Abstract
Small cell neuroendocrine carcinoma (NEC) that originates in the tonsil is extremely rare and carries a poor prognosis. Only a few cases of this tumor have been reported so far and the standard treatment protocol remains uncertain. Here we describe a 74-year-old woman presented with throat pain for about 2 months. Computed tomography (CT) scan revealed a 3.4 × 1.8 cm tumor with moderate enhancement in the left tonsil and a 1.3 × 1.0 cm neck mass in left level II. A biopsy of the tonsillar mass was performed and histologic examination revealed small round to oval tumor cells were arranged in cords or nests, containing hyperchromatic nuclei and scant cytoplasm. Mitotic figures were readily identified. Immunohistochemical staining showed that tumor cells were strongly positive for CD56, focally positive for PCK and negative for LCA. A diagnosis of primary small cell NEC of the left tonsil was obtained. The patient was treated by six cycles of cisplatin combined with etoposide and the masses showed initial complete response. But recurrence in the left neck was found 9 months after initial diagnosis and the patient refused any further treatment. With a review of the literature, the nomenclature, clinicopathological characteristics and treatment modalities of this rare tumor are discussed.
Keywords: Neuroendocrine carcinoma, small cell carcinoma, tonsil, oropharynx, immunohistochemistry
Introduction
Tonsil is one of the most common regions from where primary oropharyngeal tumors originated. The predominant tumor type in this area is squamous cell carcinoma, while minor salivary tumors, lymphomas, melanoma and sarcomas are also included [1]. Neuroendocrine carcinoma (NEC) is malignant epithelial neoplasm with neuroendocrine morphology. Small cell NECs are poorly differentiated neuroendocrine tumors that commonly originated in pulmonary, gastrointestinal tract and genitourinary system, although it has been reported that they can occur in many organs throughout the body [2]. In head and neck region, the incidence of primary small cell NEC is low. Larynx is the relative most commonly involved site, followed by salivary glands, nasal cavity and paranasal sinuses [3]. Small cell NECs of the tonsil are extremely rare and only a few cases were added since it was firstly reported by Koss et al. in 1972 [4]. Thus far, the therapeutic strategy has not been properly formulated due to the paucity of data. Here we present a rare case of primary small cell NEC arising from the tonsil and the clinical and pathological features of this case were described.
Case report
A 74-year-old woman, with no history of smoking or alcohol consumption, was referred to our department complaining of mild throat pain for nearly two months. History of dysphagia or dyspnea was denied but odynophagia had been presented before her referring. The initial treatment with antibiotics was administered by her primary care physician but showed ineffective. Physical examination revealed an enlarged left palatine tonsil with ulcerating mucosa and fullness of his anterior tonsillar pillar. The right palatine tonsil and other pharyngeal mucosal surfaces were normal. A 1.3 cm firm mass was found in left level II on careful neck palpation. The remainder examination of head and neck was negative. Her medical history included hypertension and type II diabetes mellitus. Contrast-enhanced computed tomography (CT) scan of the neck showed a 3.4 × 1.8 cm tumor with moderate enhancement in the left tonsil. Bilateral lymphadenopathy was also observed with the imaging study and a 1.3 cm × 1.0 cm left internal jugular node was the largest one (Figure 1). In addition, chest, brain and abdominal CT scans were performed and indicated there were no other lesions. Thus the left palatine tonsil was confirmed as the primary lesion and there was no evidence of distant metastasis. A biopsy of the tonsillar mass was then performed under local anesthesia. Histologic examination revealed small round to oval tumor cells were arranged in cords or nests, containing hyperchromatic nuclei and scant cytoplasm. Mitotic figures were readily identified. Immunohistochemical staining showed that tumor cells were strongly positive for neural cell adhesion molecule (CD56), focally positive for pancytokeratin (PCK) and negative for leukocyte common antigen (LCA) (Figure 2). The patient was diagnosed as primary small cell NEC of the left tonsil.
Figure 1.
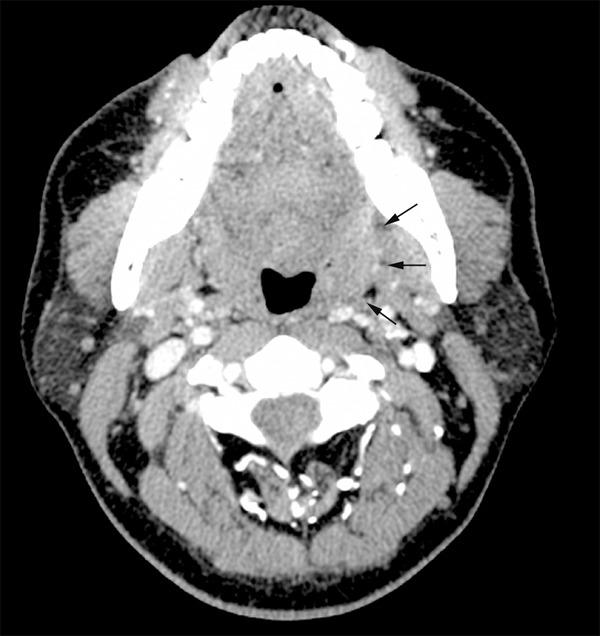
CT scan shows the contrast-enhancing mass originating from the left tonsil (arrows).
Figure 2.
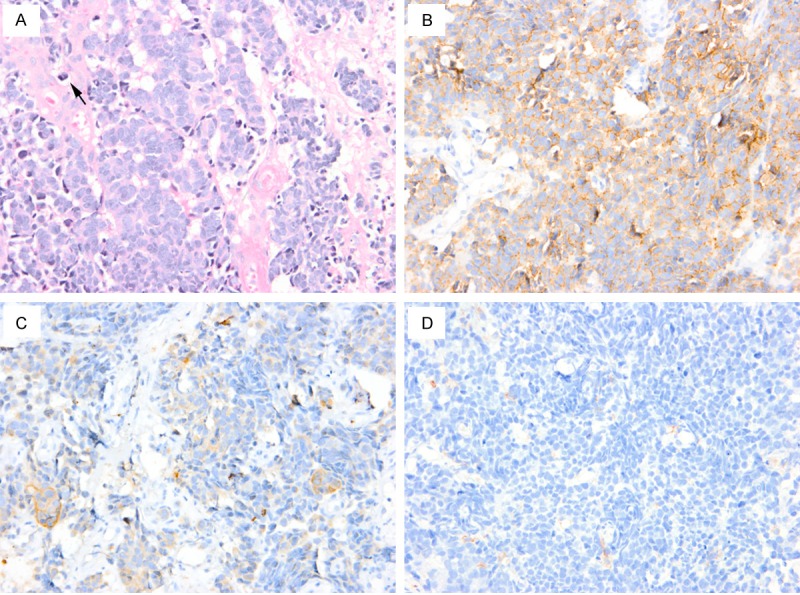
H. E. staining shows small round to oval tumor cells with scant cytoplasm, hyperchromatic nuclei and unobvious nucleoli. Mitoses (arrow) and necrosis are identified (A). Immunohistochemical study shows that tumor cells are strongly positive for CD56 (B), focally positive for PCK (C) and negative for LCA (D). Original magnification × 400.
As she refused surgery or radiotherapy, six cycles of cisplatin combined with etoposide were given and the masses showed initial complete response at that time. Recurrence in the left neck was found 9 months after initial diagnosis. The patient refused any further treatment and was lost to follow up.
Discussion
Neuroendocrine tumors are composed of heterogeneous neoplasms. In the broad sense, it can be divided into two types owning to its neural or epithelial origin. NEC is epithelial malignancy with neuroendocrine differentiation which has been reported in many organs throughout the body. Although the terminology refers to NECs varies in different anatomic sites and the classification is controversial, NECs are basically divided into two categories: well or poor-differentiated. According to the latest WHO classification of head and neck tumors (2005), typical (grade I) and atypical carcinoid (grade II) are well-differentiated NECs, and small cell NEC (grade III) is classified as poor-differentiated tumor [5]. Large cell NEC is also poor-differentiated which has been well-established in the pulmonary neuroendocrine tumors, but whether it should be defined as a distinct entity in neuroendocrine tumors of the head and neck is still under debate [6].
Small cell NECs are most commonly seen in lungs and have been reported to occur in some extra pulmonary sites, mainly in alimentary and genitourinary system [2]. A limited number of reports have described small cell NECs arising from the head and neck region and shows the relative propensity for larynx, followed by salivary glands and sinonasal region [3]. Small cell NEC that primarily occurs in tonsil is extremely rare. In light of its rarity, the latest WHO classification system included no category for small cell NEC in oropharyngeal tumors while this tumor type was specified in its relative commonly involved subsites as mentioned above. Thus, the diagnosis of small cell NEC of the present case was based on criteria established for its laryngeal counterpart which is the most wildly studied tumor subsite in head and neck region. In light microscopy, hallmarks of small cell NEC include small round to oval cells packed in sheets, cords or ribbons with hyperchromatic nuclei, sparse cytoplasm, high nuclear/cytoplasmic ratio and frequent necrosis and mitosis. In immunohistochemistry, cytokeratins and epithelial membrane antigen (EMA) are immunoreactive, and positive staining of general neuroendocrine markers including synaptophysin, CD56 and chromogranin can provide evidences of neuroendocrine differentiation of tumor cells. In the present case, the tumor cells met these criteria in histology and with strongly positive for CD56 and focally positive for PCK.
A large number of terms have been used when referred to small cell NEC. Synonyms of this tumor include small cell carcinoma, oat cell carcinoma, anaplastic small cell carcinoma and poorly differentiated (grade III) neuroendocrine carcinoma. Three cases of small cell NEC of the tonsil were initially reported by Koss et al. in 1972 [4]. During the past 40 years, there have been only 14 cases added in the English literature [7-14]. Two cases were excluded due to lack of powerful evidence of primary tonsillar origin or adequate data in pathology [8,12]. Thus, 12 of these cases, with sufficient clinicopathologic data, are summarized in Table 1. In our review, most small cell NECs of the tonsil occurred in patients between 50 and 66 years of age (range, 49 to 78 years), with the male/female ratio 1.75: 1. Of the patients reported to date, cardinal symptom of this tumor was neck mass. The rapid clinical course (from 2 weeks to 3 months) and progressive enlargement of the neck mass may suggest the malignant behavior of this tumor. Notably, in all of the 9 cases (locations of neck nodes were not given in 3 cases), cervical metastatic lymphadenopathy were from the ipsilateral primary lesions. Nevertheless, we failed to extract further information about the regularity of the neck sublevel involvement. Other symptoms included throat pain and odynophagia which were also presented in our case.
Table 1.
Review of literature describing cases of small cell NEC of the tonsil
| Literature | Case No. | Age (yrs)/sex | Initial symptom | Tumor site and size | Treatment | Results |
|---|---|---|---|---|---|---|
| Koss et al. 1972 [4] | 1 | 70/F | N.G. | Tonsil (5.0 cm) | RT | DOD 8 mos (MET) |
| 2 | 60/M | N.G. | Tonsil (occult) | RND + RT (primary and neck) | DOD 18 mos (Neck failure) | |
| 3 | 54/M | N.G. | Tonsil (3.5 cm) | Local excision + RND | DOD 1.5 yrs (MET: lung and liver) | |
| Abedi and Sismanis 1987 [7] | 4 | 67/F | Left neck mass (2 × 3 cm) | Left tonsil (N.G.) | RT + CT (for MET) | DOD 6 mos (MET) |
| Heimann et al. 1989 [8] | 5 | 78/N.G. | Left neck mass (5 × 3 × 3 cm) | Left tonsil (N.G.) | Left RND | Die of cardiac arrest 2 yrs after RND |
| Bawa and wax 1995 [9] | 6 | 53/M | Tonsil pain Odynophagia Left neck mass (5 cm in level II and 2 cm in level V) | Left tonsil (N.G.) | CT + RT (for neck REC) | DOD 15 mos (MET) |
| Weng et al. 2008 [10] | 7 | 53/M | Right neck mass (level II-VI) | Right tonsil (N.G.) | CT + RT | DOD 6 mos (MET: adrenal and bone) |
| Hatoum et al. 2009 [11] | 8 | 49/M | N.G. | Reported as T1N3 | CRT | DOD 2.5 yrs (MET) |
| 9 | 50/M | N.G. | Reported as T3N1 | CRT | Alive with NED >9 yrs | |
| 10 | 50/F | N.G. | Reported as T4N2b | CRT | Alive with NED >1.5 yrs | |
| Segawa et al. 2011 [13] | 11 | 65/M | Sore throat Left neck mass (N.G.) | Left tonsil (3.2 × 3.0 cm) | RT + CT | DOD 2 yrs (MET: liver) |
| Sehdev et al. 2012 [14] | 12 | 53/F | Right neck mass (3.5 × 2.8 cm in level II) | Right tonsil (N.G.) | CRT | Alive with NED >3 yrs |
Abbreviations: yrs: years; F: female; M: male; N.G.: not given; RT: radiotherapy; DOD: die of disease; mos: months; MET: metastases; RND: radical neck dissection; CT: chemotherapy; REC: recurrence; CRT: chemoradiotherapy; NED: no evidence of disease.
Although apparent lesions in tonsil as asymmetric swelling and ulcerating mucosa can be well recognized by physical examination, small submucosal tumors (case No. 2, for instance) might be missed without radiographic evaluation. Fine-cut imaging with CT and/or MRI can evaluate the tumor size and its infiltration depth. In addition, it is necessary to confirm the tumor is not metastasis of other distant primary, especially from the lung [15]. The diagnosis of small cell NEC is based on its pathological and immunohistochemical features which have been mentioned above. Differential diagnosis includes paraganglioma (which is positive staining for S-100 but negative for cytokeratin) and malignant lymphoma (which is immunoreactive for LCA but negative for neuroendocrine markers) [16].
Owing to its rarity, recommendations for management of small cell NEC of the tonsil have not been established. In general, it is wildly accepted that small cell NEC of the head and neck is aggressive and prone to develop early regional or distant metastasis. Based on comparative treatment for small cell NEC of the larynx and lung, various modalities including surgical resection, radiotherapy, chemotherapy or some combination of them have been indicated. In terms of local control, current opinion favors the use of radiotherapy to the primary tumor site and neck rather than surgery, or their combination, on account of their indistinctive outcome [3]. With limited controversy, many investigators suggested that chemotherapy should be considered in all patients with small cell NEC of the head and neck by reason of its propensity for early metastasis. Among all the chemotherapeutic agents, platinum-based regimens such as CDDP and etoposide are most commonly used in recent years. Use of new chemotherapeutic agents such as irinotecan, which has shown encouraging effect against small cell lung cancer, has also been reported in one case of small cell NEC arising from the tonsil [13].
Despite multimodality treatment, the outcome of patients with small cell NEC of the head and neck is dismal. By our review of the 12 cases, recurrence or distant metastases was found in 66.7% of them and these patients ultimately died of disease in 2.5 years with the median overall survival time of 18.0 months. The sites of distant metastases were the lung, liver, bone and one unusual location, the adrenal. The present case received six cycles of cisplatin combined with etoposide without local control modalities, as she refused surgery or radiotherapy. Although the tumor showed initial complete response at that time, recurrence in her left neck developed 9 months after initial diagnosis.
In conclusion, small cell NEC of the tonsil is extremely rare and highly aggressive with poor prognosis. Here we present the 13 case of this tumor with a review of its nomenclature, clinicopathological characteristics and treatment modalities. With a paucity of studies, standard treatment protocol remains uncertain while radiotherapy combined with chemotherapy seems to be the relative appropriate option.
Disclosure of conflict of interest
None.
References
- 1.Stambuk HE, Karimi S, Lee N, Patel SG. Oral cavity and oropharynx tumors. Radiol Clin North Am. 2007;45:1–20. doi: 10.1016/j.rcl.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Jang H, Yuk SM, Kim JO, Han DS. A rare case of primary malignant small cell carcinoma combined with urothelial cell carcinoma in the ureter. World J Surg Oncol. 2013;11:181. doi: 10.1186/1477-7819-11-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Renner G. Small cell carcinoma of the head and neck: a review. Semin Oncol. 2007;34:3–14. doi: 10.1053/j.seminoncol.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 4.Koss LG, Spiro RH, Hajdu S. Small cell (oat cell) carcinoma of minor salivary gland origin. Cancer. 1972;30:737–741. doi: 10.1002/1097-0142(197209)30:3<737::aid-cncr2820300322>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 5.Barnes L. Neuroendocrine Tumors. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. Pathology and Genetics of Head and Neck Tumors (World Health Organization Classification of Tumors) Lyon: IARC; 2005. pp. 135–139. [Google Scholar]
- 6.Kao HL, Chang WC, Li WY, Chia-Heng LA, Fen-Yau LA. Head and neck large cell neuroendocrine carcinoma should be separated from atypical carcinoid on the basis of different clinical features, overall survival, and pathogenesis. Am J Surg Pathol. 2012;36:185–192. doi: 10.1097/PAS.0b013e318236d822. [DOI] [PubMed] [Google Scholar]
- 7.Abedi E, Sismanis A. Extrapulmonary oat-cell carcinoma of the tonsil. Ear Nose Throat J. 1987;66:112–115. [PubMed] [Google Scholar]
- 8.Heimann R, Dehou MF, Lentrebecq B, Faverly D, Simonet ML, Dor P, Chanoine F. Anaplastic small cell (oat cell) carcinoma of the tonsils: report of two cases. Histopathology. 1989;14:67–74. doi: 10.1111/j.1365-2559.1989.tb02115.x. [DOI] [PubMed] [Google Scholar]
- 9.Bawa R, Wax MK. Small cell carcinoma of the tonsil. Otolaryngol Head Neck Surg. 1995;113:328–333. doi: 10.1016/S0194-5998(95)70131-1. [DOI] [PubMed] [Google Scholar]
- 10.Weng CT, Chu PY, Liu MT, Chen MK. Small cell carcinoma of the head and neck: a single institution’s experience and review of the literature. J Otolaryngol Head Neck Surg. 2008;37:788–793. [PubMed] [Google Scholar]
- 11.Hatoum GF, Patton B, Takita C, Abdel-Wahab M, LaFave K, Weed D, Reis IM. Small cell carcinoma of the head and neck: the university of Miami experience. Int J Radiat Oncol Biol Phys. 2009;74:477–481. doi: 10.1016/j.ijrobp.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 12.Rudman KL, King E, Poetker DM. Extrapulmonary small cell carcinoma metastasis to the external auditory canal with facial nerve paralysis. Am J Otolaryngol. 2011;32:343–345. doi: 10.1016/j.amjoto.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Segawa Y, Nakashima T, Shiratsuchi H, Tanaka R, Mitsugi K, Komune S. Small cell carcinoma of the tonsil treated with irinotecan and Cisplatin: a case report and literature review. Case Rep Oncol. 2011;4:587–591. doi: 10.1159/000335218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sehdev A, Zhao Y, Singh AK, Sharma N. Primary small cell carcinoma of the tonsil: a case report and review of the literature. Case Rep Oncol. 2012;5:537–541. doi: 10.1159/000343676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong W, Wang X, Yu XM, Chen B, Ding GJ, Zhang YP. Palatine tonsillar metastasis of lung cancer during chemotherapy. Int J Clin Exp Pathol. 2012;5:468–471. [PMC free article] [PubMed] [Google Scholar]
- 16.Warrick JI, Brink DS, Mitchell RB. Paraganglioma of the palatine tonsil. Ear Nose Throat J. 2012;91:E29–E31. [PubMed] [Google Scholar]


