Abstract
NEDD9, a member of Crk-associated substrate (CAS) family, is highly expressed in multiple cancer types and involved cancer cell adhesion, migration, invasion. The prognostic value of NEDD9 has not been evaluated before. The aim of this study was to evaluate the association between NEDD9 expression and survival in colorectal cancer (CRC) patients. NEDD9 expression was analyzed by immunohistochemistry in 92 patients with CRC. Patients were followed-up annually by telephone or at outpatient clinic. The results revealed that high expression of NEDD9 in 68/92 CRC samples, compared with 12/92 normal tissues (P<0.01). Correlation analysis showed high level of expression of NEDD9 was significantly correlated with advanced TNM stage (P=0.014), pT grade (P=0.009), pN (P=0.013) and pM status (P=0.047). Patients with a higher NEDD9 expression had a significantly shorter overall survival (OS) (P<0.01). The multivariate analysis revealed that NEDD9 expression could serve as an independent predictive factor of OS. Our finding demonstrated the potential value of NEDD9 expression level as a prognostic molecular marker and a target for new therapies for CRC patients.
Keywords: NEDD9, colorectal cancer, immunohistochemistry, EMT, prognosis
Introduction
NEDD9, also called HEF1 and Cas-L, initially identified in neuronal precursor cells in 1992, was down-regulated during the development of early embryonic mouse central nervous system [1]. NEDD9 protein belonging to a member of Crk-associated substrate (CAS) family, is also an integral player in pathological cell biology. In the past several years, studies have identified elevated scaffolding protein NEDD9 has emerged as contributing to cancer metastasis in multiple cancer types, such as breast cancer, melanoma, glioblastoma, head and neck squamous cell carcinoma (HNSCC), lung cancer, liver cancer, and cervical cancer [2-10]. Although the mechanisms have not been fully elucidated, it is believed that NEDD9 regulates cell adhesion, migration, invasion, and epithelial-mesenchymal transition (EMT) [11-14]. Hence NEDD9 was identified as a metastasis marker in cancer patients.
The prognostic role of NEDD9 has been elucidated in some cancers. In non-small cell lung cancer, NEDD9 expression strongly correlates with recurrence-free survival or overall survival of the patients with NSCLC. And results suggest that NEDD9 is a useful biomarker for the prognosis of NSCLC [15]. Although highly expressed in colorectal cancer, the prognostic value of NEDD9 has not been evaluated before. Hereby, in this study, we showed the expression of NEDD9 in colorectal cancer and the relationship between the pathological characteristics and prognosis.
Materials and methods
Clinical specimens and patient data
92 colorectal cancer of different stages between January 2007 and June 2008 were collected from the tumor database of general surgery department in our hospital. And the tissue samples used in this study were collected from the tissue bank of general surgery department. Tissue samples were routinely fixed in 10% buffered neutral formalin. Cancer tissues were cut in wedge shapes and normal tissues were cut at least 5 cm away from tumor margin. All of the CRC patients were clinically and pathologically proven, without having received preoperative chemotherapy or radiotherapy. All specimens were collected with the informed consents of the patients and the protocols used in the study were approved by the Ethical Committee of Qingdao Municipal Hospital. TNM staging and clinicopathologic classification were determined according to the National Comprehensive Cancer Network (NCCN) classification. Demographic and clinicopathological parameters were prospectively recorded by chart review. Patients were followed-up annually by telephone or at outpatient clinic till June, 2013 or death.
Immunohistochemistry and scoring
Before staining, paraffin embedded tissue blocks were cut at 4 μm thicknesses. Sections were deparaffinized in the oven at 60°C for 2 h and rehydrated by two and three changes of xylene and ethanol, respectively. Antigen retrieval was performed with microwave retrieval method; endogenous peroxidase activity was quenched by incubation with 3% hydrogen peroxide for 10 minutes at room temperature. Nonspecific binding was blocked by incubating sections with 10% normal goat serum in PBS for 30 minutes at room temperature. Without washing, sections were incubated with rabbit polyclonal antibody against human NEDD9 (1:100; Abcam, Cambridge, MA, USA) at 4°C overnight, followed by incubation with horseradish peroxidase (HRP)-conjugated secondary goat anti-rabbit antibody (Abcam, San Francisco, USA) for 1 h at room temperature. Sections were then washed with PBS and treated with the Metal Enhanced DAB Substrate Kit (Thermo Scientific, USA) to visualize the antigen-antibody complex. Scoring of each section was separately performed by two researchers (Zhu and Zhou) who were unaware of clinicopathological status of the specimens. The percentage of stained cells on each section was scored as: 0 (less than 5%), 1 (5%-25%), 2 (26%-50%), and 3 (>50%) accordingly. Staining intensity was scored as: 0 (no staining), 1 (weak staining), 2 (moderate staining), and 3 (strong staining). The final score of each specimen was calculated by multiplying stained cells score with staining intensity score and thus ranged from 0 to 9. Low NEDD9 expression was defined as a final score of <4 and ≥4 as high NEDD9 expression.
Statistical analysis
All statistical analyses were carried out with the SPSS 18.0 software (SPSS Inc., Chicago, USA). Correlation between NEDD9 expression and clinical parameters were analyzed using Χ2 and Fisher’s exact tests. Overall survival was plotted with the Kaplan-Meier method and differences of the high and low expression of NEDD9 cases were compared by log-rank test. Analyses of prognostic factors for OS were determined by univariate and multivariate Cox proportional hazards regression method. P value<0.05 was considered as statistically significant.
Results
NEDD9 is highly expressed in colorectal cancer tissues
The clinicopathological characteristics of the 92 patients in this study are shown in Table 1. The mean age of all patients was 62 (range, 23-82 years), and 40 patients were female. Of all tumors, 77 cases were tubular adenocarcinoma, 12 were mucinous ones, and 3 were papillary adenocarcinoma; 57 tumors were located in rectum and sigmoid, 26 in right colon, and 9 in left colon. The numbers of I, II, III, and IV stage cancer were 15, 30, 37, and 10, respectively. The expression of NEDD9 in CRC tissues and matched adjacent non-tumor tissues were examined by immunohistochemistry. High expression of NEDD9 was detected in 68/92 CRC samples and 12/92 normal tissues (P<0.01). NEDD9 was located predominantly on cytoplasm and cancers of different stages showed different staining intensities (Figure 1).
Table 1.
Demographic and clinicopathological parameters of CRC patients
| Parameters | Number (%) |
|---|---|
| Age (years) | |
| <60 | 38 (41.3%) |
| ≥60 | 54 (58.7%) |
| Gender | |
| Male | 52 (56.5%) |
| Female | 40 (43.5%) |
| Histological type | |
| Tubular adenocarcinoma | 77 (83.7%) |
| Mucinous adenocarcinoma | 12 (13.0%) |
| Papillary adenocarcinoma | 3 (3.3%) |
| Tumor site | |
| Rectum and sigmoid | 57 (62.0%) |
| Right colon | 26 (28.3%) |
| Left colon | 9 (9.7%) |
| TNM stage | |
| I | 15 (16.3%) |
| II | 30 (32.6%) |
| III | 37 (40.2%) |
| IV | 10 (10.9%) |
| pT | |
| T1 | 8 (8.7%) |
| T2 | 15 (16.3%) |
| T3 | 32 (34.8%) |
| T4 | 37 (40.2%) |
| pN | |
| N0 | 45 (48.9%) |
| N1 | 28 (30.4%) |
| N2 | 19 (20.7%) |
| pM | |
| M0 | 82 (89.1%) |
| M1 | 10 (10.9%) |
Figure 1.
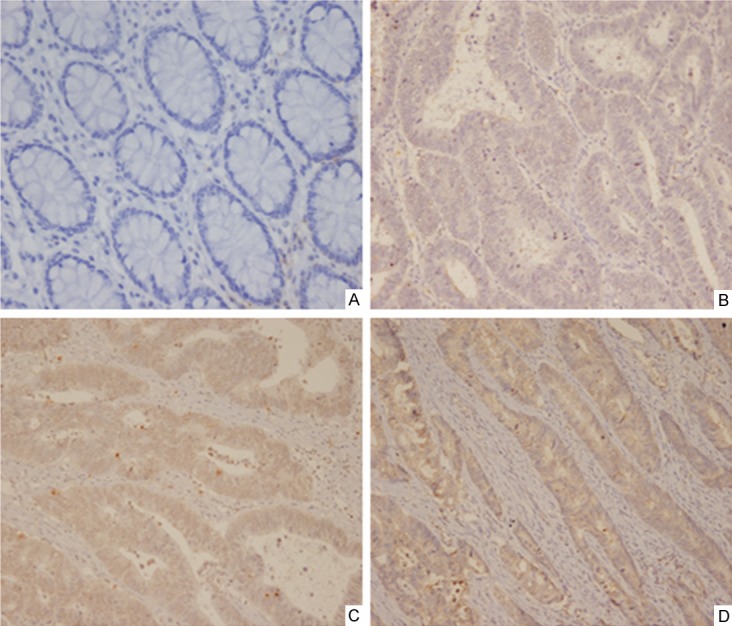
Expression of NEDD9 by immunohistochemistry in clinical specimens. (A)×200: NEDD9 staining of normal mucosa; (B)×200, (C)×200, (D)×200: NEDD9 staining in different stages of CRC tissues.
The correlation of NEDD9 with different clinicopathologic parameters
We analyzed the correlation between NEDD9 expression level of CRC samples and a set of clinicopathologic parameters, including age, gender, histological type, tumor site, and TNM stage (Table 2). High NEDD9 expression was found to be significantly correlated with TNM stage (P=0.014), pT grade (P=0.009), pN (P=0.013) and pM status (P=0.047). Other characteristics, like age (P=0.967), gender (P=0.787), histological type (P=0.956), and tumor site (P=0.857) were not associated with NEDD9 expression level.
Table 2.
Correlation of NEDD9 with different clinicopathologic parameters
| Parameters | NEDD9 | expression | Χ2 value | P value | |
|---|---|---|---|---|---|
|
|
|||||
| Low | High | ||||
| Age (years) | <60 | 10 | 28 | 0.002 | 0.967 |
| ≥60 | 14 | 40 | |||
| Gender | Male | 13 | 39 | 0.073 | 0.787 |
| Female | 11 | 29 | |||
| Histology | Tubular | 20 | 57 | 0.090 | 0.956 |
| Mucinous | 3 | 9 | |||
| Papillary | 1 | 2 | |||
| Site | Rectum & sigmoid | 16 | 41 | 0.308 | 0.857 |
| Right colon | 6 | 20 | |||
| Left colon | 2 | 7 | |||
| TNM stage | I | 8 | 7 | 10.630 | 0.014 |
| II | 9 | 21 | |||
| III | 7 | 30 | |||
| IV | 0 | 10 | |||
| pT | T1 | 4 | 4 | 11.476 | 0.009 |
| T2 | 8 | 7 | |||
| T3 | 7 | 25 | |||
| T4 | 5 | 32 | |||
| pN | N0 | 17 | 28 | 8.628 | 0.013 |
| N1 | 5 | 23 | |||
| N2 | 1 | 18 | |||
| pM | M0 | 16 | 41 | 0.308 | 0.047 |
| M1 | 0 | 10 | |||
Correlation between NEDD9 expression levels and patient overall survival
The 5-year overall survival indicated by Kaplan-Meier survival curve of low and high NEDD9 expression was shown in Figure 2. Patients with low NEDD9 had a longer OS compared with patients showing high expression (P<0.01); the 5-year OS for low and high NEDD9 expression patients were 62.5% and 35.3%. Furthermore, univariate analysis and multivariate analysis indicate that both advanced clinical stage and high NEDD9 expression independently predict poor prognosis of CRC (Table 3).
Figure 2.
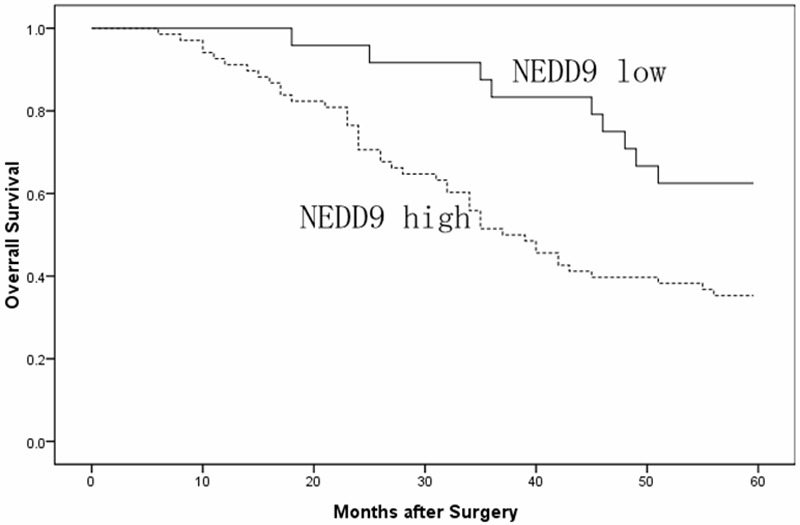
Overall survival analysis between patients with low and high NEDD9 expression (P<0.01).
Table 3.
Univariate and multivariate analysis of clinicopathologic parameters with OS by Cox proportional hazards regression
| Factors | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| OR | 95% CI | P | OR | 95% CI | P | |
| OS | ||||||
| Age | 1.003 | 0.968-1.021 | 0.838 | |||
| Gender | 1.322 | 0.974-1.033 | 0.326 | |||
| Histology | 1.435 | 0.590-3.490 | 0.98 | |||
| Site | 0.730 | 0.387-1.376 | 0.330 | |||
| TNM stage | 1.504 | 1.033-1.376 | 0.044 | 1.472 | 1.018-2.130 | 0.040 |
| NEDD9 | 2.191 | 1.020-4.708 | 0.033 | 2.435 | 1.120-5.295 | 0.025 |
Discussion
NEDD9 is a focal adhesion scaffolding protein related to p130CAS (Crk-associated substrate). Despite the reports of its initial functional analysis is in 1996 [16,17]. Up to now, NEDD9 was found to participate in cancer metastasis in breast cancer, melanoma, glioblastoma and lung cancer [2-6,8]. Currently, it is believed that NEDD9 protein is associated with migration, invasion and metastasis of cancer cells [11-14]. Kim M determined that NEDD9 was identified a bona fide melanoma metastasis gene. NEDD9 enhanced invasion in vitro and metastasis in vivo of both normal and transformed melanocytes [5]. KONG C found that NEDD9 was overexpressed in human aggressive breast cancers and promoted invasion in triple-negative breast cancer. Moreover, ectopic expression of NEDD9 upregulated the expression of the EMT, and promoted their interactions in vivo with the E-cadherin promoter [3]. In our study, we detected and compared the NEDD9 expression by immunohistochemical analysis in 92 colorectal cancer samples and 92 adjacent normal tissues. Our results revealed that NEDD9 protein was high expressed in most colorectal carcinoma tissues compared with normal tissues. Our results were similar to those reported by Kong C et al [3] in breast cancer and by Kim M et al [5] in melanoma and suggested that high expression of NEDD9 may involve in progression and metastasis of colorectal cancer.
The tumor of invasion and metastasis is a complex continuous process. the mechanism of NEDD9 in it is still not clear, may be through the phosphorylation of FAK with SRC and EMT joint action. FAK is one of the most important associated proteins of NEDD9 especially in the focal adhesion [15]. Studies found that the interaction between NEDD9 and focal adhesion kinase (FAK) for cell migration and invasion is very important [18]. Natarajan M et al thought that NEDD9 acted as specific downstream effectors of FAK that promoted glioblastoma cell migration and invasion [5]. Sima found overexpressed NEDD9 promotes migration and invasion in cervical carcinoma cells, probably via a positive feedback loop of tyrosine phosphorylation between NEDD9 and FAK or SRC [10]. Currently, the consensus by many groups suggests a mechanism in which cell attachment triggers the interaction of FAK, NEDD9, and SRC: overexpression of one of these proteins can also propel complex formation. These interactions lead to extensive tyrosine phosphorylation of NEDD9, creating binding sites for effector proteins with SH2 domains. Tumor invasiveness often requires EMT, during which cells lose lateral attachments and become more motile. One stamp of EMT is downregulation of the cell-cell adhesion protein E-cadherin, resulting in instability of the adherens junctions (AJs) that connect cells [19], but also revealed the role of NEDD9 in the EMT [14]. Nadezhda established that NEDD9 through SRC kinase promoted E-cadherin removal from cell junctions and its lysosomal degradation in mammalian cells [4]. Consequently, we found that NEDD9 was highly expressed in colorectal cancer tissues compared with adjacent noncancerous tissues, and significantly correlated with high TNM stage, which in turn supports the findings that NEDD9 is involved FAK tyrosine phosphorylation and EMT regulation of colorectal cancer.
The prognostic value of NEDD9 had been validated in human lung cancer, high expression of NEDD9 was a promising biomarker for the prognosis of NSCLCs [15]. Consistently, we found for the first time that the CRC patients with a high expression were associated with a shorter OS; furthermore, both univariate and multivariate Cox proportional hazards regression revealed that high NEDD9 expression was an independent poor prognostic factor. Thus, it’s worthy to further detect its value as a prognosis marker for the benefits of CRC patients.
Conclusively, the findings in this study showed NEDD9 was associated with poor prognosis of CRC patients. Based on our results, NEDD9 might serve as a prognosis marker for CRC patients with high levels of NEDD9. The practical value of this new biomarker and its underlying mechanisms in regulation of colorectal cancer progression require further evaluation.
Disclosure of conflict of interest
None.
References
- 1.Kumar S, Tomooka Y, Noda M. Identification of a set of genes with developmentally down-regulated expression in the mouse brain. Biochem Biophys Res Commun. 1992;185:1155–61. doi: 10.1016/0006-291x(92)91747-e. [DOI] [PubMed] [Google Scholar]
- 2.Izumchenko E, Singh MK, Plotnikova OV, Tikhmyanova N, Little JL, Serebriiskii IG, Seo S, Kurokawa M, Egleston BL, Klein-Szanto A, Pugacheva EN, Hardy RR, Wolfson M, Connolly DC, Golemis EA. NEDD9 promotes oncogenic signaling in mammary tumor development. Cancer Res. 2009;69:7198–7206. doi: 10.1158/0008-5472.CAN-09-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kong C, Wang C, Wang L, Ma M, Niu C, Sun X, Du J, Dong Z, Zhu S, Lu J, Huang B. NEDD9 is a positive regulator of epithelial-mesenchymal transition and promotes invasion in aggressive breast cancer. PLoS One. 2011;6:e22666. doi: 10.1371/journal.pone.0022666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tikhmyanova N, Golemis EA. NEDD9 and BCAR1 negatively regulate E-cadherin membrane localization, and promote E-cadherin degradation. PLoS One. 2011;6:1025–48. doi: 10.1371/journal.pone.0022102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim M, Gans JD, Nogueira C, Wang A, Paik JH, Feng B, Brennan C, Hahn WC, Cordon-Cardo C, Wagner SN, Flotte TJ, Duncan LM, Granter SR, Chin L. Comparative oncogenomics identifies NEDD9 as a melanoma metastasis gene. Cell. 2006;125:1269–81. doi: 10.1016/j.cell.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Natarajan M, Stewart JE, Golemis EA, Pugacheva EN, Alexandropoulos K, Cox BD, Wang W, Grammer JR, Gladson CL. HEFI is a necessary and specific downstream effector of FAK that promotes the migration of glioblastoma cell. Oncogene. 2006;25:1721–32. doi: 10.1038/sj.onc.1209199. [DOI] [PubMed] [Google Scholar]
- 7.Lucas JT Jr, Salimath BP, Slomiany MG, Rosenzweig SA. Regulation of invasive behavior by vascular endothelial growth factor is HEF1-dependent. Oncogene. 2010;29:4449–4459. doi: 10.1038/onc.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang JX, Gao F, Zhao GQ, Zhang GJ. Expression and clinical significance of NEDD9 in lung tissues. Med Oncol. 2012;29:2654–60. doi: 10.1007/s12032-012-0213-0. [DOI] [PubMed] [Google Scholar]
- 9.Budhu A, Forgues M, Ye QH, Jia HL, He P, Zanetti KA, Kammula US, Chen Y, Qin LX, Tang ZY, Wang XW. Prediction of venous metastases, recurrence, and prognosis in hepatocellar cacinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10:99–111. doi: 10.1016/j.ccr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Sima N, Cheng X, Ye F, Ma D, Xie X, Lu W. The Overexpression of Scaffolding Protein NEDD9 Promotes Migration and Invasion in Cervical Cancer via Tyrosine Phosphorylated FAK and SRC. PLoS One. 2013;8:e74594. doi: 10.1371/journal.pone.0074594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh M, Cowell L, Seo S, O’Neill G, Golemis E. Molecular basis for HEF1/NEDD9/Cas-L action as a multifunctional co-ordinator of invasion, apoptosis and cell cycle. Cell Biochem Biophys. 2007;48:54–72. doi: 10.1007/s12013-007-0036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Law SF, O’Neill GM, Fashena SJ, Einarson MB, Golemis EA. The docking protein HEFI is an apoptotic mediator at focal adhesion sites. Mol Cell Biol. 2000;20:5184–95. doi: 10.1128/mcb.20.14.5184-5195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fashena SJ, Einarson MB, O’Neill GM, Patriotis C, Golemis EA. Dissection of HEF1-dependent functions in motility and transcriptional regulation. J Cell Sci. 2002;115:99–111. doi: 10.1242/jcs.115.1.99. [DOI] [PubMed] [Google Scholar]
- 14.Bui LC, Tomkiewicz C, Chevallier A, Pierre S, Bats AS, Mota S, Raingeaud J, Pierre J, Diry M, Transy C, Garlatti M, Barouki R, Coumoul X. Nedd9/Hef1/Cas-L mediates the effects of environmental pollutants on cell migration and plasticity. Oncogene. 2009;28:3642–51. doi: 10.1038/onc.2009.224. [DOI] [PubMed] [Google Scholar]
- 15.Kondo S, Iwata S, Yamada T, Inoue Y, Ichihara H, Kichikawa Y, Katayose T, Souta-Kuribara A, Yamazaki H, Hosono O, Kawasaki H, Tanaka H, Hayashi Y, Sakamoto M, Kamiya K, Dang NH, Morimoto C. Impact of the integrin signaling adaptor protein NEDD9 on prognosis and metastatic behavior of human lung cancer. Clin Cancer Res. 2012;18:6326–38. doi: 10.1158/1078-0432.CCR-11-2162. [DOI] [PubMed] [Google Scholar]
- 16.Law SF, Estojak J, Wang B, Mysliwiec T, Kruh G, Golemis EA. Human enhancer of filamentation1, a novel p130cas-like docking protein, associates with focal adhesion kinaseand induces pseudohyphal growth in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:3327–37. doi: 10.1128/mcb.16.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minegishi M, Tachibana K, Sato T, Iwata S, Nojima Y, Morimoto C. Structure and function of Cas-L, a 105-kD Crk-associated substrate-related protein that is involved in beta-1 integrin-mediated signaling in lymphocytes. J Exp Med. 1996;184:1365–75. doi: 10.1084/jem.184.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Seventer GA, Salmen HJ, Law SF, O’Neill GM, Mullen MM, Franz AM, Kanner SB, Golemis EA van Seventer JM. Focal adhesion kinase regulates beta1 integrin-dependent T cell migration through an HEF1 effector pathway. Eur J Immunol. 2001;31:1417–27. doi: 10.1002/1521-4141(200105)31:5<1417::AID-IMMU1417>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 19.Giehl K, Menke A. Microenvironmental regulation of E-cadherin mediated adherens junctions. Front Biosci. 2008;13:3975–85. doi: 10.2741/2985. [DOI] [PubMed] [Google Scholar]


