4).
Figure 3.
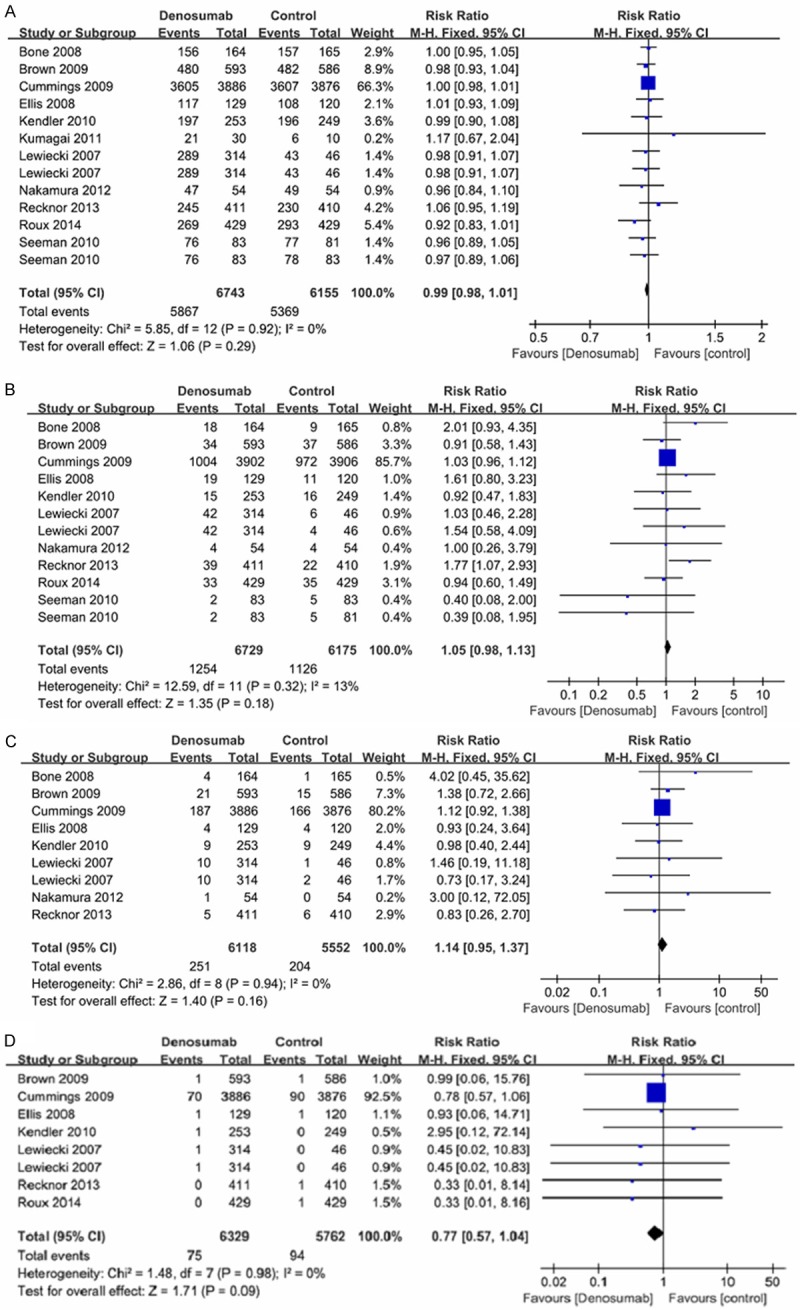
Forest plots of the risk of any adverse events, serious adverse events, neoplasm/cancer and deaths. A: Forest plot of the risk of any adverse events, B: Forest plot of the risk of serious adverse events, C: Forest plots of the risk of neoplasm/cancer, D: Forest plots of deaths.
Figure 4.
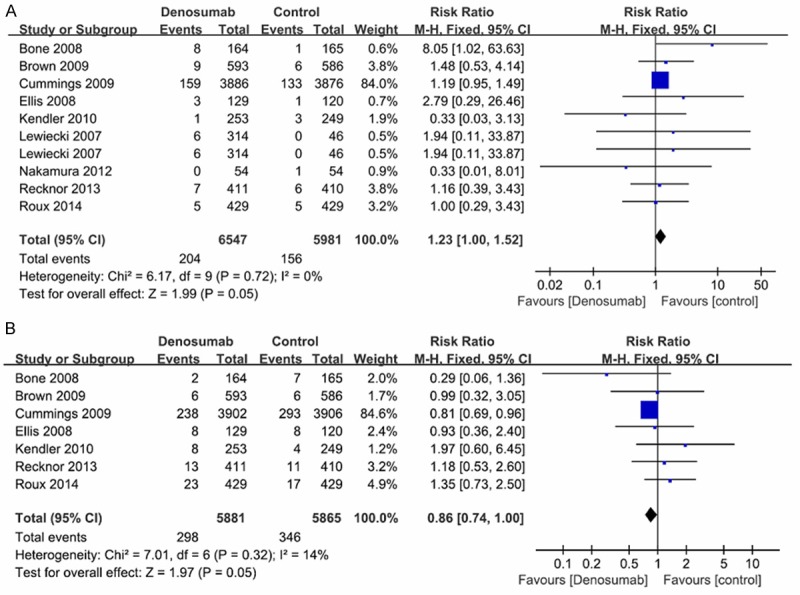
Forest plots of the risk of SAE related to infection and non-vertebral fracture. A: Forest plot of the risk of SAE related to infection, B: Forest plot of the risk of non-vertebral fracture.
Subgroup analysis
In subgroup analysis, the pooled data (AAE: RR=1.00, 95% CI=0.99-1.01; SAE: RR=1.05, 95% CI=0.97-1.13; SAE related to infection: RR=1.25, 95% CI=1.00-1.56; non-vertebral fracture: RR=0.80, 95% CI=0.68-0.95; neoplasm/ cancer: RR=1.13, 95% CI=0.93-1.38; deaths: RR=0.77, 95% CI=0.57-1.05) of denosumab vs. placebo showed the consistent results with the overall analysis. The results of denosumab vs. bisphosphonates indicated that there was no evidence to prove the significant differences between denosumab and bisphosphonates group in all the risk indicators (AAE: RR=0.98, 95% CI=0.95-1.02; SAE: RR=1.06, 95% CI=0.84-1.34; SAE related to infection: RR=1.13, 95% CI=0.63-2.03; neoplasm/cancer: RR=1.17, 95% CI=0.73-1.87; non-vertebral fracture: RR=1.31, 95% CI=0.87-1.98; deaths: RR=0.72, 95% CI=0.20-2.59) (Table 2).
Table 2.
Summary of subgroup analysis and sensitivity analysis
| Outcomes | Overall effect | Sensitivity analysis | Subgroup analysis | ||
|---|---|---|---|---|---|
|
| |||||
| Method 1 | Method 2 | Denosumab vs. placebo | Denosumab vs. bisphosphonates | ||
| AAE | 0.99 (0.98, 1.01) | 0.99 (0.96, 1.02) | 0.99 (0.98, 1.01) | 1.00 (0.99, 1.01) | 0.98 (0.95, 1.02) |
| SAE | 1.05 (0.98, 1.13) | 1.15 (0.94, 1.41) | 1.05 (0.97, 1.13) | 1.05 (0.97, 1.13) | 1.06 (0.84, 1.34) |
| SAE related to infection | 1.23 (1.00, 1.52) | 1.45 (0.87, 2.41) | 1.23 (1.00, 1.51) | 1.25 (1.00, 1.56) | 1.13 (0.63, 2.03) |
| Neoplasm (Cancer) | 1.14 (0.95, 1.37) | 1.20 (0.80, 1.81) | 1.14 (0.95, 1.38) | 1.13 (0.93, 1.38) | 1.17 (0.73, 1.87) |
| Non-vertebral fracture | 0.86 (0.74, 1.00) | 1.12 (0.78, 1.61) | 0.86 (0.74, 1.00) | 0.80 (0.68, 0.95) | 1.31 (0.87, 1.98) |
| Deaths | 0.77 (0.57, 1.04) | 0.72 (0.24, 2.13) | 0.78 (0.58, 1.05) | 0.77 (0.57, 1.05) | 0.72 (0.20, 2.59) |
Sensitivity analysis
Based on the characteristic of the included studies, two methods were used to do sensitivity analysis. First, the study of Cummings et al. [17] was eliminated because of great weight (66.3%~92.5%) (Figures 3, 4). After eliminating the study of Cummings et al., the pooled data showed an inconsistent result with the result of the overall analysis in non-vertebral fracture (RR=1.12, 95% CI=0.78-1.61). The result indicated no significant difference between denosumab and control group. For the other risk indicators (AAE: RR=0.99, 95% CI=0.96-1.02; SAE: RR=1.15, 95% CI=0.94-1.41; SAE related to infection: RR=1.45, 95% CI=0.87-1.41; neoplasm/ cancer: RR=1.20, 95% CI=0.80-1.81; deaths: RR=0.72, 95% CI=0.24-2.13), the results were similar with the results of the overall analysis. Second, the study of Kumagai et al. [19] and Lewiecki et al. [20] were omitted to detect the stability of the results. Because multiple doses of denosumab were used in the two studies, while single-dose (60 mg/6 month) was used in the other studies. After omitting the study of Kumagai et al. and Lewiecki et al., the similar results (AAE: RR=0.99, 95% CI=0.98-1.01; SAE: RR=1.05, 95% CI=0.97-1.13; SAE related to infection: RR=1.23, 95% CI=1.00-1.51; non-vertebral fracture: RR=0.86, 95% CI=0.74-1.00; neoplasm/cancer: RR=1.14, 95% CI=0.95-1.38; deaths: RR=0.78, 95% CI=0.58-1.05) with overall analysis was obtained (Table 2).
Statistically similar results with overall analysis were obtained in sensitivity analysis. It evinced the stability of this meta-analysis.
Publication bias
In this meta-analysis, no evidence of publication bias was detected in the risk indicator of SAE related to infection by the funnel plot in Figure 5.
Figure 5.
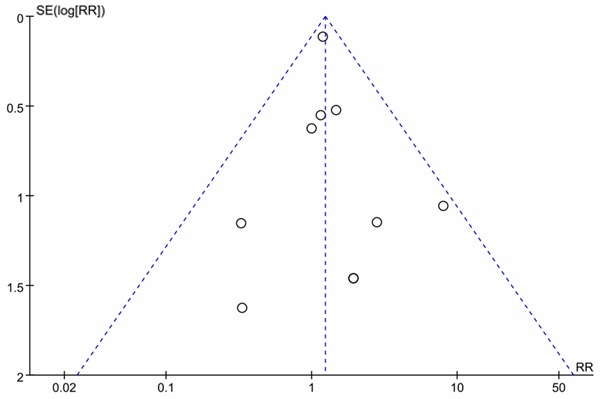
Funnel plot of detection of publication bias.
Discussion
In this meta-analysis, we pooled data from 11 studies. Based on the results of statistical analysis, we concluded that there was no significant difference in AAE, SAE, neoplasm/cancer and deaths between denosumab and control group. Compared to control group, denosumab treatment increased the risk of SAE related to infection but reduced the risk of non-vertebral fracture. Nevertheless, the increase in the risk of SAE related to infection and the reduction in the risk of non-vertebral fracture were not demonstrated in the results of denosumab vs. bisphosphonates in the subgroup analysis.
At present, the phase III studies of denosumab were carried on [27]. Although many studies reported that denosumab was well tolerated [28-30], there were many potential theoretical safety concerns. A concern regarding the long-term use of denosumab relates to its possible effects on the immune system, increasing the risk of infections and cancer [27,31]. In this meta-analysis, the result showed the increase in the risk of SAE related to infection. However, no significant effect on the risk of cancer was found. Another theoretical safety concern was that over-suppression of bone remodeling might increase fracture [32]. However, the risk of non-vertebral fracture was reduced when patients were treated by denosumab in this meta-analysis. Further studies need to be done to verify the result of this meta-analysis and explain the reason that why the results were inconsistent with the theoretical speculation.
Bisphosphonates have been widely, efficiently, and safely used for the treatment of osteoporosis [33]. No difference was found between the safety of denosumab and bisphosphonates in this meta-analysis. Thus, denosumab was safe as bisphosphonates in treating osteoporosis.
Anastasilakis et al. [34] reported a similar meta-analysis in 2009. Compared to that one, there were basically three reasons that constitute the primary advantages of this meta-analysis. First, the included studies were updated. Eight studies [17-19,21-24,26] that published after 2009 were included. Second, the safety of denosumab was assessed by comparing with placebo in the study of Anastasilakis et al. However, in this meta-analysis, the safety of denosumab was assessed by comparing with placebo or bisphosphonates in the overall analysis, and subgroup-analysis by the type of drugs used in the control group was performed. Third, there was no heterogeneity among the included studies in this meta-analysis.
Some limitations of this meta-analysis should be paid attention to. First, the sample size in the included studies was small except the study of Cummings et al. Thus, the stability and reliability of the result in this meta-analysis need to be verified by large sample studies. Second, the efficacy of denosumab for increasing BMD did not analysis in this meta-analysis due to the limitation of the data in the included studies. The following study need to be done to perfect the study of denosumab.
In conclusion, compared to placebo, denosumab treatment significantly reduced the risk of non-vertebral fracture but increased the risk of SAE related to infection in the postmenopausal women with osteoporosis or low BMD. However, there was no difference between the safety of denosumab and bisphosphonates. Denosumab is a valuable new option for the treatment of postmenopausal osteoporosis in women and may be used as a first-line treatment in future. However, due to the existence of the unstable factors, furthermore studies need to be done to verify the result of this study.
Disclosure of conflict of interest
The authors have declared that no competing interests exist.
References
- 1.Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rapuri PB, Gallagher JC, Haynatzka V. Protein intake: effects on bone mineral density and the rate of bone loss in elderly women. Am J Clin Nutr. 2003;77:1517–1525. doi: 10.1093/ajcn/77.6.1517. [DOI] [PubMed] [Google Scholar]
- 3.Bock O, Felsenberg D. Bisphosphonates in the management of postmenopausal osteoporosis– optimizing efficacy in clinical practice. Clin Interv Aging. 2008;3:279. doi: 10.2147/cia.s2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jang SN, Choi YH, Choi MG, Kang SH, Jeong JY, Choi YJ, Kim DH. Prevalence and associated factors of osteoporosis among postmenopausal women in Chuncheon: Hallym Aging Study (HAS) J Prev Med Public Health. 2006;39:389–396. [PubMed] [Google Scholar]
- 5.Shao M, Liu QS. Quality of life in patients with postmenopausal osteoporosis. J Osteoporos. 2000;6:58–58. [Google Scholar]
- 6.Kurth A, Pfeilschifter J. [Diagnosis and treatment of postmenopausal osteoporosis and osteoporosis in men. German Guidelines Update 2006] . Der Orthopade. 2007;36:683. doi: 10.1007/s00132-007-1106-3. [DOI] [PubMed] [Google Scholar]
- 7.Geusens P, Reid D. Newer drug treatments: Their effects on fracture prevention. Best Pract Res Clin Rheumatol. 2005;19:983–989. doi: 10.1016/j.berh.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Hughes DE, Wright KR, Uy HL, Sasaki A, Yoneda T, Roodman DG, Mundy GR, Boyce BF. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J Bone Miner Res. 1995;10:1478–1487. doi: 10.1002/jbmr.5650101008. [DOI] [PubMed] [Google Scholar]
- 9.Kostenuik PJ, Nguyen HQ, McCabe J, Warmington KS, Kurahara C, Sun N, Chen C, Li L, Cattley RC, Van G. Denosumab, a Fully Human Monoclonal Antibody to RANKL, Inhibits Bone Resorption and Increases BMD in Knock-In Mice That Express Chimeric (Murine/Human) RANKL. J Bone Miner Res. 2009;24:182–195. doi: 10.1359/jbmr.081112. [DOI] [PubMed] [Google Scholar]
- 10.Lewiecki EM. RANK ligand inhibition with denosumab for the management of osteoporosis. Expert Opin Biol Ther. 2006;6:1041–50. doi: 10.1517/14712598.6.10.1041. [DOI] [PubMed] [Google Scholar]
- 11.Delavallée L, Assier E, Semerano L, Bessis N, Boissier MC. Emerging applications of anticytokine vaccines. Expert Rev Vaccines. 2008;7:1507–17. doi: 10.1586/14760584.7.10.1507. [DOI] [PubMed] [Google Scholar]
- 12.Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther. 2007;9:S1. doi: 10.1186/ar2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Green S, Collaboration C. Wiley Online Library. 2008. Cochrane handbook for systematic reviews of interventions. [Google Scholar]
- 15.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bone HG, Bolognese MA, Yuen CK, Kendler DL, Wang H, Liu Y, San Martin J. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women. J Clin Endocrinol Metab. 2008;93:2149–57. doi: 10.1210/jc.2007-2814. [DOI] [PubMed] [Google Scholar]
- 17.Cummings SR, Martin JS, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 18.Kendler DL, Roux C, Benhamou CL, Brown JP, Lillestol M, Siddhanti S, Man HS, Martin JS, Bone HG. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res. 2010;25:72–81. doi: 10.1359/jbmr.090716. [DOI] [PubMed] [Google Scholar]
- 19.Kumagai Y, Hasunuma T, Padhi D. A randomized, double-blind, placebo-controlled, single-dose study to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of denosumab administered subcutaneously to postmenopausal Japanese women. Bone. 2011;49:1101–1107. doi: 10.1016/j.bone.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Lewiecki EM, Miller PD, McClung MR, Cohen SB, Bolognese MA, Liu Y, Wang A, Siddhanti S, Fitzpatrick LA. Two-Year Treatment With Denosumab (AMG 162) in a Randomized Phase 2 Study of Postmenopausal Women With Low BMD. J Bone Miner Res. 2007;22:1832–1841. doi: 10.1359/jbmr.070809. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura T, Matsumoto T, Sugimoto T, Shiraki M. Dose–response study of denosumab on bone mineral density and bone turnover markers in Japanese postmenopausal women with osteoporosis. Osteoporos Int. 2012;23:1131–1140. doi: 10.1007/s00198-011-1786-8. [DOI] [PubMed] [Google Scholar]
- 22.Recknor C, Czerwinski E, Bone HG, Bonnick SL, Binkley N, Palacios S, Moffett A, Siddhanti S, Ferreira I, Ghelani P. Denosumab Compared With Ibandronate in Postmenopausal Women Previously Treated With Bisphosphonate Therapy: A Randomized Open-Label Trial. Obstet Gynecol. 2013;121:1291–1299. doi: 10.1097/AOG.0b013e318291718c. [DOI] [PubMed] [Google Scholar]
- 23.Roux C, Hofbauer L, Ho P, Wark J, Zillikens M, Fahrleitner-Pammer A, Hawkins F, Micaelo M, Minisola S, Papaioannou N. Denosumab compared with risedronate in postmenopausal women suboptimally adherent to alendronate therapy: Efficacy and safety results from a randomized open-label study. Bone. 2014;58:48–54. doi: 10.1016/j.bone.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Seeman E, Delmas PD, Hanley DA, Sellmeyer D, Cheung AM, Shane E, Kearns A, Thomas T, Boyd SK, Boutroy S. Microarchitectural deterioration of cortical and trabecular bone: differing effects of denosumab and alendronate. J Bone Miner Res. 2010;25:1886–1894. doi: 10.1002/jbmr.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellis GK, Bone HG, Chlebowski R, Paul D, Spadafora S, Smith J, Fan M, Jun S. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J. Clin. Oncol. 2008;26:4875–4882. doi: 10.1200/JCO.2008.16.3832. [DOI] [PubMed] [Google Scholar]
- 26.Brown JP, Prince RL, Deal C, Recker RR, Kiel DP, de Gregorio LH, Hadji P, Hofbauer LC, Álvaro-Gracia JM, Wang H. Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res. 2009;24:153–161. doi: 10.1359/jbmr.0809010. [DOI] [PubMed] [Google Scholar]
- 27.Moen MD, Keam SJ. Denosumab. Drugs Aging. 2011;28:63–82. doi: 10.2165/11203300-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 28.Body JJ, Facon T, Coleman RE, Lipton A, Geurs F, Fan M, Holloway D, Peterson MC, Bekker PJ. A study of the biological receptor activator of nuclear factor-kappaB ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clin Cancer Res. 2006;12:1221–1228. doi: 10.1158/1078-0432.CCR-05-1933. [DOI] [PubMed] [Google Scholar]
- 29.McClung M, Lewiecki E, Geller M, Bolognese M, Peacock M, Weinstein R, Ding B, Rockabrand E, Wagman R, Miller P. Effect of denosumab on bone mineral density and biochemical markers of bone turnover: 8-year results of a phase 2 clinical trial. Osteoporos Int. 2013;24:227–235. doi: 10.1007/s00198-012-2052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller PD, Wagman RB, Peacock M, Lewiecki EM, Bolognese MA, Weinstein RL, Ding B, San Martin J, McClung MR. Effect of denosumab on bone mineral density and biochemical markers of bone turnover: six-year results of a phase 2 clinical trial. J Clin Endocrinol Metab. 2011;96:394–402. doi: 10.1210/jc.2010-1805. [DOI] [PubMed] [Google Scholar]
- 31.Canalis E. New treatment modalities in osteoporosis. Endocr Pract. 2010;16:855–863. doi: 10.4158/EP10048.RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Somma C. Interventional Neuroradiology of the Spine: Clinical Features, Diagnosis, and Therapy. 2013. Medical Therapy in Porotic Patients 10; p. 117. [Google Scholar]
- 33.Yoneda T, Hagino H, Sugimoto T, Ohta H, Takahashi S, Soen S, Taguchi A, Toyosawa S, Nagata T, Urade M. Bisphosphonate-related osteonecrosis of the jaw: position paper from the allied task force committee of Japanese society for bone and mineral research, Japan osteoporosis society, Japanese society of periodontology, Japanese society for oral and maxillofacial radiology, and Japanese society of oral and maxillofacial surgeons. J Bone Miner Metab. 2010;28:365–383. doi: 10.1007/s00774-010-0162-7. [DOI] [PubMed] [Google Scholar]
- 34.Anastasilakis AD, Toulis KA, Goulis DG, Polyzos SA, Delaroudis S, Giomisi A, Terpos E. Efficacy and safety of denosumab in postmenopausal women with osteopenia or osteoporosis: a systematic review and a meta-analysis. Horm Metab Res. 2009;41:721–729. doi: 10.1055/s-0029-1224109. [DOI] [PubMed] [Google Scholar]


