Abstract
CTRP3, discovered as novel adipokines, is a member of the C1q tumor necrosis factor (TNF) related protein (CTRP) super-family. CTRP3 is found to function as adipokines that display diverse biological activities in metabolic and cardiovascular diseases. Recent study demonstrated that CTRP3 was protective against pathological cardiac remodeling in mice. Nevertheless, the effect of CTRP3 on vascular remodeling remains undefined. Our present study aimed to explore the effects of adipokine CTRP3 on the activation of adventitial fibroblasts (AFs) induced by TGF-β1. Immunofluorescent staining, real-time PCR and Western blot were conducted to evaluate the expression of α-smooth muscle-actin (α-SMA) and collagen I. The expression of CTGF was evaluated by enzymelinked immunosorbent assay (ELISA), while the proliferation and migration of adventitial fibroblasts were detected by using cell counting kit-8 (CCK-8) assay and Transwell technique, respectively. Functional analysis showed that CTRP3 inhibited TGF-β1 inducing AFs phenotypic conversion, collagen synthesis, proliferation and migration. The secretion of CTGF was also inhibited by CTRP3. Our findings suggest that CTRP3 may be beneficial to the prevention of cardiovascular diseases and provide a promising therapeutic strategy to attenuate vascular remodeling.
Keywords: Adventitial fibroblasts, myofibroblasts, vascular remodeling, CTRP3, phenotypic conversion
Introduction
Cardiovascular disease is the major reason of morbidity and mortality in the industrialized world [1]. Vascular remodeling, which represents any enduring changes in the size and/or composition of an adult blood vessel is a common feature of various cardiovascular diseases, such as atherosclerosis, restenosis after angioplasty and hypertension [2]. As the importance of pathological vascular remodeling in cardiovascular diseases is increasingly recognized, the prevention of inappropriate remodeling has become a heated research topic in the field of cardiology. It is recently reported that studies targeted on modifying TGF-β1 may give rise to promising strategies in the treatment of pathological vascular remodeling [3]. Nevertheless, presently no drugs are specifically targeted on attenuating the pathological vascular remodeling in clinic.
Traditionally, adventitial was regarded as a supporting connective tissue that provide nourishment and support. Researches about vascular remodeling have long concentrated on the intimal and medial layers [4]. In the last few decades, the capacity of adventitial fibroblasts (AFs) to infl uence vascular remodeling has received growing attention. Nowadays, considerable evidences indicate that adventitial fibroblasts act as a biological processing center in the process of inappropriate of vascular remodeling and neointima formation. During response to infl ammatory, hormonal and other environmental stresses adventitial fibroblasts are the first vascular wall cells that exhibit evidences of “activation” [5]. Such activation of adventitial fibroblasts is denoted by increase in cell proliferation and migration, up-regulated the expression of α-smooth muscle-actin (α-SMA) and extracellular matrix (ECM) proteins, as well as in the secretion of cytokines and chemokines that capable of modulating vascular wall cells growth and initiating infl ammation through infl uencing vascular structure and function. Therefore, the study of AFs bioactivity and the possible mechanisms underlying adventitial activation may provide a potential therapeutic strategy for vascular diseases. It is well established that transforming growth factor-β1 (TGF-β1) is one of the most key regulator of vascular fibrosis by inducing the activation of the fibroblasts [6]. Furthermore, TGF-β1 contributes to constructive remodeling through direct effects or via induction of CTGF [3,7].
Most recently, C1q/TNF-related proteins (CTRPs), a structural and functional adiponectin paralogs, have been discovered. Some members of CTRPs are found to display diverse biological activities in metabolic and cardiovascular diseases. CTRP3 was first identified as a secreted protein induced in a murine mesenchymal stem cell line. This novel protein plays an important role in regulating cartilage development and adiponectin secretion [8,9]. Besides, CTRP3 also exerts an impact on the differentiation and growth of several types of cells, including endothelial, osteosarcoma and vascular smooth muscle cells [10,11]. Moreover, CTRP3 displays diverse biological activities in the context of metabolic and cardiovascular diseases. CTRP3 also has been reported to improve glucose metabolism in obese mice by suppressing gluconeogenesis and activating Akt signaling in the liver [12]. Recent researches have showed that, like adiponectin, circulating CTRP3 level was reduced in diet-induced obese mice [13]. Yi et al [14] discovered that the adipokine CTRP3 was protective against pathological cardiac remodeling and CTRP3 replenishment dramatically increased the ratio of myocytes to fibrotic cells in the ischemic zone and significantly attenuated interstitial fibrosis. In addition, Li et al [15] found that the expression of CTRP3 gene was up-regulated after balloon-injure of rat carotid artery tissue. Taken together, these studies highlight a novel and significant role for CTRP3 in modulating cardiovascular functions.
A recent research has revealed that some CTRPs members, such as CTRP9, play a protective role in the heart against ischemia injury [16,17], as well as attenuating vascular remodeling in response to vascular injury [18]. However, whether CTRP3, a key member of the newest adipokine family, may function as an inhibitor of pathological vascular remodeling has never been investigated. In the current study, we examined the effect of CTRP3 on the phenotypic conversion of adventitial fibroblasts to myfibroblasts, collagen production, cell proliferation, migration and the expression of CTGF induced by TGF-β1 in vitro to explore the potent effects of CTRP3 on arterial remodeling. These findings provide new insights about the prevention and treatment of vascular remodeling.
Materials and methods
Cell culture
The adventitial fibroblasts were cultured as described by Zhang et al and Kim et al. [19,20]. Briefl y, Sprague–Dawley rats were anesthetized with chloral hydrate and rapidly decapitated. Adventitia was carefully removed from rat thoracic aorta and minced into small pieces (1-2 mm2). The adventitia tissue pieces were then placed on culture fl asks and cultured with Dulbecco’s modified Eagle’s medium (pH 7.2-7.4) containing 20% fetal calf, and maintained in a humidified incubator with 95% air and 5% CO2 at 37°C. Fibroblasts migrated from the edge of tissues within 7-10 days. Once reached confl uence, the isolated cells were harvested and used for experiments at passages 3 to 5. Before each experiment, cells were placed in DMEM containing 0.5% fetal bovine serum (FBS) for 4 h for serum starvation. AFs were pretreated by recombinant CTRP3 protein (10 μg/ml)or vehicle for 16 h, followed by stimulation with DMEM containing 10% FBS with or without TGF-β1 (10 ng/ml) for 24 h. All experimental protocols were approved by Institutional Animal Care and Use Committee (the Shanghai Jiao Tong University).
Immunofluorescent staining for α-SMA
AFs were cultured on glass cover slips for two days. The cells were fixed with 4% paraformaldehyde for 15 minutes at room temperature, washed with PBS 3 times and permeabilized with PBS containing 0.25% Triton X-100. After rinsed with PBS 3 times, the cells were incubated with 10% normal goat serum for 1 h at room temperature. Next, the cover slips were incubated with mouse anti-rat α-SMA monoclonal antibody in a humid chamber overnight at 4°C. After 3 washes with PBS, the cells were incubated with FITC-conjugated anti-mouse IgG secondary antibody for 1 h at room temperature. Finally, cell cover slips were labeled by DAPI. The fl uorescent images were captured using a confocal laser scanning microscope.
Real-time reverse transcriptase–polymerase chain reaction
Total RNA was isolated from cultured cells with RNeasy Mini Kit (Qiagen), according to the manufacturer’s protocol. The concentration of the total RNA was detected. Total RNA (1 ug) were reverse-transcribed into cDNA in 20 μl reaction volumes using the Prime Script RT reagent kit (Takara, China). The Real-time PCR was performed with a Stratagene Mx3000p (Agilent Technologies, La Jolla, CA) detection system, using SYBR Green reaction mix (Takara, China) in the 20 μl reaction mixtures. The mRNA levels were normalized by GAPDH housekeeping gene. The PCR primers used were as follows: α-SMA forward: 5’-GGA GTG ATG GTT GGA ATG G-3’ and reverse: 5’-ATG ATG CCG TGT TCT ATC G-3’; COL-I forward: 5’-TGC CGT GAC CTC AAG ATG TG-3’ and reverse: 5’-CAC AAG CGT GCT GTA GGT GA-3’; GAPDH forward: 5’-GAA CGG GAA GCT CAC TGG C-3’ and reverse: 5’-GCA TGT CAG ATC CAC AAC GG-3’. The reaction was run at 95°C for 30 s, followed by 40 cycles of 30 sec at 95°C and 30 sec at 60°C.
Western blot analysis
After appropriate treatments, cells were harvested with lysis buffer. Protein concentrations of cell lysates were measured by BCA Protein Assay Kit (Pierce, IL, USA). Equal protein amounts from each sample were separated by SDS-PAGE on 10% polyacrylamide gels, followed by transferring onto nitrocellulose membranes. The membranes were blocked in TBST containing 5% milk for 1 h at room temperature, and then the membrane was incubated with α-SMA, collagen I monoclonal antibodies overnight at 4°C. Membranes were washed three times with TBST, and then incubated for 2 h at room temperature with a horseradish-coupled secondary antibody. Bands were detected using the ECL Western blotting detection kit. Signals were analyzed with the image analysis software Image Pro Plus 6.0 [21].
Cell proliferation assay
AFs were added into 96-well plates at 5.0×104/ml cells per well in a total volume of 100 μl culture medium. The effect of CTRP3 on AFs viability after various treatments was determined by cell counting kit-8 (CCK-8, Dojindo, Japan) assay. According to the protocol, 10 μl of CCK-8 was added to each well and cultured for 4 h at 37°C. The absorbance value at 450 nm was measured, using a microplate reader (Bio-Tek, VT, USA).
Migration assay
Cell migration assay was performed using a transwell system (Corning, New York, USA), as described previously [22], which allows cells to migrate through a polycarbonate membrane with 8-um pore size. In brief, AFs pretreated with or without recombinant CTRP3 protein for 16 h were plated into the upper chamber, while the bottom chamber filled with 500 μl of DMEM containing 10% FBS with or without TGF-β1. After 24 h of incubation, the cells that had migrated through the pores were stained with DAPI. Quantification was performed by counting five random fields under the microscope.
Elisa analysis
Detection of CTGF with ELISA was performed using CTGF enzyme linked immunosorbent assay kit (Biovalue, China). After incubation, the samples were collected and centrifuged, and the levels of CTGF were measured. OD value was read at 450 nm by ELISA reader and the concentration of CTGF was calculated from a standard curve diagram. Dates were displayed as mean of CTGF concentration (μg/ml).
Statistical analysis
All measures were expressed as the mean ± SD. The software of SPSS version 19.0 was used to analyze the data. The results were analyzed by using one-way analysis of variance followed by t-test. A value of P < 0.05 was considered to be statistically significant.
Results
CTRP3 inhibits TGF-β1 induced phenotypic conversion of fibroblasts
The expression levels of a-smooth muscle-actin (α-SMA), a symbol of fibroblasts phenotypic conversion stimulated by TGF-β1, were measured. To evaluate the role of CTRP3 in fibroblasts differentiating to myofibroblast, Immunofl uorescence staining, Western blot and Real-time PCR were utilized to examine the expression of α-SMA. The expression of α-SMA was weakly stained in adventitia fibroblasts. As shown in Figure 1A, the immunofl uorescence levels of α-SMA was increased after treatment of TGF-β1, which was significantly reduced by CTRP3 pretreatment. The similar results were also observed by analysis the mRNA and protein expression levels of α-SMA. It appears that the treatment of adventitia fibroblasts with CTRP3 could significantly reduced the expression levels of α-SMA mRNA and protein induced by TGF-β1.
Figure 1.
CTRP3 attenuated TGF-β1-induced adventitial fibroblasts α-SMA expression. A: Immunofl uorescence analysis of myofibroblast differentiation by staining with an α-smooth muscle actin (α-SMA) antibody. B: Quantitative analyses of α-SMA positive cells are presented. C: Western blotting was performed to determine α-SMA expression. D: Representative western blot images of α-SMA proteins and the relative content of α-SMA proteins after normalization to GAPDH expression. E: Real-time quantitative PCR was performed to determine mRNA levels. The data are presented as the mean ± SD. *P < 0.05 versus the control group; #P < 0.05 versus TGF-β1 group.
CTRP3 attenuated TGF-β1 induced adventitial fibroblasts proliferation and migration
The proliferation of AFs was induced by the TGF-β1 stimulation. The OD values were significantly higher in the TGF-β1 group compared with the control group (P < 0.05). However, compared with the TGF-β1 group, CCK-8 assay showed that the OD values in the CTRP3 pretreated group were decreased markedly (Figure 2A). The effect of CTRP3 on AFs migration was analyzed by Trans-well migration assay (Figure 2B). CTRP3 reduced AFs migration induced by TGF-β1 than those treated with TGF-β1 alone. These results suggested a significant contribution of CTRP3 to lessening TGF-β1 induced of AFs proliferation and migration.
Figure 2.

CTRP3 attenuated TGF-β1-induced adventitial fibroblasts proliferation and migration. A: CTRP3 inhibit the proliferation of fibroblasts. B: CTRP3 inhibits fibroblasts migration. Each bar represents the mean 6 SD of the OD values. *P < 0.05 versus the control group; #P < 0.05 versus TGF-β1 group.
Effect of CTRP3 on type I collagen expression of adventitial fibroblasts
The effects of CTRP3 on collagen I mRNA and protein expression were evaluated by Real-time PCR and Western blot. We found that TGF-β1 remarkably increased collagen I mRNA and protein levels. In contrast, the pretreated adventitia fibroblasts with CTRP3 exhibited a down-regulation of collagen I mRNA and protein levels (Figure 3).
Figure 3.
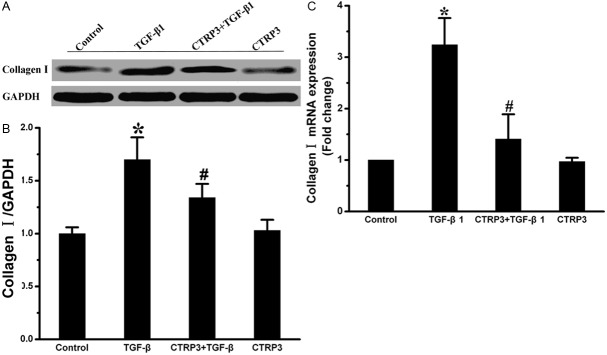
CTRP3 weakens TGF-β1-induced collagen synthesis. A: Collagen I protein levels were assessed by Western blot. GAPDH was a loading control. B: Level of quantification of Collagen I as a ratio of GAPDH in densitometric units was presented. C: Collagen I mRNA levels were assessed by quantitative real-time PCR. *P < 0.05 versus the control group; #P < 0.05 versus TGF-β1 group.
CTRP3 suppressed the expression of CTGF induced by TGF-β1
The levels of CTGF were detected by ELISA analysis. Results are depicted in Figure 4. The results demonstrated that the content of CTGF in the four groups were 117.44 ± 6.33, 454.30 ± 13.85, 320.87 ± 13.07, and 114.7 ± 8.04 ng/mL, respectively. The content of CTGF in the CTRP3 pretreatment group was markedly lower than that in TGF-β1 group (P < 0.05).
Figure 4.

ELISA assay was used to determine the expression of CTGF. Results are presented as the mean ± SD. *P < 0.05 versus the control group; #P < 0.05 versus TGF-β1 group.
Discussion
Pathological vascular remodeling is a keystone of diverse cardiovascular diseases including hypertension, atherosclerosis and post-angioplasty restenosis [23]. Thus, it is necessary to find effective methods to prevent and reverse the pathological vascular remodeling. Recent study demonstrates that the adipokine CTRP3 possesses protective properties on cardiac remodeling. However, there is little known about the effect of CTRP3 on vascular remodeling. Our present investigation provided the first evidence that CTRP3 could directly regulate the activation of AFs stimulated by TGF-β1. The results showed that CTRP3 suppressed phenotypic conversion of AFs to MFs and inhibited collagen synthesis and the proliferation and migration of AFs induced by TGF-β1, elucidating a protective role of CTRP3 in pathological vascular remodeling by modulating the responses of adventitial fibroblasts. In addition, CTRP3 exerts an effect on the secretion of CTGF, which implies a role for CTGF in vessel protection induced by CTRP3.
Subsequent evidences showed that vascular remodeling was an “adventitia-based” process. The adventitia is considered as the most sensitive cell layer to blood pressure and other stimulus [24]. As the primary cell type of adventitia, AFs exist various structural and functional behaviors responding to stimulus. Almost immediately after injury, AFs are activated and transform into MFs with increased expression of α-SMA and collagen [4,25], in which TGF-β1 acts as a crucial modulator. TGF-β1 can be induced by various heart stress factors that are involved in cardiovascular disease, including mechanical stress, high glucose [26,27]. It regulates a variety of pathophysiological and physiological processes including cell proliferation, migration and differentiation. Under the influence of high levels of TGF-β1, adventitial fibroblasts can be activated and contribute to vascular remodeling. In experimental models, down expression of the TGF-β1 gene could attenuate these events and thereby prevent constrictive remodeling and neointima hyperplasia after vascular injury [28,29]. Therefore, antagonism of vascular TGF-β1 signaling is considered as a putative therapeutic target in disorders of the blood-vessel interface.
The activated adventitial fibroblasts, known as myofibroblasts, play important roles in the pathological vascular remodeling [30]. The switched phenotypic of adventitial fibroblasts to myofibroblasts is considered as a hallmark of vascular remodeling following vascular injury and is critical in fibroblasts aviation induced by TGF-β1 [31,32]. Several studies have documented that the activated myofibroblasts could elevate the proliferation, migration and extracellular matrix deposition. Myofibroblasts are characterized by the appearance of α-SMA and production of ECM components [33,34]. They are metabolically active cells and can perform multiple cellular functions in response to changes in local environments [35]. The accumulation of myofibroblasts can contribute to changes in the structure and tone of the vessel wall under pathophysiologic conditions [36,37]. Collagen is a major component of ECM and accounts for more than fifty percent of vascular wall with stenosis [38]. Among all the collagen subtypes, type I collagen is considered to be most relevant to arterial remodeling. Synthesis and accumulation of collagen are essential for vascular homeostasis, development, and wound healing. However, excessive collagen expression and deposition can lead to fibrotic disorders. AS an active component of the vessel wall, ECM plays an important role in the pathophysiology of vascular diseases. Considerable studies have demonstrated TGF-β1 could induce fibroblasts into myofibroblasts by stimulating α-SMA expression [39] and promoting the secretion of ECM proteins, which have been found to play a vital role in the process of pathological vascular remodeling. In accordance with previous findings, the α-SMA mRNA and protein expression levels is obviously up-regulated in AFs stimulated by TGF-β1. Moreover, we observed that pretreatment of adventitial fibroblasts with CTRP3 markedly decreased the expression of α-SMA and type I collagen, which were induced by TGF-β1. These findings suggest that CTRP3 could blunt vascular remodeling by inhibiting the differentiation of adventitial fibroblasts and secretion of type I collagen.
The proliferative and migratory responses of cells in the adventitia contribute to vascular remodeling in restenosis [24]. The proliferation and migration of myofibroblasts are critical cellular events in the development of constructive remodeling [40]. TGF-β1 is reported to be one of the most key factors that induce the proliferation and migration of AFs in response to vascular injury. Previous researches demonstrated blockage of TGF-β1 signaling pathway could markedly prevent fibroblast proliferation [41]. Previous studies showed that adventitia cell migration play a more vital role than cell proliferation in vascular remodeling [22]. Thus, we investigated the effects of CTRP3 on AFs proliferation and migration. Our present study revealed that TGF-β1 indeed up-regulated cell proliferation and migration in adventitial fibroblasts, which was consistent with former studies. Interestingly, we found all these changes were noticeable attenuated by treatment with CTRP3. Taken together, these results suggested a significant effect for CTRP3 in regulating fibroblasts growth and migration during vascular remodeling.
To further examine whether CTGF is also involved in the process, we studied the secretion of CTGF in TGF-β1 induced AFs pretreated with or without CTRP3. CTGF, which is believed to be a mediator of the profibrogenic effects of TGF-β1, plays a critical role in the architectural changes, followed by the vessel injury [42]. CTGF modulates different signal transduction pathways by interacting with various molecules, including cytokines and receptors, matrix proteins and growth factors, which result in different changes in cellular responses. In vitro studies have shown CTGF not only induces the activation of fibroblasts and enhances extracellular matrix production but also regulates the activity of TGF-β1 [43], which all lead to vascular remodeling and fibrosis. It has been reported that the maintenance of vascular function was modulated by interplay of TGF-β1 and CTGF [44]. CTGF could regulate the TGF-β1 signal pathway and contribute to the activation of AFs [42,45]. Serving as an extracellular adapter protein, CTGF activates TGF-β signals by direct binding to TGF-β1 receptors in the extracellular space and then helps to present them to their receptors to stimulate cellular response. The previous research found that CTRP3 exerts potent anti-fibrotic and anti-infl ammatory effects in Human primary colonic lamina propria fibroblasts by inhibiting the expression of collagen I and CTGF. Our study showed the similar results that CTRP3 obviously decreased the content of CTGF protein in cultured AFs induced by TGF-β1, although it was unable to suppress the basal level of CTGF protein expression. Added to previous findings that CTGF can induce fibroblasts activation and have a close interaction with TGF-β1, the findings above suggest that, CTRP3 may suppress the activation of AFs provoked by TGF-β1, at least in part, by directly inhibiting the CTGF protein secretion or by inducing genes that are involved in the suppression of CTGF. Our present investigation reveals a model of molecular crosstalk and support the active role for CTGF in vascular remodeling.
In conclusion, this study fi rst demonstrated the effect of CTRP3 on the activation of adventitial fibroblasts. CTRP3 could prevent TGF-β1 ind-uced adventitial fibroblasts proliferation, migration, phenotypic conversion, collagen synthesis and the expression of CTGF. The discoveries above have raised the prospect that CTRP3 might ameliorate pathological vascular remodeling by manipulating the responses of adventitial fibroblasts, in which CTGF may be involved. Collectively, the approaches aimed at enhancing CTRP3 levels may be a logical strategy for therapies in the alleviation of vascular remodeling. However, further investigation is required to prove the viability of therapeutic approach in humans.
Acknowledgements
This project was supported by the Science and Technology Commission of Shanghai Municipality (10JC1408902).
Disclosure of conflict of interest
None.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med. 1994;330:1431–1438. doi: 10.1056/NEJM199405193302008. [DOI] [PubMed] [Google Scholar]
- 3.Goel SA, Guo LW, Liu B, Kent KC. Mechanisms of post-intervention arterial remodelling. Cardiovasc Res. 2012;96:363–371. doi: 10.1093/cvr/cvs276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sartore S, Chiavegato A, Faggin E, Franch R, Puato M, Ausoni S, Pauletto P. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ Res. 2001;89:1111–1121. doi: 10.1161/hh2401.100844. [DOI] [PubMed] [Google Scholar]
- 5.Stenmark KR, Nozik-Grayck E, Gerasimovskaya E, Anwar A, Li M, Riddle S, Frid M. The adventitia: Essential role in pulmonary vascular remodeling. Compr Physiol. 2011;1:141–161. doi: 10.1002/cphy.c090017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorescu D. Smad3 mediates angiotensin II- and TGF-beta1-induced vascular fibrosis: Smad3 thickens the plot. Circ Res. 2006;98:988–989. doi: 10.1161/01.RES.0000221824.87718.c0. [DOI] [PubMed] [Google Scholar]
- 7.Lan TH, Huang XQ, Tan HM. Vascular fibrosis in atherosclerosis. Cardiovasc Pathol. 2013;22:401–407. doi: 10.1016/j.carpath.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Maeda T, Jikko A, Abe M, Yokohama-Tamaki T, Akiyama H, Furukawa S, Takigawa M, Wakisaka S. Cartducin, a paralog of Acrp30/adiponectin, is induced during chondrogenic differentiation and promotes proliferation of chondrogenic precursors and chondrocytes. J Cell Physiol. 2006;206:537–544. doi: 10.1002/jcp.20493. [DOI] [PubMed] [Google Scholar]
- 9.Akiyama H, Furukawa S, Wakisaka S, Maeda T. Cartducin stimulates mesenchymal chondroprogenitor cell proliferation through both extracellular signal-regulated kinase and phosphatidylinositol 3-kinase/Akt pathways. FEBS J. 2006;273:2257–2263. doi: 10.1111/j.1742-4658.2006.05240.x. [DOI] [PubMed] [Google Scholar]
- 10.Yokohama-Tamaki T, Maeda T, Tanaka TS, Shibata S. Functional analysis of CTRP3/cartducin in Meckel’s cartilage and developing condylar cartilage in the fetal mouse mandible. J Anat. 2011;218:517–533. doi: 10.1111/j.1469-7580.2011.01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maeda T, Wakisaka S. CTRP3/cartducin is induced by transforming growth factor-beta1 and promotes vascular smooth muscle cell proliferation. Cell Biol Int. 2010;34:261–266. doi: 10.1042/CBI20090043. [DOI] [PubMed] [Google Scholar]
- 12.Peterson JM, Wei Z, Wong GW. C1q/TNF-related protein-3 (CTRP3), a novel adipokine that regulates hepatic glucose output. J Biol Chem. 2010;285:39691–39701. doi: 10.1074/jbc.M110.180695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoo HJ, Hwang SY, Hong HC, Choi HY, Yang SJ, Choi DS, Baik SH, Bluher M, Youn BS, Choi KM. Implication of progranulin and C1q/TNF-related protein-3 (CTRP3) on infl ammation and atherosclerosis in subjects with or without metabolic syndrome. PLoS One. 2013;8:e55744. doi: 10.1371/journal.pone.0055744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yi W, Sun Y, Yuan Y, Lau WB, Zheng Q, Wang X, Wang Y, Shang X, Gao E, Koch WJ, Ma XL. C1q/tumor necrosis factor-related protein-3, a newly identified adipokine, is a novel antiapoptotic, proangiogenic, and cardioprotective molecule in the ischemic mouse heart. Circulation. 2012;125:3159–3169. doi: 10.1161/CIRCULATIONAHA.112.099937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li JM, Zhang X, Nelson PR, Odgren PR, Nelson JD, Vasiliu C, Park J, Morris M, Lian J, Cutler BS, Newburger PE. Temporal evolution of gene expression in rat carotid artery following balloon angioplasty. J Cell Biochem. 2007;101:399–410. doi: 10.1002/jcb.21190. [DOI] [PubMed] [Google Scholar]
- 16.Kambara T, Ohashi K, Shibata R, Ogura Y, Maruyama S, Enomoto T, Uemura Y, Shimizu Y, Yuasa D, Matsuo K, Miyabe M, Kataoka Y, Murohara T, Ouchi N. CTRP9 protein protects against myocardial injury following ischemia-reperfusion through AMP-activated protein kinase (AMPK)-dependent mechanism. J Biol Chem. 2012;287:18965–18973. doi: 10.1074/jbc.M112.357939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su H, Yuan Y, Wang XM, Lau WB, Wang Y, Wang X, Gao E, Koch WJ, Ma XL. Inhibition of CTRP9, a novel and cardiac-abundantly expressed cell survival molecule, by TNFalpha-initiated oxidative signaling contributes to exacerbated cardiac injury in diabetic mice. Basic Res Cardiol. 2013;108:315. doi: 10.1007/s00395-012-0315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uemura Y, Shibata R, Ohashi K, Enomoto T, Kambara T, Yamamoto T, Ogura Y, Yuasa D, Joki Y, Matsuo K, Miyabe M, Kataoka Y, Murohara T, Ouchi N. Adipose-derived factor CTRP9 attenuates vascular smooth muscle cell proliferation and neointimal formation. FASEB J. 2013;27:25–33. doi: 10.1096/fj.12-213744. [DOI] [PubMed] [Google Scholar]
- 19.Zhang YG, Li J, Li YG, Wei RH. Urotensin II induces phenotypic differentiation, migration, and collagen synthesis of adventitial fibroblasts from rat aorta. J Hypertens. 2008;26:1119–1126. doi: 10.1097/HJH.0b013e3282fa1412. [DOI] [PubMed] [Google Scholar]
- 20.Kim DK, Huh JE, Lee SH, Hong KP, Park JE, Seo JD, Lee WR. Angiotensin II stimulates proliferation of adventitial fibroblasts cultured from rat aortic explants. J Korean Med Sci. 1999;14:487–496. doi: 10.3346/jkms.1999.14.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu H, Lin S, Xiao D, Zheng X, Gu Y, Guo S. Evaluation of the Wound Healing Potential of Resina Draconis (Dracaena cochinchinensis) in Animal Models. Evid Based Complement Alternat Med. 2013;2013:709865. doi: 10.1155/2013/709865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L, Zhu DL, Shen WL, Gao PJ. Increased migration of vascular adventitial fibroblasts from spontaneously hypertensive rats. Hypertens Res. 2006;29:95–103. doi: 10.1291/hypres.29.95. [DOI] [PubMed] [Google Scholar]
- 23.Heeneman S, Sluimer JC, Daemen MJ. Angiotensin-converting enzyme and vascular remodeling. Circ Res. 2007;101:441–454. doi: 10.1161/CIRCRESAHA.107.148338. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz SM, Reidy MA, O’Brien ER. Assessment of factors important in atherosclerotic occlusion and restenosis. Thromb Haemost. 1995;74:541–551. [PubMed] [Google Scholar]
- 25.Shi Y, O’Brien JE Jr, Fard A, Zalewski A. Transforming growth factor-beta 1 expression and myofibroblast formation during arterial repair. Arterioscler Thromb Vasc Biol. 1996;16:1298–1305. doi: 10.1161/01.atv.16.10.1298. [DOI] [PubMed] [Google Scholar]
- 26.Li JH, Huang XR, Zhu HJ, Johnson R, Lan HY. Role of TGF-beta signaling in extracellular matrix production under high glucose conditions. Kidney Int. 2003;63:2010–2019. doi: 10.1046/j.1523-1755.2003.00016.x. [DOI] [PubMed] [Google Scholar]
- 27.Li JH, Huang XR, Zhu HJ, Oldfield M, Cooper M, Truong LD, Johnson RJ, Lan HY. Advanced glycation end products activate Smad signaling via TGF-beta-dependent and independent mechanisms: implications for diabetic renal and vascular disease. FASEB J. 2004;18:176–178. doi: 10.1096/fj.02-1117fje. [DOI] [PubMed] [Google Scholar]
- 28.Nikol S, Isner JM, Pickering JG, Kearney M, Leclerc G, Weir L. Expression of transforming growth factor-beta 1 is increased in human vascular restenosis lesions. J Clin Invest. 1992;90:1582–1592. doi: 10.1172/JCI116027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Majesky MW, Lindner V, Twardzik DR, Schwartz SM, Reidy MA. Production of transforming growth factor beta 1 during repair of arterial injury. J Clin Invest. 1991;88:904–910. doi: 10.1172/JCI115393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powell DW, Miffl in RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol. 1999;277:C1–9. doi: 10.1152/ajpcell.1999.277.1.C1. [DOI] [PubMed] [Google Scholar]
- 31.Forte A, Della Corte A, De Feo M, Cerasuolo F, Cipollaro M. Role of myofibroblasts in vascular remodelling: focus on restenosis and aneurysm. Cardiovasc Res. 2010;88:395–405. doi: 10.1093/cvr/cvq224. [DOI] [PubMed] [Google Scholar]
- 32.Coen M, Gabbiani G, Bochaton-Piallat ML. Myofibroblast-mediated adventitial remodeling: an underestimated player in arterial pathology. Arterioscler Thromb Vasc Biol. 2011;31:2391–2396. doi: 10.1161/ATVBAHA.111.231548. [DOI] [PubMed] [Google Scholar]
- 33.Swaney JS, Roth DM, Olson ER, Naugle JE, Meszaros JG, Insel PA. Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase. Proc Natl Acad Sci U S A. 2005;102:437–442. doi: 10.1073/pnas.0408704102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 36.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 37.Shi-Wen X, Chen Y, Denton CP, Eastwood M, Renzoni EA, Bou-Gharios G, Pearson JD, Dashwood M, du Bois RM, Black CM, Leask A, Abraham DJ. Endothelin-1 promotes myofibroblast induction through the ETA receptor via a rac/phosphoinositide 3-kinase/Akt-dependent pathway and is essential for the enhanced contractile phenotype of fibrotic fibroblasts. Mol Biol Cell. 2004;15:2707–2719. doi: 10.1091/mbc.E03-12-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu P, Wang S, Cai W, Sheng J. Role of TGF-beta1/Smad3 signaling pathway in secretion of type I and III collagen by vascular smooth muscle cells of rats undergoing balloon injury. J Biomed Biotechnol. 2012;2012:965953. doi: 10.1155/2012/965953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou HY, Chen WD, Zhu DL, Wu LY, Zhang J, Han WQ, Li JD, Yan C, Gao PJ. The PDE1A-PKCalpha signaling pathway is involved in the upregulation of alpha-smooth muscle actin by TGF-beta1 in adventitial fibroblasts. J Vasc Res. 2010;47:9–15. doi: 10.1159/000231716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wernig F, Xu Q. Mechanical stress-induced apoptosis in the cardiovascular system. Prog Biophys Mol Biol. 2002;78:105–137. doi: 10.1016/s0079-6107(02)00008-1. [DOI] [PubMed] [Google Scholar]
- 41.Kuwahara F, Kai H, Tokuda K, Kai M, Takeshita A, Egashira K, Imaizumi T. Transforming growth factor-beta function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overloaded rats. Circulation. 2002;106:130–135. doi: 10.1161/01.cir.0000020689.12472.e0. [DOI] [PubMed] [Google Scholar]
- 42.Kundi R, Hollenbeck ST, Yamanouchi D, Herman BC, Edlin R, Ryer EJ, Wang C, Tsai S, Liu B, Kent KC. Arterial gene transfer of the TGF-beta signalling protein Smad3 induces adaptive remodelling following angioplasty: a role for CTGF. Cardiovasc Res. 2009;84:326–335. doi: 10.1093/cvr/cvp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grotendorst GR, Duncan MR. Individual domains of connective tissue growth factor regulate fibroblast proliferation and myofibroblast differentiation. FASEB J. 2005;19:729–738. doi: 10.1096/fj.04-3217com. [DOI] [PubMed] [Google Scholar]
- 44.Beddy D, Mulsow J, Watson RW, Fitzpatrick JM, O’Connell PR. Expression and regulation of connective tissue growth factor by transforming growth factor beta and tumour necrosis factor alpha in fibroblasts isolated from strictures in patients with Crohn’s disease. Br J Surg. 2006;93:1290–1296. doi: 10.1002/bjs.5431. [DOI] [PubMed] [Google Scholar]
- 45.Holmes A, Abraham DJ, Sa S, Shiwen X, Black CM, Leask A. CTGF and SMADs, maintenance of scleroderma phenotype is independent of SMAD signaling. J Biol Chem. 2001;276:10594–10601. doi: 10.1074/jbc.M010149200. [DOI] [PubMed] [Google Scholar]



