Abstract
Backgroud and aim: Podoplanin (D2-40) is a specific marker for lymphatic endothelium. The vast majority of previous studies on podoplanin immunostaining in esophageal squamous cell carcinoma (ESCC) focused on identifying lymphatic vessel invasion (LVI) and counting lymphatic vessel density (LVD) and had contradictory results. Recent studies show podoplanin expression on cancer cells or tumor stroma in several cancers, which have specific significance; but the status in ESCC remains unclear. Therefore, the aim of this study was to further study and summarize the clinicopathological significance of podoplanin immunoreactivity in ESCC. Materials and methods: We examined podoplanin expression in tissue specimens from 107 patients with ESCC by immunohistochemistry. Podoplanin positive lymphatic vessels in intratumoral and peritumoral tissues and podoplanin positive expression in cancer cells and tumor stroma were analyzed, and correlated with clinicopathologic parameters and three-year overall and free-disease survival. Results: 34 (31.8%) and 28 (26.2%) of 107 specimens had podoplanin positive expression in cancer cells and tumor stroma, respectively. Logistic regression analysis showed high intratumoral lymphatic vessel density (I-LVD) and podoplanin positivity in cancer cells were increased risks of lymph node metastasis (LNM) (OR = 2.45, P = 0.03; OR = 0.35, P = 0.01, respectively). Survival analysis showed that I-LVD was a significant factor related to poor three-year overall and free-disease survival (P = 0.04, P = 0.03, respectively). Conclusions: Previous data and our results show that podoplanin seems to be a useful marker to predict LNM, recurrence, and worse prognosis in ESCC; in particular, LVI, high I-LVD, and podoplanin positivity in cancer cells are associated with LNM, recurrence and overall survival.
Keywords: Podoplanin, esophagus, immunohistochemistry, squamous cell carcinoma
Introduction
In China, esophageal cancer ranks 4th in terms of incidence and mortality among all cancers, with an estimated 259,235 new cases and 211,084 deaths in 2008 [1]. While esophageal adenocarcinom (EAC) has emerged as the major type in some western countries, esophageal squamous cell carcinoma (ESCC) is the predominant type in China [2]. Compared with other gastrointestinal tract cancers, ESCC is characterized by early lymphatic metastasis. The presence of lymph node metastasis (LNM) is a poor prognostic indicator for patients with ESCC [3]. After surgery, the five-year survival rate is less than 30% with positive lymph nodes (LNs), whereas it is greater than 60% in patients without LNM [4].
Podoplanin (D2-40) is a specific marker for lymphatic endothelium, allowing for the identification of lymphatic vessels (LVs) and assessment of lymphatic vessel density (LVD) [5,6]. Recent studies also found podoplanin expression in cancer cells and tumor stroma and demonstrated it to be involved in tumor progression and poor outcome [7-12]. In ESCC, most previous studies on D2-40 immunostaining investigated lymphatic status including lymphatic vessel invasion (LVI) and LVD [13-18], and few studies concerned about podoplanin expression in cancer cells or tumor stroma [19-21] (Table 1). Moreover, they had contradictory results. Thus, the clinicopathologic significance of podoplanin immunoreactivity in ESCC needs to be further investigated and summarized.
Table 1.
Previous studies on podoplanin immunostaining in esophageal squamous cell carcinoma
| Assessment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| LVI | LVD | Cancer cells expression | Stroma expression | |||||||
|
|
||||||||||
| Refs. | Sample size | pT | LNM | Prognosis | LNM | Prognosis | LNM | Prognosis | LNM | Prognosis |
| Tomita et al. [13] | 115 | T1 | √ | — | — | — | — | — | — | — |
| Gockel et al. [14] | 21 | T1 | √ | — | — | — | — | — | — | — |
| Our previous study. [15] | 107 | T1-4 | √ | — | — | — | — | — | — | — |
| Mori et al. [16] | 46 | T1-3 | √ | — | √ | — | — | — | — | — |
| Inoue et al. [17] | 81 | T1-3 | — | — | √ | √ | — | — | — | — |
| Imamura et al. [18] | 159 | Tis-T3 | √ | √ | √ | √ | — | — | — | — |
| Rahadiani et al. [19] | 61 | T1-3 | — | — | — | — | √ | √ | — | — |
| Chuang WY et al. [20] | 59 | T1-4 | √ | — | — | — | √ | √ | — | — |
| Tong L. et al. [21] | 56 | T1-4 | — | — | √ | √ | √ | √ | — | — |
| Current study | 107 | T1-4 | — | — | √ | √ | √ | √ | √ | √ |
Note: LVI, lymphatic vessel invasion; LVD, lymphatic vessel density; LNM, lymph node metastasis.
In the present study, we examined 107 ESCC tissue specimens by immunohistochemistry (IHC) with podoplanin monocolonal antibody. Podoplanin positive LVs in intratumoral and peritumoral tissues; podoplanin positive expression in cancer cells and tumor stroma were analyzed, and correlated with clinicopathologic parameters and three-year overall and free-disease survival.
Materials and methods
Patients and specimens
The subjects included 107 patients with thoracic ESCC who underwent radical surgical dissection and lymph node dissection without any preoperative therapy at the Department of Thoracic Surgery, QiLu Hospital of ShanDong University, from 1st January to 31th December 2010. The cases with other organ metastasis were excluded from this study. The cancer tissue specimens were collected from them. They all provided their informed consent regarding this study, and the protocol was approved by the Ethics Committee of QiLu Hospital of Shandong University. Patient, tumor, and treatment characteristics were retrieved from the Medical Records Room, which are summarized and shown in Table 2 (according to 7th edition UICC TNM stage).
Table 2.
Clinicopathologic parameters in 107 patients with esophageal squamous cell carcinoma
| Parameters | Total NO. (%) | |
|---|---|---|
| Gender | Male | 85 (79.4) |
| Female | 22 (20.6) | |
| Age | < 60 | 57 (53.3) |
| ≥ 60 | 50 (46.7) | |
| Location | Upper 1/3 | 11 (10.3) |
| Middle 1/3 | 60 (56.1) | |
| Lower 1/3 | 36 (33.6) | |
| Length | < 30 mm | 36 (33.6) |
| ≥ 30 mm | 71 (66.4) | |
| Differentiation | Well (G1) | 25 (23.4) |
| Moderate (G2) | 53 (49.5) | |
| Poor (G3) | 29 (27.1) | |
| Radicality | R0 | 88 (92.6) |
| R1 | 5 (5.3) | |
| R2 | 2 (2.1) | |
| pT | T1 | 14 (13.1) |
| T2 | 23 (21.5) | |
| T3 | 62 (57.9) | |
| T4 | 8 (7.5) | |
| LNM | N0 | 69 (64.5) |
| N1 | 38 (35.5) | |
| TNM stage | I | 17 (15.9) |
| II | 53 (49.5) | |
| III | 37 (34.6) | |
Note: LNM, lymph node metastasis; TNM, tumor node metastasis classification.
IHC staining
Formalin-fixed, paraffin-embedded tissue blocks were retrieved, cut into 5 μm-thick sections, and mounted on glass slides. These sections were deparaffinized in xylene, dehydrated in graded ethanol, microwaved in 10 mM citrate buffer (pH 6.0) for 20 min to unmask the epitopes and treated with 3% hydrogen peroxide for 10 min. They were then incubated with the ready-to-use anti-human D2-40 monoclonal antibody (Dako, Carpinteria, CA) at 4°C for 12 h. After washing, horseradish peroxidase/Fab polymer conjugate (EnVision Plus/HRP peroxidase kit; Dako, Carpinteria, CA) was applied to the sections at 37°C for 30 min. Finally, the sections were incubated with diaminobenzidine (DAB) for 1 min to develop the signals. Each series included negative control sections incubated without the primary antibody. Positive controls were paraffin-embedded sections of squamous cell carcinoma in cervix previously shown to react with the primary antibody.
Evaluation of podoplanin immunostaining
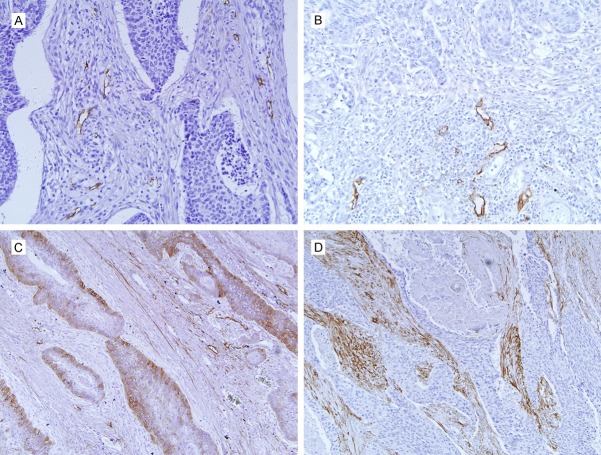
Intratumoral LVs were defined as the vessels within the main tumor mass, surrounded by tumor cells. Peritumoral LVs were defined as the vessels within 2 mm of the tumor margin which was bordered along tumor cells under microscopic observation. Only the vessels exhibiting a typical morphology (lumen) were considered LVs (Figure 1A and 1B). LVD was assessed as described previously [22]. Briefly, the most vascularized intratumoral and peritumoral areas (hot spot areas) were identified under low magnification (40× or 100×). The number of immunostained LVs found in 5 hot spot areas was counted at 200× magnification. The LVD in each case was expressed as means (total number of vessels in 5 hot spot areas/5).
Figure 1.
Podoplanin positive expression in esophageal squamous cell carcinoma. Strong podoplanin staining for lymphatics in intratumoral (A) and peritumoral (B) locations; the blood vessels in peritumoral tissue are negative for D2-40 indicating its specificity for lymphatic vessels. D2-40 positivity is seen in membrane and cytoplasm of cancer cells, and positive cells are mainly located in the periphery of cancer nests (C). (D) Shows stromal podoplanin staining with high amount of podoplanin expressing cancer-associated fibroblasts. Original magnification, ×200.
Cellular immunoreaction was considered to be negative if it involved less than 5% of cancer cells and positive if it involved greater than 5%; stromal immunoreaction was considered to be negative if it involved less than 10% of stroma and positive if it involved greater than 10% [8].
Stained histologic sections were analyzed using standard light microscopy (Olympus, BS51). Evaluation of podoplanin immunostaining was performed independently by two pathologists (Drs. Tingguo Zhang and Xiaoqing Yang) without knowledge of any of the patients’ clinicopathological features.
Statistical analysis
Statistical analysis was performed by using the Kolmogorov-Smirnov normality test for all parameters considered.
The Student test and chi-square test (χ2 test) were used as appropriate. Logistic regression analysis was used for the relationship between considered risk factors and LNM. The Kaplan-Meier method was applied in the survival analysis and its statistical significance was calculated by using the log-rank test. Univariate and multivariate analyses were performed by using a Cox proportional hazards model. Variables with a probability value of less than 0.05 in the univariate analysis were introduced in a multivariate stepwise proportional-hazards analysis (Cox model) to identify which variable had an independent effect on prognosis. The results are given as hazard ratios with their corresponding 95% confidence interval. P values < 0.05 (two-sided) were considered statistically significant. All analyses were performed using SPSS 16 (SPSS Inc., Chicago, IL).
The effect of intratumoral LVD (I-LVD) and peritumoral LVD (P-LVD) on the survival rate was determined using the median (4.39 and 5.40) as the cutoff value. For the analysis of three-year overall survival, events were defined as death from any cause. For the analysis of three-year disease-free survival, events were defined as first loco-regional or distant tumor relapse or death from any cause.
Results
Lymph node status and clinicopathologic parameters
Postoperative follow-up data were obtained from all patients until death or March 2013. The median follow-up time was 40 months (range, 4 months to 50 months); the median age of the patients was 60 years (range 42-77 years). The clinicopathologic parameters of tumors are shown in Table 2.
There were 69 patients without LNM (N0) and 38 patients with LNM (N+).
Mean three-year overall survival rate was 53.3%, with significant difference between N0 and N+ patients (63.8% vs. 34.2%; P = 0.003, log-rank test); mean three-year disease-free survival rate was 54.2%, with significant difference between N0 and N+ patients (62.3% vs. 39.5%; P = 0.003, log-rank test).
Podoplanin expression
Podoplanin immunopositive expression was observed in LV endothelia, cancer cells, and tumor stroma (Figure 1).
LVD and clinicopathologic parameters
Lymphatic endothelium staining was strong and distinct when present in tumor tissue. We found that most intratumoral lymphatics were small and flattened, in contrast with the widely open lymphatics in peritumoral regions. Also, intratumoral lymphatics were not accompanied by preexisting structures such as smooth muscles, fibres, or large blood vessels (Figure 1A and 1B).
Podoplanin positive LVs were seen in peritumoral tissue from all specimens and intratumoral tissue from 93 (90.7%) specimens. The number of intratumoral and peritumoral LVs ranged respectively from 0 to 19 (median, 4.39; mean ± SD, 5.09 ± 4.13), 2 to 23 (median, 5.40; mean ± SD, 7.65 ± 6.16). Mean P-LVD was significantly higher than I-LVD (P = 0.00, Student t test) (Figure 2A).
Figure 2.
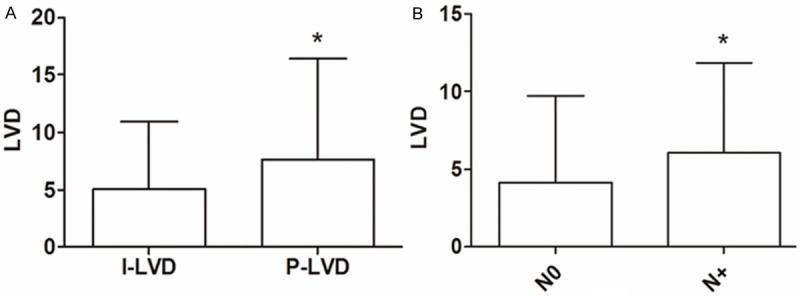
Comparison of lymphatic vessel density (LVD) in different groups. A: Peritumoral LVD (P-LVD) was greater than intratumoral LVD (I-LVD). B: Intratumoral LVD was higher in patients with LNM (N+) than in patients without LNM (N0).
High I-LVD (> 4.39) was correlated significantly with depth of invasion (P = 0.00, χ2 test), LNM (P = 0.02, χ2 test) and clinical stage (P = 0.01, χ2 test), whereas P-LVD had no significant association with any clinicopathological parameters.
I-LVD was higher in N+ patients (mean ± SD, 6.09 ± 4.06) than in N0 patients (mean ± SD, 4.15 ± 3.92) (P = 0.01, Student t test) (Figure 2B). Logistic regression analysis showed high I-LVD was an increased risk of LNM (OR = 2.45, P = 0.03). On the other hand, tumor differentiation and depth of invasion were related with LNM (OR = 0.38, P = 0.04; OR = 3.31, P = 0.02, respectively).
Podoplanin expression in cancer cells and clinicopathologic parameters
34 (31.8%) of 107 specimens had podoplanin positive expression in tumor cells. IHC results showed strong membrane and cytoplasmatic podoplanin staining, and the positive cells were mainly located in the periphery of cancer nests (Figure 1C).
Podoplanin positivity in cancer cells was found in 47.4% and 23.2% of patients with LNM and without LNM, respectively (P = 0.01, χ2 test). No significant correlation was observed between it and any other parameters. Logistic regression analysis showed podoplanin positivity in cancer cells was also an increased risk of LNM (OR = 0.35, P = 0.01).
Podoplanin expression in tumor stroma and clinicopathologic parameters
Stromal podoplanin immunostaining was mainly in tumor-associated fibroblasts (Figure 1D). 28 (26.2%) of 107 specimens had podoplanin positive expression in tumor stroma; 26.3% and 26.1% of patients with LNM and without LNM had podoplanin positivity, respectively (P = 0.097, χ2 test). It had no significant association with any other parameters.
Survival analysis
The three-year overall and free-disease survival rate were 59.1% and 60.6% in the low I-LVD group (< 4.39); 43.9% and 43.9% in the high I-LVD group, respectively. Both three-year overall and free-disease survival rate in the low I-LVD group were significantly better than those in the high I-LVD group (P = 0.04, P = 0.03, respectively) (Figure 3). There were no statistically difference between low and high P-LVD (P = 0.60, P = 0.40, respectively).
Figure 3.
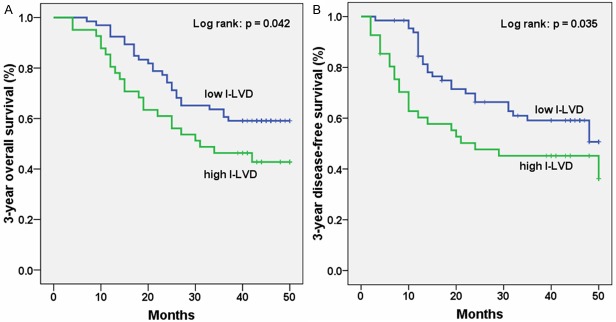
Kaplan-Meier analysis of three-year survival according to intratumoral lymphatic vessel density (I-LVD). For three-year overall (A) and free-disease (B) survival, high I-LVD was significantly associated with poor outcomes (P = 0.042, P = 0.035, respectively).
In the univariate analysis, I-LVD, radicality, depth of invasion, LNM and TNM stage were all found to be significantly related to worse prognosis (all P < 0.05, log-rank test). The multivariate analysis showed that radicality (P = 0.01), depth of invasion (P = 0.03), LNM (P = 0.00) and TNM stage (P = 0.00) were the significant independent prognostic factors for three-year overall survival rate; the significant independent prognostic factors for three-year free-disease survival rate were only radicality, depth of invasion and LNM (P = 0.01, P = 0.04 and P = 0.01, respectively) (Table 3).
Table 3.
Cox analysis for three-year survival in 107 patients with esophageal squamous cell carcinoma
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| 3-Yr OS | 3-Yr DFS | 3-Yr OS | 3-Yr DFS | |||||
|
|
||||||||
| Parameters | P Value | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value |
| Length (mm) | 0.68 | 0.89 | — | — | ||||
| < 30 vs. ≥ 30 | ||||||||
| Differentiation | 0.71 | 0.77 | — | — | ||||
| Well vs. Moderate, Poor | ||||||||
| Radicality | 0.00* | 0.00* | 0.29 | 0.11-0.73 | 0.01* | 0.33 | 0.140-0.78 | 0.01* |
| R0 vs. R1, R2 | ||||||||
| Depth of invasion | 0.01* | 0.01* | 0.39 | 0.16-0.94 | 0.03* | 2.09 | 0.95-4.05 | 0.04* |
| T1, 2 vs. T3, 4 | ||||||||
| LNM | 0.00* | 0.00* | 2.73 | 1.08-5.04 | 0.00* | 2.34 | 1.09-3.30 | 0.01* |
| N0 vs. N+ | ||||||||
| TNM stage | 0.00* | 0.01 | 2.63 | 1.50-4.06 | 0.00* | 1.41 | 0.34-5.81 | 0.63 |
| I, II vs. III, IV | ||||||||
| I-LVD | 0.04* | 0.04* | 0.63 | 0.33-1.20 | 0.16 | 0.87 | 0.48-1.26 | 0.69 |
| Low vs. High | ||||||||
| P-LVD | 0.60 | 0.40 | — | — | ||||
| Low vs. High | ||||||||
| Cancer cells | 0.58 | 0.60 | — | — | ||||
| (+) vs. (-) | ||||||||
| Stroma | 0.44 | 0.09 | — | — | ||||
| (+) vs. (-) | ||||||||
Note: OS, overall survival; DFS, disease-free survival; LVD, lymphatic vessel density; LNM, lymph node metastasis; TNM, tumor node metastasis classification; HR, hazard ratio; CI, confidence interval.
Statistically significant.
Discussion
The lymphatic system is the primary pathway of metastasis for most human cancers, and the extent of LN involvement is a crucial factor for clinical treatments and patient prognosis. Especially, monocolonal antibody D2-40 (podoplanin) specific for lymphatic endothelium has provided important new insights into metastasis of cancer cells in lymphatic system [23]. In addition, the high expression of podoplanin itself in cancer cells and tumor stroma also attract attention [24]. Therefore, we further investigated and summarized the clinicopathological significance of podoplanin immunostaining in surgical specimens from 107 patients with ESCC.
Clinical and pathological data show that the presence of tumor cells in LVs and regional LNs is an important early event in the metastasis of carcinoma [25]. However, there were contradictory results regarding the channels through which tumor cells enter into the lymphatic system, the preexisting lymphatics or the newly formed tumor-associated lymphatics in various malignant neoplasia [26-29]. More and more studies have indicated that cancer metastasis and tumor lymphangiogenesis have complex mechanisms that can differ significantly in tumors of different types or anatomic locations [30-34]. Regarding to ESCC, it seems that I-LVD, not P-LVD, is associated with LNM and worse prognosis according to the existing literatures including this study [16-18,21]. These results demonstrate that I-LVD would be useful in daily pathological or clinical practice. We recommend that the ESCC patients with LNM or without LNM, but with high I-LVD (the data should be further unified) should be followed up carefully. And earlier and longer treatments might need to be seriously considered. Contrasting with the widely open peritumoral lymphatics, most intratumoral lymphatics were smaller, flattened, and more difficult to see owing to high interstitial pressure in tumors [35]. We hold the view that the increase of permeability in newly formed lymphatics lead to tumor cells into microvessels, which is resulted from structural defects such as incomplete basement membrane and loose cell links compared to complete peritumoral lymphatics.
Podoplanin has been suggested to play a role in lymphangiogenesis, since podoplanin deficient mice were found to have dilated malfunctioning lymphatic vessels and lymphedema [36,37]. High podoplanin expression in tumor cells was found to correlate with LNM in patients with cutaneous squamous cell carcinoma [11]. The more significant finding of this study was D2-40 cellular positivity in 31.8% ESCC specimens and it was an increased risk of LNM, which was in accordance with previous results [19-21]. But there is no consensus about podoplanin cellular expression in different cancers. High podoplanin expression in cancer cells predicts lower incidence of LN metastasis in patients with lung squamous cell carcinoma [38]. Schoppmann et al [8] observed podoplanin expression in eight (5.6%) gastrointestinal stromal tumors and it was not associated with clinical risk factors or patient survival. In addition, the association between podoplanin cellular positivity and patient survival was negative in our study, but positive in former researches [19-21]. Although they had positive results, their sample size was smaller and divided into more subgroups (+, ++, +++ and ++++), which might lead to various conclusions.
The significance of podoplanin expression in tumor stromal remains also controversial. Some researchers described the stromal expression of podoplanin as an adverse prognostic factor in lung carcinomas [39] and invasive ductal breast carcinoma [8], while other investigators found podoplanin expression to be associated with a better prognosis in patients with colorectal carcinomas [40]. In our study, the percentage of podoplanin positivity in stroma was 26.2% and it did not correlate with any parameters or survival, which raised doubts on the above results. Podoplanin positive expression rate in stroma from 1350 cases of 14 common cancer types was an average of 43% [41]. A lower percentage of Podoplanin positive expression in ESCC stroma might cause these negative results. To the best of our knowledge, this is the first study on stromal D2-40 expression in ESCC.
Many studies including our former report have indicated that podoplanin is useful in identifying the presense of LVI in various malignant neoplasms [15,42]. Podoplanin staining increased the detection rate of LV by 15% and 16% over hematoxylin-eosin (HE) staining in EAC and melanoma, respectively [43,44]. Furthermore, two recent meta-analyses suggest that LV is a predictor of LNM and/or poorer outcome in primary melanoma and non-small cell lung cancer [45,46]. As the relationship between LVI and prognosis/LNM has become evident in various cancers, it seems that podoplanin immunostaining, LVI, should be first used in guiding clinical treatments and assessing prognosis.
Podoplanin immunostaining cells were located in the periphery of cancer nests in the present study (Figure 1C). A similar pattern of expression has been reported in squamous cell carcinoma of the head and neck, esophagus and uterine cervix [19,47,48]. The exclusive expression of podoplanin suggests that some growth factors may influence podoplanin expression. In fact, podoplanin expression is induced by epidermal growth factor, basic fibroblast growth factor, and tumor necrosis factor α in MCF7 breast cancer cells, and by bradykinin in 3T3 fibroblasts [49,50].
D2-40 is a monoclonal antibody that was initially developed against the M2A antigen, which is a fetal testis-related antigen, and is now better known as podoplanin [37,51]. Previous studies support the concept that podoplanin is a cancer stem cell marker for squamous cell carcinoma [50,52,53]. The findings of our study, systematic description for the podoplanin expression in ESCC, may be related to the biological and functional complexity of podoplanin, especially to its role in enhancing cell migration and lymphatic spread to regional lymph nodes.
Our data suggested podoplanin was frequently expressed in ESCC; high I-LVD, not high P-LVD; and podoplanin positivity in cancer cells, not in tumor stroma were increased risks of LNM; and only increased I-LVD was negatively related with recurrence and overall survival. To sum up, podoplanin seems to be a useful marker to predict LNM, recurrence, and worse prognosis in ESCC; in particular, high I-LVD, LVI and podoplanin positivity in cancer cells were associated with LNM, recurrence and overall survival.
Acknowledgements
The authors thank Drs Tingguo Zhang and Xiaoqing Yang from the Department of Pathology, Shandong University School of Medicine, China, for his expert suggestions and technical assistance. This work was supported by China Postdoctoral Science Fund (No. 2011M500531) and Science and Technology development planning of Shandong province (No. 2012GGE27088).
Disclosure of conflict of interest
None.
References
- 1.Lin Y, Totsuka Y, He Y, Kikuchi S, Qiao Y, Ueda J, Wei W, Inoue M, Tanaka H. Epidemiology of esophageal cancer in Japan and China. J Epidemiol. 2013;23:233–242. doi: 10.2188/jea.JE20120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shibata A, Matsuda T, Ajiki W, Sobue T. Trend in incidence of adenocarcinoma of the esophagus in Japan, 1993-2001. Jpn J Clin Oncol. 2008;38:464–468. doi: 10.1093/jjco/hyn064. [DOI] [PubMed] [Google Scholar]
- 3.Izbicki JR, Hosch SB, Pichlmeier U, Rehders A, Busch C, Niendorf A, Passlick B, Broelsch CE, Pantel K. Prognostic value of immunohistochemically identifiable tumor cells in lymph nodes of patients with completely resected esophageal cancer. N Engl J Med. 1997;337:1188–1194. doi: 10.1056/NEJM199710233371702. [DOI] [PubMed] [Google Scholar]
- 4.Wang LS, Chow KC, Chi KH, Liu CC, Li WY, Chiu JH, Huang MH. Prognosis of esophageal squamous cell carcinoma: analysis of clinicopathological and biological factors. Am J Gastroenterol. 1999;94:1933–1940. doi: 10.1111/j.1572-0241.1999.01233.x. [DOI] [PubMed] [Google Scholar]
- 5.Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154:385–394. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi’s sarcoma and a subset of angiosarcomas. Mod Pathol. 2002;15:434–440. doi: 10.1038/modpathol.3880543. [DOI] [PubMed] [Google Scholar]
- 7.Minardi D, d’Anzeo G, Lucarini G, Filosa A, Zizzi A, Simonetti O, Polito M Jr, Offidani AM, Di Primio R, Montironi R, Muzzonigro G. D2-40 immunoreactivity in penile squamous cell carcinoma: a marker of aggressiveness. Hum Pathol. 2011;42:1596–1602. doi: 10.1016/j.humpath.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 8.Schoppmann SF, Berghoff A, Dinhof C, Jakesz R, Gnant M, Dubsky P, Jesch B, Heinzl H, Birner P. Podoplanin-expressing cancer-associated fibroblasts are associated with poor prognosis in invasive breast cancer. Breast Cancer Res Treat. 2012;134:237–244. doi: 10.1007/s10549-012-1984-x. [DOI] [PubMed] [Google Scholar]
- 9.Hoshino A, Ishii G, Ito T, Aoyagi K, Ohtaki Y, Nagai K, Sasaki H, Ochiai A. Podoplanin-positive fibroblasts enhance lung adenocarcinoma tumor formation: podoplanin in fibroblast functions for tumor progression. Cancer Res. 2011;71:4769–4779. doi: 10.1158/0008-5472.CAN-10-3228. [DOI] [PubMed] [Google Scholar]
- 10.Pula B, Jethon A, Piotrowska A, Gomulkiewicz A, Owczarek T, Calik J, Wojnar A, Witkiewicz W, Rys J, Ugorski M, Dziegiel P, Podhorska-Okolow M. Podoplanin expression by cancer-associated fibroblasts predicts poor outcome in invasive ductal breast carcinoma. Histopathology. 2011;59:1249–1260. doi: 10.1111/j.1365-2559.2011.04060.x. [DOI] [PubMed] [Google Scholar]
- 11.Toll A, Gimeno-Beltran J, Ferrandiz-Pulido C, Masferrer E, Yebenes M, Jucgla A, Abal L, Marti RM, Sanmartin O, Baro T, Casado B, Gandarillas A, Barranco C, Costa I, Mojal S, Garcia-Patos V, Pujol RM. D2-40 immuno- histochemical overexpression in cutaneous squamous cell carcinomas: a marker of metastatic risk. J Am Acad Dermatol. 2012;67:1310–1318. doi: 10.1016/j.jaad.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Funayama A, Cheng J, Maruyama S, Yamazaki M, Kobayashi T, Syafriadi M, Kundu S, Shingaki S, Saito C, Saku T. Enhanced expression of podoplanin in oral carcinomas in situ and squamous cell carcinomas. Pathobiology. 2011;78:171–180. doi: 10.1159/000324926. [DOI] [PubMed] [Google Scholar]
- 13.Tomita N, Matsumoto T, Hayashi T, Arakawa A, Sonoue H, Kajiyama Y, Tsurumaru M. Lymphatic invasion according to D2-40 immunostaining is a strong predictor of nodal metastasis in superficial squamous cell carcinoma of the esophagus: algorithm for risk of nodal metastasis based on lymphatic invasion. Pathol Int. 2008;58:282–287. doi: 10.1111/j.1440-1827.2008.02224.x. [DOI] [PubMed] [Google Scholar]
- 14.Gockel I, Domeyer M, Sgourakis GG, Schimanski CC, Moehler M, Kirkpatrick CJ, Lang H, Junginger T, Hansen T. Prediction model of lymph node metastasis in superficial esophageal adenocarcinoma and squamous cell cancer including D2-40 immunostaining. J Surg Oncol. 2009;100:191–198. doi: 10.1002/jso.21336. [DOI] [PubMed] [Google Scholar]
- 15.Bai B, Ma W, Wang K, Ha S, Wang JB, Tan BX, Wang NN, Yang SS, Jia YB, Cheng YF. Detection of D2-40 monoclonal antibody-labeled lymphatic vessel invasion in esophageal squamous cell carcinoma and its clinicopathologic significance. Cancer Biol Med. 2013;10:81–85. doi: 10.7497/j.issn.2095-3941.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mori D, Yamasaki F, Shibaki M, Tokunaga O. Lateral peritumoral lymphatic vessel invasion can predict lymph node metastasis in esophageal squamous cell carcinoma. Mod Pathol. 2007;20:694–700. doi: 10.1038/modpathol.3800786. [DOI] [PubMed] [Google Scholar]
- 17.Inoue A, Moriya H, Katada N, Tanabe S, Kobayashi N, Watanabe M, Okayasu I, Ohbu M. Intratumoral lymphangiogenesis of esophageal squamous cell carcinoma and relationship with regulatory factors and prognosis. Pathol Int. 2008;58:611–619. doi: 10.1111/j.1440-1827.2008.02279.x. [DOI] [PubMed] [Google Scholar]
- 18.Imamura Y, Watanabe M, Nagai Y, Baba Y, Hirashima K, Karashima R, Iwatsuki M, Yoshida N, Kinoshita K, Kurashige J, Iyama K, Baba H. Lymphatic vessel invasion detected by the D2-40 monoclonal antibody is an independent prognostic factor in node-negative esophageal squamous cell carcinoma. J Surg Oncol. 2012;105:277–283. doi: 10.1002/jso.22079. [DOI] [PubMed] [Google Scholar]
- 19.Rahadiani N, Ikeda J, Makino T, Tian T, Qiu Y, Mamat S, Wang Y, Doki Y, Aozasa K, Morii E. Tumorigenic role of podoplanin in esophageal squamous-cell carcinoma. Ann Surg Oncol. 2010;17:1311–1323. doi: 10.1245/s10434-009-0895-5. [DOI] [PubMed] [Google Scholar]
- 20.Chuang WY, Yeh CJ, Wu YC, Chao YK, Liu YH, Tseng CK, Chang HK, Liu HP, Hsueh C. Tumor cell expression of podoplanin correlates with nodal metastasis in esophageal squamous cell carcinoma. Histol Histopathol. 2009;24:1021–1027. doi: 10.14670/HH-24.1021. [DOI] [PubMed] [Google Scholar]
- 21.Tong L, Yuan S, Feng F, Zhang H. Role of podoplanin expression in esophageal squamous cell carcinoma: a retrospective study. Dis Esophagus. 2012;25:72–80. doi: 10.1111/j.1442-2050.2011.01211.x. [DOI] [PubMed] [Google Scholar]
- 22.Birner P, Schindl M, Obermair A, Breitenecker G, Kowalski H, Oberhuber G. Lymphatic microvessel density as a novel prognostic factor in early-stage invasive cervical cancer. Int J Cancer. 2001;95:29–33. doi: 10.1002/1097-0215(20010120)95:1<29::aid-ijc1005>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 23.Kalof AN, Cooper K. D2-40 immunohistochemistry--so far! Adv Anat Pathol. 2009;16:62–64. doi: 10.1097/PAP.0b013e3181915e94. [DOI] [PubMed] [Google Scholar]
- 24.Chuang WY, Chang YS, Yeh CJ, Wu YC, Hsueh C. Role of podoplanin expression in squamous cell carcinoma of upper aerodigestive tract. Histol Histopathol. 2013;28:293–299. doi: 10.14670/HH-28.293. [DOI] [PubMed] [Google Scholar]
- 25.Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics, 2001. CA Cancer J Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 26.Gao P, Zhou GY, Zhang QH, Xiang L, Zhang SL, Li C, Sun YL. Clinicopathological significance of peritumoral lymphatic vessel density in gastric carcinoma. Cancer Lett. 2008;263:223–230. doi: 10.1016/j.canlet.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 27.Zhou M, He L, Zu X, Zhang H, Zeng H, Qi L. Lymphatic vessel density as a predictor of lymph node metastasis and its relationship with prognosis in urothelial carcinoma of the bladder. BJU Int. 2011;107:1930–1935. doi: 10.1111/j.1464-410X.2010.09725.x. [DOI] [PubMed] [Google Scholar]
- 28.El-Gendi S, Abdel-Hadi M. Lymphatic vessel density as prognostic factor in breast carcinoma: relation to clinicopathologic parameters. J Egypt Natl Canc Inst. 2009;21:139–149. [PubMed] [Google Scholar]
- 29.Goes RS, Carvalho JP, Almeida BG, Bacchi CE, Goes JC, Calil MA, Baracat EC, Carvalho FM. Intratumoral lymphatic vessel density in vulvar squamous cell carcinomas: a possible association with favorable prognosis. Int J Gynecol Pathol. 2012;31:8–14. doi: 10.1097/PGP.0b013e31821f4de2. [DOI] [PubMed] [Google Scholar]
- 30.Bevacqua SJ, Welch DR, Diez de Pinos SM, Shapiro SA, Johnston MG, Witte MH, Leong SP, Dorrance TL, Leibovitz A, Hendrix MJ. Quantitation of human melanoma, carcinoma and sarcoma tumor cell adhesion to lymphatic endothelium. Lymphology. 1990;23:4–14. [PubMed] [Google Scholar]
- 31.Padera TP, Kadambi A, di Tomaso E, Carreira CM, Brown EB, Boucher Y, Choi NC, Mathisen D, Wain J, Mark EJ, Munn LL, Jain RK. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002;296:1883–1886. doi: 10.1126/science.1071420. [DOI] [PubMed] [Google Scholar]
- 32.Franchi A, Gallo O, Massi D, Baroni G, Santucci M. Tumor lymphangiogenesis in head and neck squamous cell carcinoma: a morphometric study with clinical correlations. Cancer. 2004;101:973–978. doi: 10.1002/cncr.20454. [DOI] [PubMed] [Google Scholar]
- 33.Cunningham JE, Jurj AL, Oman L, Stonerock AE, Nitcheva DK, Cupples TE. Is risk of axillary lymph node metastasis associated with proximity of breast cancer to the skin? Breast Cancer Res Treat. 2006;100:319–328. doi: 10.1007/s10549-006-9256-2. [DOI] [PubMed] [Google Scholar]
- 34.Shayan R, Inder R, Karnezis T, Caesar C, Paavonen K, Ashton MW, Mann GB, Taylor GI, Achen MG, Stacker SA. Tumor location and nature of lymphatic vessels are key determinants of cancer metastasis. Clin Exp Metastasis. 2013;30:345–356. doi: 10.1007/s10585-012-9541-x. [DOI] [PubMed] [Google Scholar]
- 35.Van der Auwera I, Van Laere SJ, Van den Eynden GG, Benoy I, van Dam P, Colpaert CG, Fox SB, Turley H, Harris AL, Van Marck EA, Vermeulen PB, Dirix LY. Increased angiogenesis and lymphangiogenesis in inflammatory versus noninflammatory breast cancer by real-time reverse transcriptase-PCR gene expression quantification. Clin Cancer Res. 2004;10:7965–7971. doi: 10.1158/1078-0432.CCR-04-0063. [DOI] [PubMed] [Google Scholar]
- 36.Schacht V, Ramirez MI, Hong YK, Hirakawa S, Feng D, Harvey N, Williams M, Dvorak AM, Dvorak HF, Oliver G, Detmar M. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003;22:3546–3556. doi: 10.1093/emboj/cdg342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schacht V, Dadras SS, Johnson LA, Jackson DG, Hong YK, Detmar M. Up-regulation of the lymphatic marker podoplanin, a mucin-type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am J Pathol. 2005;166:913–921. doi: 10.1016/S0002-9440(10)62311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki H, Onimaru M, Koga T, Takeshita M, Yano T, Maehara Y, Nakamura S, Sueishi K. High podoplanin expression in cancer cells predicts lower incidence of nodal metastasis in patients with lung squamous cell carcinoma. Pathol Res Pract. 2011;207:111–115. doi: 10.1016/j.prp.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Kawase A, Ishii G, Nagai K, Ito T, Nagano T, Murata Y, Hishida T, Nishimura M, Yoshida J, Suzuki K, Ochiai A. Podoplanin expression by cancer associated fibroblasts predicts poor prognosis of lung adenocarcinoma. Int J Cancer. 2008;123:1053–1059. doi: 10.1002/ijc.23611. [DOI] [PubMed] [Google Scholar]
- 40.Yamanashi T, Nakanishi Y, Fujii G, Akishima-Fukasawa Y, Moriya Y, Kanai Y, Watanabe M, Hirohashi S. Podoplanin expression identified in stromal fibroblasts as a favorable prognostic marker in patients with colorectal carcinoma. Oncology. 2009;77:53–62. doi: 10.1159/000226112. [DOI] [PubMed] [Google Scholar]
- 41.Kitano H, Kageyama S, Hewitt SM, Hayashi R, Doki Y, Ozaki Y, Fujino S, Takikita M, Kubo H, Fukuoka J. Podoplanin expression in cancerous stroma induces lymphangiogenesis and predicts lymphatic spread and patient survival. Arch Pathol Lab Med. 2010;134:1520–1527. doi: 10.1043/2009-0114-OA.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kahn HJ, Marks A. A new monoclonal antibody, D2-40, for detection of lymphatic invasion in primary tumors. Lab Invest. 2002;82:1255–1257. doi: 10.1097/01.lab.0000028824.03032.ab. [DOI] [PubMed] [Google Scholar]
- 43.Saad RS, Lindner JL, Liu Y, Silverman JF. Lymphatic vessel density as prognostic marker in esophageal adenocarcinoma. Am J Clin Pathol. 2009;131:92–98. doi: 10.1309/AJCPKWUQSIPVG90H. [DOI] [PubMed] [Google Scholar]
- 44.Niakosari F, Kahn HJ, Marks A, From L. Detection of lymphatic invasion in primary melanoma with monoclonal antibody D2-40: a new selective immunohistochemical marker of lymphatic endothelium. Arch Dermatol. 2005;141:440–444. doi: 10.1001/archderm.141.4.440. [DOI] [PubMed] [Google Scholar]
- 45.Wang J, Wang B, Zhao W, Guo Y, Chen H, Chu H, Liang X, Bi J. Clinical significance and role of lymphatic vessel invasion as a major prognostic implication in non-small cell lung cancer: a meta-analysis. PLoS One. 2012;7:e52704. doi: 10.1371/journal.pone.0052704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pasquali S, van der Ploeg AP, Mocellin S, Stretch JR, Thompson JF, Scolyer RA. Lymphatic biomarkers in primary melanomas as predictors of regional lymph node metastasis and patient outcomes. Pigment Cell Melanoma Res. 2013;26:326–337. doi: 10.1111/pcmr.12064. [DOI] [PubMed] [Google Scholar]
- 47.Yuan P, Temam S, El-Naggar A, Zhou X, Liu DD, Lee JJ, Mao L. Overexpression of podoplanin in oral cancer and its association with poor clinical outcome. Cancer. 2006;107:563–569. doi: 10.1002/cncr.22061. [DOI] [PubMed] [Google Scholar]
- 48.Dumoff KL, Chu C, Xu X, Pasha T, Zhang PJ, Acs G. Low D2-40 immunoreactivity correlates with lymphatic invasion and nodal metastasis in early-stage squamous cell carcinoma of the uterine cervix. Mod Pathol. 2005;18:97–104. doi: 10.1038/modpathol.3800269. [DOI] [PubMed] [Google Scholar]
- 49.Scholl FG, Gamallo C, Vilaro S, Quintanilla M. Identification of PA2.26 antigen as a novel cell-surface mucin-type glycoprotein that induces plasma membrane extensions and increased motility in keratinocytes. J Cell Sci. 1999;112:4601–4613. doi: 10.1242/jcs.112.24.4601. [DOI] [PubMed] [Google Scholar]
- 50.Wicki A, Lehembre F, Wick N, Hantusch B, Kerjaschki D, Christofori G. Tumor invasion in the absence of epithelial-mesenchymal transition: podoplanin-mediated remodeling of the actin cytoskeleton. Cancer Cell. 2006;9:261–272. doi: 10.1016/j.ccr.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 51.Marks A, Sutherland DR, Bailey D, Iglesias J, Law J, Lei M, Yeger H, Banerjee D, Baumal R. Characterization and distribution of an oncofetal antigen (M2A antigen) expressed on testicular germ cell tumours. Br J Cancer. 1999;80:569–578. doi: 10.1038/sj.bjc.6690393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 53.Atsumi N, Ishii G, Kojima M, Sanada M, Fujii S, Ochiai A. Podoplanin, a novel marker of tumor-initiating cells in human squamous cell carcinoma A431. Biochem Biophys Res Commun. 2008;373:36–41. doi: 10.1016/j.bbrc.2008.05.163. [DOI] [PubMed] [Google Scholar]