Abstract
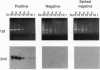
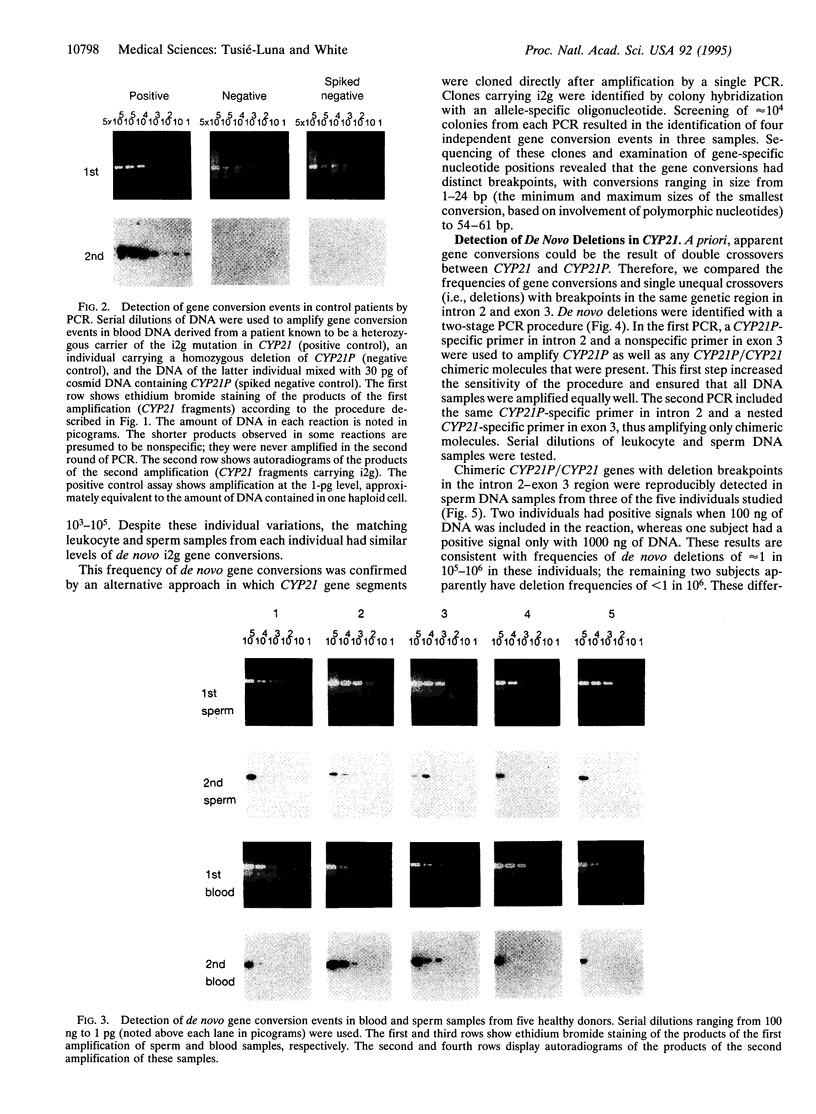
Most cases of congenital adrenal hyperplasia, the inherited inability to synthesize cortisol, are caused by mutations in the steroid 21-hydroxylase gene (CYP21). Steroid 21-hydroxylase deficiency is unusual among genetic diseases in that approximately 95% of the mutant alleles have apparently been generated by recombination between a normally active gene (CYP21) and a linked pseudogene (CYP21P). Approximately 20% of mutant alleles carry DNA deletions of 30 kb that have presumably been generated by unequal meiotic crossing-over, whereas 75% carry one or more mutations in CYP21 that are normally found in the CYP21P pseudogene. These latter mutations are termed "gene conversions," although the mechanism by which they are generated is not well understood. To assess the frequency at which these different recombination events occur, we have used PCR to detect de novo deletions and gene conversions in matched sperm and peripheral blood leukocyte DNA samples from normal individuals. Deletions with breakpoints in a 100-bp region in intron 2 and exon 3 were detected in sperm DNA samples with frequencies of approximately 1 in 10(5)-10(6) genomes but were never detected in the matching leukocyte DNA. Gene conversions in the same region occur in approximately 1 in 10(3)-10(5) genomes in both sperm and leukocyte DNA. These data suggest that whereas deletions occur exclusively in meiosis, gene conversions occur during both meiosis and mitosis, or perhaps only during mitosis. Thus, gene conversions must occur by a mechanism distinct from unequal crossing-over.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amor M., Parker K. L., Globerman H., New M. I., White P. C. Mutation in the CYP21B gene (Ile-172----Asn) causes steroid 21-hydroxylase deficiency. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1600–1604. doi: 10.1073/pnas.85.5.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M. C., Palsdottir A., Belt K. T., Porter R. R. Deletion of complement C4 and steroid 21-hydroxylase genes in the HLA class III region. EMBO J. 1985 Oct;4(10):2547–2552. doi: 10.1002/j.1460-2075.1985.tb03969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoue P. A., van Dop C., McLean R. H., White P. C., Jospe N., Migeon C. J. Gene conversion in salt-losing congenital adrenal hyperplasia with absent complement C4B protein. J Clin Endocrinol Metab. 1986 May;62(5):995–1002. doi: 10.1210/jcem-62-5-995. [DOI] [PubMed] [Google Scholar]
- Higashi Y., Hiromasa T., Tanae A., Miki T., Nakura J., Kondo T., Ohura T., Ogawa E., Nakayama K., Fujii-Kuriyama Y. Effects of individual mutations in the P-450(C21) pseudogene on the P-450(C21) activity and their distribution in the patient genomes of congenital steroid 21-hydroxylase deficiency. J Biochem. 1991 Apr;109(4):638–644. doi: 10.1093/oxfordjournals.jbchem.a123433. [DOI] [PubMed] [Google Scholar]
- Higashi Y., Tanae A., Inoue H., Hiromasa T., Fujii-Kuriyama Y. Aberrant splicing and missense mutations cause steroid 21-hydroxylase [P-450(C21)] deficiency in humans: possible gene conversion products. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7486–7490. doi: 10.1073/pnas.85.20.7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi Y., Yoshioka H., Yamane M., Gotoh O., Fujii-Kuriyama Y. Complete nucleotide sequence of two steroid 21-hydroxylase genes tandemly arranged in human chromosome: a pseudogene and a genuine gene. Proc Natl Acad Sci U S A. 1986 May;83(9):2841–2845. doi: 10.1073/pnas.83.9.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Högstrand K., Böhme J. A determination of the frequency of gene conversion in unmanipulated mouse sperm. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9921–9925. doi: 10.1073/pnas.91.21.9921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasin M., Zalamea P. Analysis of Escherichia coli beta-galactosidase expression in transgenic mice by flow cytometry of sperm. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10681–10685. doi: 10.1073/pnas.89.22.10681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Tamaki K., MacLeod A., Monckton D. G., Neil D. L., Armour J. A. Complex gene conversion events in germline mutation at human minisatellites. Nat Genet. 1994 Feb;6(2):136–145. doi: 10.1038/ng0294-136. [DOI] [PubMed] [Google Scholar]
- Li H., Cui X., Arnheim N. Direct electrophoretic detection of the allelic state of single DNA molecules in human sperm by using the polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4580–4584. doi: 10.1073/pnas.87.12.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifton R. P., Dluhy R. G., Powers M., Rich G. M., Gutkin M., Fallo F., Gill J. R., Jr, Feld L., Ganguly A., Laidlaw J. C. Hereditary hypertension caused by chimaeric gene duplications and ectopic expression of aldosterone synthase. Nat Genet. 1992 Sep;2(1):66–74. doi: 10.1038/ng0992-66. [DOI] [PubMed] [Google Scholar]
- Lipkowitz S., Garry V. F., Kirsch I. R. Interlocus V-J recombination measures genomic instability in agriculture workers at risk for lymphoid malignancies. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5301–5305. doi: 10.1073/pnas.89.12.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkowitz S., Stern M. H., Kirsch I. R. Hybrid T cell receptor genes formed by interlocus recombination in normal and ataxia-telangiectasis lymphocytes. J Exp Med. 1990 Aug 1;172(2):409–418. doi: 10.1084/jem.172.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monckton D. G., Neumann R., Guram T., Fretwell N., Tamaki K., MacLeod A., Jeffreys A. J. Minisatellite mutation rate variation associated with a flanking DNA sequence polymorphism. Nat Genet. 1994 Oct;8(2):162–170. doi: 10.1038/ng1094-162. [DOI] [PubMed] [Google Scholar]
- Mornet E., Dupont J., Vitek A., White P. C. Characterization of two genes encoding human steroid 11 beta-hydroxylase (P-450(11) beta). J Biol Chem. 1989 Dec 15;264(35):20961–20967. [PubMed] [Google Scholar]
- Pascoe L., Curnow K. M., Slutsker L., Connell J. M., Speiser P. W., New M. I., White P. C. Glucocorticoid-suppressible hyperaldosteronism results from hybrid genes created by unequal crossovers between CYP11B1 and CYP11B2. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8327–8331. doi: 10.1073/pnas.89.17.8327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnott P., Collier S., Costigan C., Dyer P. A., Harris R., Strachan T. Genesis by meiotic unequal crossover of a de novo deletion that contributes to steroid 21-hydroxylase deficiency. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2107–2111. doi: 10.1073/pnas.87.6.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speiser P. W., Dupont B., Rubinstein P., Piazza A., Kastelan A., New M. I. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am J Hum Genet. 1985 Jul;37(4):650–667. [PMC free article] [PubMed] [Google Scholar]
- Speiser P. W., Dupont J., Zhu D., Serrat J., Buegeleisen M., Tusie-Luna M. T., Lesser M., New M. I., White P. C. Disease expression and molecular genotype in congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Invest. 1992 Aug;90(2):584–595. doi: 10.1172/JCI115897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speiser P. W., New M. I., White P. C. Molecular genetic analysis of nonclassic steroid 21-hydroxylase deficiency associated with HLA-B14,DR1. N Engl J Med. 1988 Jul 7;319(1):19–23. doi: 10.1056/NEJM198807073190104. [DOI] [PubMed] [Google Scholar]
- Tusie-Luna M. T., Traktman P., White P. C. Determination of functional effects of mutations in the steroid 21-hydroxylase gene (CYP21) using recombinant vaccinia virus. J Biol Chem. 1990 Dec 5;265(34):20916–20922. [PubMed] [Google Scholar]
- Watkins W. S., Zenger R., O'Brien E., Nyman D., Eriksson A. W., Renlund M., Jorde L. B. Linkage disequilibrium patterns vary with chromosomal location: a case study from the von Willebrand factor region. Am J Hum Genet. 1994 Aug;55(2):348–355. [PMC free article] [PubMed] [Google Scholar]
- Wedell A., Thilén A., Ritzén E. M., Stengler B., Luthman H. Mutational spectrum of the steroid 21-hydroxylase gene in Sweden: implications for genetic diagnosis and association with disease manifestation. J Clin Endocrinol Metab. 1994 May;78(5):1145–1152. doi: 10.1210/jcem.78.5.8175971. [DOI] [PubMed] [Google Scholar]
- White P. C. Genetic diseases of steroid metabolism. Vitam Horm. 1994;49:131–195. doi: 10.1016/s0083-6729(08)61147-4. [DOI] [PubMed] [Google Scholar]
- White P. C., Grossberger D., Onufer B. J., Chaplin D. D., New M. I., Dupont B., Strominger J. L. Two genes encoding steroid 21-hydroxylase are located near the genes encoding the fourth component of complement in man. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1089–1093. doi: 10.1073/pnas.82.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. C., New M. I., Dupont B. Structure of human steroid 21-hydroxylase genes. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5111–5115. doi: 10.1073/pnas.83.14.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. C., Vitek A., Dupont B., New M. I. Characterization of frequent deletions causing steroid 21-hydroxylase deficiency. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4436–4440. doi: 10.1073/pnas.85.12.4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R. T., Rudders R. A., Zelenetz A., Delellis R. A., Krontiris T. G. BCL2 oncogene translocation is mediated by a chi-like consensus. J Exp Med. 1992 Jun 1;175(6):1575–1588. doi: 10.1084/jem.175.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]