Abstract
Rhynchophylline (Rhy) is the major component of Uncaria species, which is used in Chinese traditional medicine for the treatment of central nervous system disorders. However, its oral bioavailability has not been known. This study aims to investigate the intestinal permeability and related mechanisms of Rhy using cultured human epithelial Caco-2 cells. The cytotoxicity of Rhy on Caco-2 cells was evaluated with MTT assay. The effect of Rhy on the integrity of Caco-2 cell monolayer was assayed with transepithelial electrical resistance. The permeability of Rhy across cell monolayer was assayed by measuring Rhy quantity in received side with HPLC. The effect of Rhy on the expression of P-glycoprotein and MDR1 was detected with Western blot and flow cytometry, respectively. In the concentration of Rhy, which did not produce toxicity on cell viability and integrity of Caco-2 cell monolayer, Rhy crossed the monolayer with velocity 2.76~5.57×10^-6 cm/sec and 10.68~15.66×10^-6 cm/sec from apical to basolateral side and from basolateral to apical side, respectively. The permeability of Rhy was increased by verapamil, a P-glycoprotein inhibitor, or rhodamine123, a P-glycoprotein substrate. Rhy revealed an induction effect on P-glycoprotein expression in Caco-2 cells. These results demonstrate the low permeability of Rhy in intro, and suggest that P-glycoprotein may underlie the mechanism.
Keywords: Rhynchophylline, P-glycoprotein, permeability, Caco-2 cells
Introduction
Rhynchophylline (Rhy) is a natural product extracted from the stems of Uncaria rhynchophylla, a major tetracyclic oxindole alkaloid [1]. Rhy is usually used together with other traditional Chinese medicines, and has been clinically used for hundreds of years [2]. As its neuroprotective and anti-hypertensive effects, Rhy is commonly used in the treatment of cardiovascular and cerebrovascular diseases, and exhibits some good therapeutic effects [3,4]. The neuroprotective effect of Rhy has been identified though the inhibition of c-Jun kinase phosphorylation and nuclear factor-κB activity and delay A-Type K+ channel reflects [5,6].
The low oral bioavailability of drug candidates is one of the obstacles which limit them to become a new drug. Statistics showed that nearly 40% of the manufactured drugs through the world had low oral bioavailability [7]. Therefore, it is not only important but also necessary to study the oral bioavailability in the early development of new drugs. The small intestine is the organ where the vast majority of digestion takes place. Human epithelial cell line Caco-2 cell is one of the most extensively utilized cell lines for permeability and efflux screening in vitro during the development of oral drugs. Caco-2 cell monolayer is a suitable model to assay the permeability of drugs in vitro, and is used to predict the bioavailability in vivo [8]. This model has become an important tool to investigate the transport of drugs across the intestinal epithelium, actively or passively, and, if the transport is active, to identify the relevant carrier.
Caco-2 cells express some transporters with the immune efflux function, including P-glycoprotein (P-gp) and multiple drug resistance associated protein (MRP) [9]. Drug transporters are increasingly being recognized to play an important role in drug disposition. These transport carriers protect the human body from the damage of xenobiotics by suppressing intestinal absorption and increasing intestinal excretion. Meantime, these transport carriers also efflux drugs out of the human body, and reduce the effect of the drugs. P-gp (MDR1/ABCB1), expressing in the apical side of the monolayer, is the most extensively studied drug transporter, and is known to transport structurally diverse classes of compounds. P-gp is ubiquitously presence in human body, and extrudes a large number of chemicals. The presence of P-gp is an important reason causing the low oral bioavailability and preventing drug candidates becoming new clinical drugs [9,10]. Therefore, a clear interaction between chemicals and P-gp is important in the understanding of drug absorption mechanisms, and is a help to guide new drug investigation and clinical application.
The purpose of this study is to find out the transport characterizations of Rhy in the intestinal epithelial and verify whether P-gp involves the transport of Rhy.
Materials and methods
Materials
Rhy, with over 98% purity, was purchased from Shanghai Winherb Medical S & T Development Co. Ltd. (Shanghai, China). Rhodamine123 (Rh123), probenecid, 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), fetal bovine serum (FBS) and trypsin-EDTA solution were purchased from Sigma-Aldrich (St. Louis, MO, USA). Carbamazepine, verapamil and cyclosporine A (CsA) were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Minimum Essential Medium (MEM), N-hydro-xyethylpiperazine-N0-2-ethanesulfonate (HEPES, 1M), penicillin and streptomycin were purchased from Gibco Laboratories (Grand Island, NY). All reagents used in western blot were purchased from Cowin Bioscience Co., Ltd (Beijing, China). Anti-β-actin antibody, anti-P-gp antibody, horseradish peroxidase-conjugated second antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Phycoerythrin-labeled anti-human MDR1 was purchased from Biosciences (BD, USA). Acetonitrile was purchased from Fisher Co. Ltd. (Emerson, IA, USA). Triethanolamine and glacial acetic acid were purchased from the Beijing Chemical Reagent Company (Beijing, China). HPLC-grade reagents were used as the mobile phase in HPLC analysis, and all the other chemicals and reagents were commercially available and their purity was guaranteed. Milli-Q (Milford, MA, USA) water was used throughout the study.
Cell culture
The Caco-2 cells were purchased from American Type Culture Collection (ATCC, Virginia, USA). Caco-2 cells at Passage 21-60 were used in this study. Cells were grown in MEM supplemented with heat-inactivated FBS (10% v/v), 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, non-essential amino acids (1% v/v), and cultured at 37°C in an atmosphere of 5% CO2 with a relative humidity of 95%.
Cell viability assay
The effect of Rhy on the viability of Caco-2 cells was assessed using a standard MTT assay. Briefly, cells were seeded at 1×10^4 cells per well in 96-well plates and grown for 36 h. Rhy at the concentration of 12.5~400 μmol/L was added in culture medium and incubated for 24 h. Ten microliter of MTT solution (5 mg/mL in PBS) was then added to each well and incubated for another 4 h to allow MTT to be metabolized. After the MTT solution was removed, 100 μl DMSO was added. The 96-well plates were shaken for 10 min and the absorbance was detected at 570 nm on a microplate reader (Spectrafluor, TECAN, Sunrise, Austria). Cell viability was expressed as a percentage of the no-Rhy control.
Integrity assay of cell monolayer
The integrity of the Caco-2 cell monolayer was measured by the transepithelial electrical resistance (TEER) using a Millicell-ERS epithelial voltometer (Millipore, Massachusetts, USA). The cells were seeded at a density of 1×10^6 cells/cm2 on permeable polycarbonate filter supports (0.4 μm pore size, 1.12 cm2 growth area) in Transwell cell culture chambers (Corning Costar, Cambridge, MA) and cultured for 21 days to form cell monolayer. The culture medium (0.5 mL/insert and 1.5 mL/well) was replaced every two days at the first week and every day thereafter.
Before TEER measured, cell monolayers were washed twice with Hanks’ buffered salt solution (HBSS), and incubated in HBSS for 20 min at 37°C with shaking speed of 50 rpm. The TEER value was measured every 30 min in 180 min. The TEER in HBSS alone was used to confirm that the Caco-2 cell monolayer was comparable to the small intestinal epithelium [10-12].
The TEER value was calculated from the following equation.
TEER = (Rmonolayer - Rblank)×A
Rmonolayer is the resistance of the cell monolayer along with the filter membrane; Rblank is the resistance of the filter membrane itself, and A is the surface area of the membrane (1.12 cm2).
Permeability assay
Permeability assay was performed in an air shaking bath (SW 23, Julabo, Seelbach, Germany) at 37°C and 50 rpm to simulate the small intestine motility. The transport experiments were carried out in HBSS with 25 mM HEPES at pH 7.4. All the other drug solutions were adjusted pH 7.4 prior to use. Cell monolayer was washed twice with HBSS, and pre-incubated in HBSS at 37°C for 20 min in the air shaking bath. After that, TEER crossing the apical (AP) and the basolateral (BL) sides was monitored before transport studies. Those monolayer with TEER more than 800 Ω•cm2 was used in the transport studies.
In the permeability assay of Rhy (add little hydrochloric acid solution for better dissolution), HBSS solution on both sides of the cell monolayer was removed by aspiration. For the transport from AP to BL side, 500 μL of HBSS containing Rhy was added to the AP side and 1500 μL HBSS was added to the BL side. For the transport from BL to AP, 1500 μL of HBSS containing Rhy was added to the BL side and 500 μL of HBSS was added to the AP side. To test the effect of a chemical inhibitor on the bidirectional transport of Rhy, HBSS containing Rhy and inhibitor was loaded onto the donor side. The quantity of Rhy in the samples from the receiver compartment was analyzed using HPLC. The apparent permeability coefficient (Papp, cm/s) was calculated from the following equation.
Papp = (dQ / dt) / (C0×A)
dQ/dt is the Rhy transport rate (μmol/L.min), defined as the slope obtained from linear regression of Rhy transport amount. C0 is the initial concentration of Rhy on the donor side (μmol/L). A is the surface area of the filters or inserts (1.12 cm2).
The efflux ratio (ER) was calculated according to the following equation:
ER = Papp (BL→AP) / Papp (AP→BL)
Papp (BL→AP) and Papp (AP→BL) represent the apparent permeability of test compound from BL to AP side and AP to BL side of the cell monolayer, respectively.
Expression of P-gp and MDR1 in Caco-2 cells
Caco-2 cells were cultured in 6-well Transwell plates for 21 days of differentiation, then refresh medium containing Rhy or Ver (50 μmol/L) was replaced for another 2 h culture. Cells were rinsed twice with HBSS for 5 min at 37°C and the TEER of monolayer was measured. The polycarbonate membrane with the cell monolayer was excised from the Transwell insert using scalpel. The cells were then used to measure the expression of P-gp and MDR1 using Western blot and flow cytometry, respectively. In Western blot, the cells were lysed in RIPA lysis buffer containing a protease and phosphatase inhibitor cocktail. The lysates were centrifuged at 12000 g for 15 min at 4°C, and the supernatant was kept for Western blot. Protein concentration was determined with bicinchoninic acid assay. After boiling, 50 μg of protein was electroblotted on a 6% sodium dodecyl sulfate-poly-acrylamide gel. Proteins were then transferred to nitrocellulose membranes in a wet system. The membranes were blocked in Tris buffered saline containing 0.01% Tween-20 and 5% non-fat dry milk for 1 h at room temperature. The membranes were then incubated overnight at 4°C with anti-P-gp antibody (1:1000) or anti-β-actin antibody (1:5000). After washing with TBST, the membranes were incubated for 2 h at room temperature with the related 2nd antibody. Then membranes were washed and treated with ECL Western blotting reagents. The protein levels were determined by analyzing the signals captured on the nitrocellulose membranes using a Chemi-doc image analyzer (Bio-Rad, USA).
In flow cytometry analysis, the cells were adjusted to 1×10^6 cells/ml, and treated with PE-anti-MDR1 (1:200) for 15 min in the dark at room temperature. The cells were washed twice with PBS. The fluorescence was measured by flow cytometry analysis with a FACS Calibur Flow Cytometer (BD Biosciences, USA).
HPLC analysis
The quantity of Rhy from the receiver compartment was measured using a reversed-phase Shimadzu HPLC system (Waters, USA) equipped with a Waters 2695 pump/injector, a Wa|ters 996 photodiode array detection detector, a cooling autosampler, a reversed-phase C-18 column (250×4.6 mm, 5 μm; Merck KGaA, Darmstadt, Germany), and a security column (Phenomenex, Torrance, CA). The mobile phase consisted of solvent A and solvent B (65:35, v/v), and was set at a flow of 1 mL/min. Solvent A was composed of acetonitrile, while solvent B consisted of 0.15% triethylamine (pH 7). The column temperature was maintained at 25°C, and the sample temperature was maintained at 15°C. After centrifugation at 12,000 g for 5 min, 30 μL carbamazepine solution (0.2 μg/ml), served as the internal standard, was added to all the supernatant samples to yield adequate volumes for analysis. Synthesized Rhy was determined by HPLC at 243 nm. A 10 μL aliquot of each sample was injected into the HPLC apparatus for analysis. All solvents for HPLC were filtered through 0.45 μm filter membranes before injecting into the system.
Statistical analysis
All Caco-2 experiments were performed in triplicates, and the results are presented as Mean ± S.D. Data were analyzed using independent sample t-test and one-way analysis of variance (ANOVA) with Dunnett’s T3 post hoc test (SPSS 15.0, SPSS, Inc.). Probability values of <0.05 were considered statistically significant.
Results
Effect of Rhy on cell viability
As the cytotoxicity may affect the permeability result, we first measured whether Rhy damaged cell viability. The MTT assay is a colorimetric assay for assessing cell viability. MTT, a yellow tetrazole, is reduced to purple formazan in living cells. DMSO is added to dissolve the insoluble purple formazan product into a colored solution. In MTT assay, compared to the no-Rhy control, Rhy treatment at the concentration range of 12.5~400 μmol/L in 24 h did not produce significant effect on cell viability. No significant difference of viability was seen between these different concentrations of Rhy (Figure 1). The result confirms Rhy is non-toxic to Caco-2 cells, at least in indicated concentrations and time.
Figure 1.
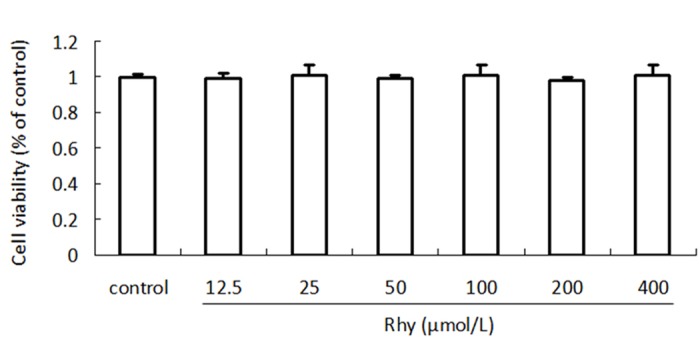
Effect of Rhy on Caco-2 cells viability. Rhy Caco-2 cells were treated with Rhy for 24 h at indicated concentrations, and cell viability was measured with MTT assay. Data are shown as percentage of no Rhy-treated control (n = 4).
Effect of Rhy on integrity of cell monolayer
We next examined whether Rhy affected the integrity of cell monolayer by measuring the TEER values. As shown in Figure 2, The TEER values were not obviously affected by HBSS in 3 h. Rhy (100 μmol/L) did not significantly change the TEER values in 2 h, although it decreased the TEER values at 2.5 h and 3 h, however this phenomenon was not appeared in HBSS. It might be caused by the PH value or toxicity of RHY solution. Thus, the Rhy transport studies were performed in 2 h to prevent the interference of damaged cell monolayer integrity.
Figure 2.
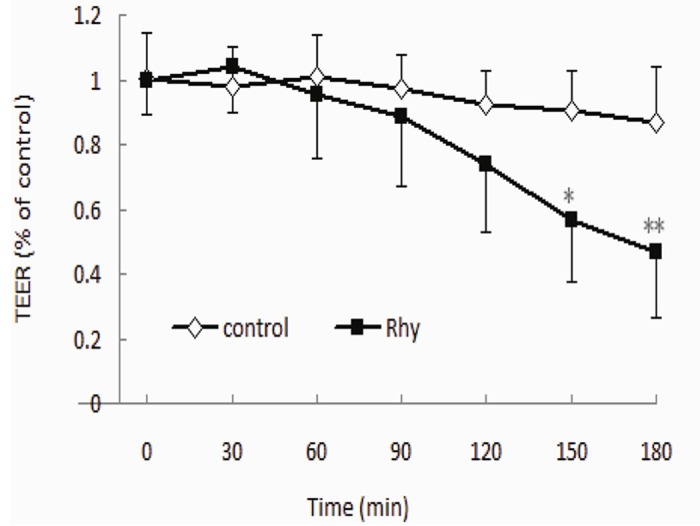
Effect of Rhy on cell monolayer integrity. Caco-2 cells were cultured for 21 days to form cell monolayer. HBSS containing Rhy (100 μmol/L) or HBSS alone (served as control) was added in cells. TEER was measured every 30 min in 180 min. Data are shown as percentage of the initial TEER (n = 3).
Permeability assay
The absorptive and secretory transport profiles of Rhy across cell monolayers in vitro are shown in Figure 3, and the Papp values were listed in Table 1. The bi-directional transport of Rhy across Caco-2 cell monolayer at a different concentration (25, 50, or 100 μmol/L) and different time points (0~120 min) showed a consistent trend, the transport accumulation of Rhy increased with time. The Papp (A→B) values was 2.76~5.57×10^-6, the Papp (B→A) values ranged 10.68~15.66×10^-6. The ER value of Rhy was 2.81~3.87, which indicated more efflux than absorbance.
Figure 3.
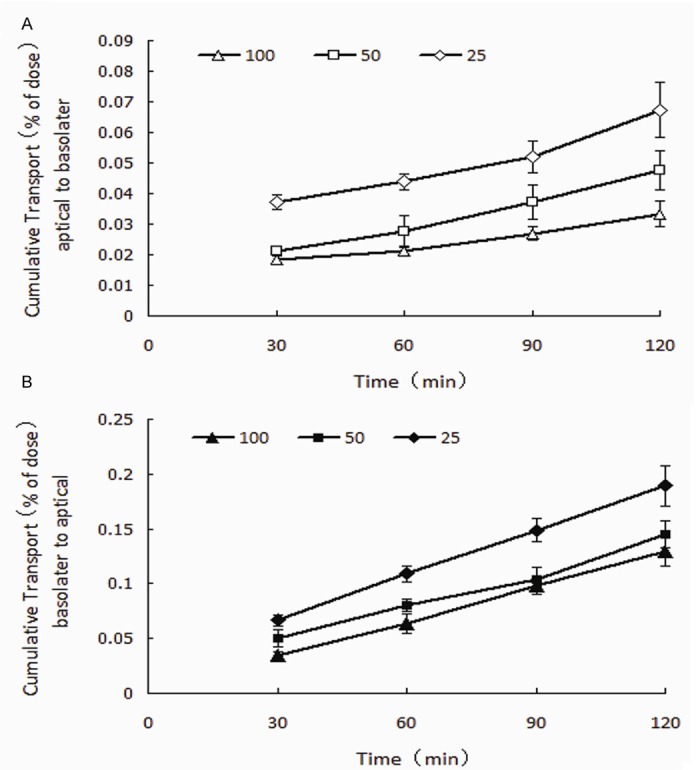
Bidirectional transport of Rhy. Rhy was added into donor side of Caco-2 cell monolayers. Samples were taken from the received sides at different time points (30, 60, 90 and 120 min), and measured with HPLC. Results are expressed as percent of initial dose (n = 3).
Table 1.
Apparent permeability (Papp) and the efflux ratio (ER) of Rhy (n = 3)
| Concentration (μmol/L) | 25 | 50 | 100 |
|---|---|---|---|
| Papp (AP→BL) | 5.57 ± 0.75 | 3.93 ± 0.53 | 2.76 ± 0.36 |
| Papp (BL→AP) | 15.66 ± 1.51 | 12.01 ± 1.00 | 10.68 ± 1.08 |
| ER | 2.81 | 3.06 | 3.87 |
Effects of transporters inhibitors on the transport of Rhy
To identify which efflux transporter was involved in the efflux transport of Rhy across Caco-2 cell monolayers, we applied the inhibitors against P-gp (verapamil), against MRP (probenecid), or against both (CsA), two important transporters on the intestinal epithelial cells.
We added 20 μmol/L of probenecid, verapamil or CsA in donor side in the transport medium, Figure 5. Effect of Rhy on Rhodamine123 transport. Five μmol/L Rhodamine123 (rh123) and indicated concentration of Rhy were added into donor side of cell monolayer. Samples from the received compartment were collected at 120.and then examined the bidirectional transport of Rhy (Figure 4). Compared to the no-inhibitor control, verpamil or CsA increased significantly transport from AP to BL side. In contrast, probenecid did not reveal a significant influence on the transport. All the three inhibitors did not significantly change the transport form BL to AP side. These data indicated that P-gp, but not MRP played a main role in transport of Rhy across Caco-2 monolayer.
Figure 5.
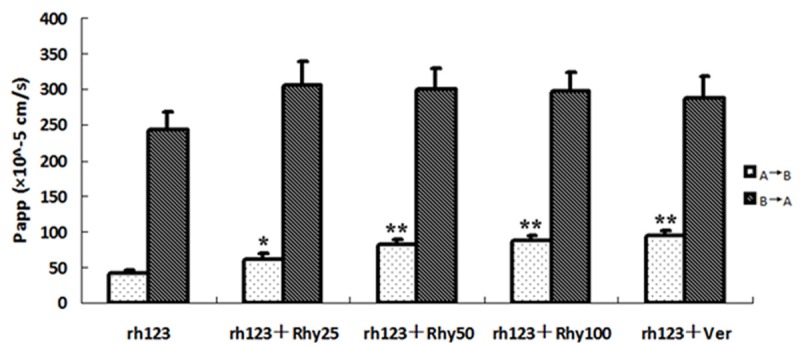
Effect of Rhy on Rhodamine123 transport. Five μmol/L Rhodamine123 (rh123) and indicated concentration of Rhy were added into donor side of cell monolayer. Samples from the received compartment were collected at 120.
Figure 4.
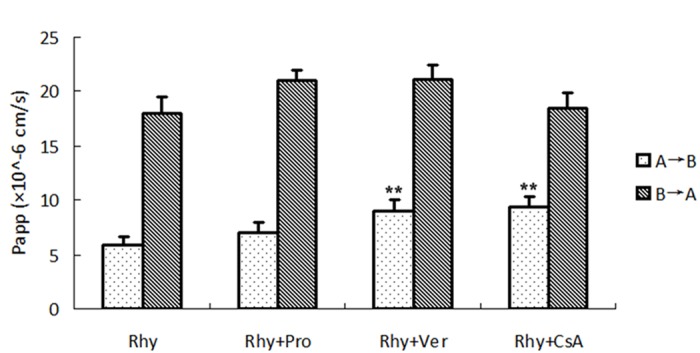
Effect of transport inhibitors on the bidirectional transport of Rhy. Twenty μmol/L transport inhibitor verapamil (Ver), probencid (Pro) or CsA and Rhy (50 μmol/L) were added to donor side of cell monolayer. Samples from the received compartment were collected at 120 min, and Rhy was measured with HPLC system. Papp was calculated (n = 3).
Effects of Rhy on rhodamine123 transport
Rhodamine123, a specific substrate of P-gp, was identified that its transport across Caco-2 cell monolayer was mediated by P-gp [10]. Thereby we investigated whether rhodamine123 and Rhy competed with the binding site of P-gp and interfered in their transport each other. As shown in Figure 5, Rhy (25, 50, 100 μmol/L) concentration-dependently increased the rhodamine123 transport from AP to BL side. Verapamil (20 μmol/L), the P-gp inhibitor, also showed significant increase of rhodamine123 from AP to BL side. There was no significant change of the Rhodamine123 transport from BL to AP side by Rhy or verapamil.
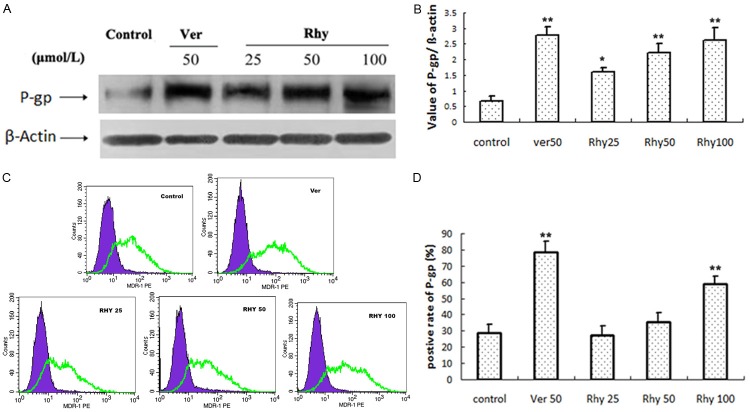
Effects of Rhy on the expression of P-gp and MDR1
We nest investigated whether Rhy affect the expression of P-gp and MDR1, a member of P-gp. The Western blot analysis showed that 24 h treatment of Rhy (25, 50, 100 μmol/L) concentration-dependently increased P-gp expression (Figure 6A, 6B). Verapamil (50 μmol/L) also increased significantly P-gp expression.
Figure 6.
Effect of Rhy on the expression of P-gp and MDR1. Caco-2 cells were treated with indicated concentration of RhyA or verapamil (Ver, 50 μmol/L) for 24 h. The cells were collected to detect protein expression of P-gp and MDR1 with Western blot (A) and flow cytometry (B), respectively (n = 3).
In the flow cytometry (Figure 6C, 6D), 24 h of Rhy treatment significantly elevated the MDR1 expression at high concentration, 100 μmol/L. No significant increase was seen at 25 and 50 μmol/L. Verapamil (50 μmol/L) also increased significantly MDR1 expression.
Discussion
Rhy, an important tetracyclic oxindole alkaloids isolated from Uncaria species, such as Uncaria Rhy (MIQ) Jackson and Uncaria sinensis (Oliv.) Havil, has been long used in traditional Chinese medicine [13]. As a kind of exogenous material, Rhy has been well documented to possess a great variety of cardiovascular pharmacological properties, including antihypertension, brachycardia, antiarrhythmia, vascular dementia and sedation [14]. These pharmacological properties contribute to Rhy a new drug candidate and a significant market potential. The previous studies have shown that the half-life period of Rhy is very short, and the oral bioavailability is very low [15,16], and we determined the stability, accuracy, precision under the applied experimental condition to verify the feasibility of our research [17].
As the body immune efflux of the exogenous materials is a key factor for the low oral drug bioavailability, clarifying the efflux mechanism of Rhy, as well as enhancing its bioavailability, is important pathway to solve the low bioavailability [17]. Based on the Caco-2 cell monolayer model, in this study, we found that Rhy had low transmembrane transport, and P-gp, but not MRP participated in the low level of transport.
Exogenous substances must be passed the human body though four process: absorption, distribution, metabolism and excretion. Absorption is the first process of exogenous substances into the body, and very importance for predicting druggability. Caco-2 cells exhibit many features of absorptive intestinal cells. Caco-2 cell monolayer has been used as a model of human intestinal absorption of drugs and other compounds, especially in drug discovery [18,19].
It has been suggested that apparent permeability coefficients in the intestinal is related with oral drug bioavailability, and directly reflects the drug absorption, big or small. Caco-2 cells express a variety of transport proteins including P-gp, MRP2, and breast cancer resistance protein (BCRP) [20-22]. P-gp, MRP and BCRP belong to the transporters of the adenosine triphosphate (ATP)-binding cassette (ABC) family.
In this study, the Caco-2 cells formed a proper monolayer with efficient tight junctions. This tight monolayer led to TEER value more than 800 Ω•cm2, and allowed the passage of the Rhy only through transcellular route. The micro morphological characteristics were observed by transmission electron microscope in our early studies [23]. The monolayer integrity was damaged in longer treatment of Rhy (2.5 h, 3 h). It is inferred that Rhy may increase the permeability by opening intercellular tight junctions. Besides the TEER test, another validation of monolayer integrity was two permeability markers ANT and FSD [24,25].
The Caco-2 permeability model system was used to determine the permeability of Rhy. According to the previous reports involving drugs crossing Caco-2 cell monolayer, apparent permeability coefficients (Papp) of 14.0×10^-6 cm/s or more were considered highly permeable, while Papp values of less than 5.0×10^-6 cm/s were characteristic of low permeability [15,26]. In this study, the Papp (AP→BL) values ranged 2.76~5.57×10^-6 and the Papp (BL→AP) values ranged 10.68~15.66×10^-6. The results showed that Rhy exhibited a low permeability in the AP→BL transport. When calculating the efflux ratio, the value was more than 2. The high ER value indicates a net efflux of the drug.
Among the efflux transporters, P-gp and MRP are the most important factors on the intestinal epithelial cells. P-gp is one of the efflux transporters which exist in the brush border membrane of intestinal cells, basolateral membrane of liver cells, blood-brain barrier [10]. It is generally accepted that co-administration of drugs that interact with P-gp (as a substrate, inhibitor or inducer) can result in drug–drug interactions (P-gp-mediated DDI) that affect the PK/PD of the co-administered drugs [27,28]. In contrast, the MRP are also highly expressed in Caco-2 cells. Substrates of MRP2 have been reported to be large compounds with medium lipophilicity carrying at least one negative charge, and having a large polar surface area [29].
In this study, we saw that P-gp inhibitors verapamil and CsA increased transport of Rhy from AP to BL sides, but MRP inhibitor probenecid did not. All the three inhibitors did not affect the transport from BL to AP sides. Thus, these results further confirmed that P-gp existed at AP side, and suggest that Rhy is a substrate of transporter P-gp.
Previous studies reported that Rhodamine123 was a specific substrate of P-gp in Caco-2 cells [30]. Different substrates compete with the binding site of same transporter. Indeed, in our result, Rhy increased significantly the transport of Rhodamine123 from AP to BL side. The data indicated that Rhy bound to P-gp, and reduced Rhodamine123 efflux.
A variety of oral drugs, which are subjected to efflux affect transporters expression, which further increases drug efflux. In this study, 24-h of Rhy treatment significant induced P-gp and MDR1 expression. The classical inhibitor, verapamil, also revealed induction effect on P-gp and MDR1 expression. However, the effect of verapamil on P-gp expression is being in dispute [31,32]. The induction effect of Rhy on P-gp may produce very bad consequence: lower absorbance and bioavailability.
In conclusion, the present study shows that Rhy subjected to high efflux in Caco-2 cell monolayer model, and Rhy also induced P-gp expression, which may further increase the efflux. P-gp inhibitor is an efficacious way to improve Rhy efflux. These results provide new understanding of oral bioavailability of Rhy.
Acknowledgements
This work was supported by research grants from Technology Pillar Program in the National Science (2008BAI51B02), major national Drug Discovery Scientific and Technological Projects (2012ZX095001001; 2012ZX09301002-001), China Postdoctoral Science Foundation (2012M510360).
Disclosure of conflict of interest
None.
References
- 1.Laus G, Teppner H. The alkaloids of Uncaria rhynchophylla (Rubiaceae-Coposapelteae) Phyton (Austria) 1996;36:185–196. [Google Scholar]
- 2.Zhixian Mo, Dandan Xu. New clinical research progress of Uncaria rhynchophylla. Lishizhen Medicine and Materia Medica Research. 2006;17:684–685. [Google Scholar]
- 3.Zhou JY, Zhou SW. Antihypertensive and neuroprotective activities of rhynchophylline: the role of rhynchophylline in neurotransmission and ion channel activity. J Ethnopharmacol. 2010;132:15–27. doi: 10.1016/j.jep.2010.08.041. [DOI] [PubMed] [Google Scholar]
- 4.Zhang WB, Chen CX, Sim SM, Kwan CY. In vitro vasodilator mechanisms of the indole alkaloids rhynchophylline and isorhynchophylline, isolated from the hook of Uncaria rhynchophylla (Miquel) Naunyn Schmiedebergs Arch Pharmacol. 2004;369:232–238. doi: 10.1007/s00210-003-0854-9. [DOI] [PubMed] [Google Scholar]
- 5.Chou CH, Gong CL, Chao CC, Lin CH, Kwan CY, Hsieh CL, Leung YM. Rhynchophylline from Uncaria rhynchophylla Functionally turns delayed rectifiers into A-Type K+ Channels. J Nat Prod. 2009;72:830–834. doi: 10.1021/np800729q. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh CL, Ho TY, Su SY, Lo WY, Liu CH, Tang NY. Uncaria rhynchophylla and Rhynchophylline inhibit c-Jun N-Terminal Kinase Phosphorylation and Nuclear Factor-κB Activity in Kainic Acid-Treated Rats. Am J Chin Med. 2009;37:351–360. doi: 10.1142/S0192415X09006898. [DOI] [PubMed] [Google Scholar]
- 7.Liu JY, Lee KF, Sze CW, Tong Y, Tang SC, Ng TB, Zhang YB. Intestinal absorption and bioavailability of traditional Chinese medicines: a review of recent experimental progress and implication for quality control. J Pharm Pharmacol. 2012;65:621–633. doi: 10.1111/j.2042-7158.2012.01608.x. [DOI] [PubMed] [Google Scholar]
- 8.Netzel M, Netzel G, Zabaras D, Lundin L, Day L, Addepalli R, Osborne SA, Seymour R. Release and absorption of carotenes from processed carrots (Daucus carota) using in vitro digestion coupled with a Caco-2 cell trans-well culture model. Food Res Int. 2011;44:868–874. [Google Scholar]
- 9.Artursson P, Palm K, Luthman K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv Drug Deliv Rev. 2001;46:27–43. doi: 10.1016/s0169-409x(00)00128-9. [DOI] [PubMed] [Google Scholar]
- 10.Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL, Chang G. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–1722. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watts T, Berti I, Sapone A, Gerarduzzi T, Not T, Zielke R, Fasano A. Role of the intestinal tight junction modulator zonulin in the pathogenesis of type I diabetes in BB diabetic-prone rats. Proc Natl Acad Sci U S A. 2005;102:2916–2921. doi: 10.1073/pnas.0500178102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ibrahim S, Peggins J, Knapton A, Licht T, Aszalos A. Influence of antipsychotic, antiemetic, and Ca(2+) channel blocker drugs on the cellular accumulation of the anticancer drug daunorubicin: P-glycoprotein modulation. J Pharmacol Exp Ther. 2000;295:1276–83. [PubMed] [Google Scholar]
- 13.Kang TH, Murakami Y, Takayama H, Kitajima M, Aimi N, Watanabe H, Matsumoto K. Protective effect of rhynchophylline and isorhynchophylline on in vitro ischemia-induced neuronal damage in the hippocampus: putative neurotransmitter receptors involved in their action. Life Sci. 2004;76:331–343. doi: 10.1016/j.lfs.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Li PY, Zeng XR, Cheng J, Wen J, Inoue I, Yang Y. Rhynchophylline-induced vasodilation in human mesenteric artery is mainly due to blockage of L-type calcium channels in vascular smooth muscle cells. Naunyn-Schmiedeberg’s Arch Pharmacol. 2013;386:973–982. doi: 10.1007/s00210-013-0888-6. [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Ma CM, Hattori M. Metabolism and pharmacokinetics of Rhynchophyline in rats. Biol Pharm Bull. 2012;33:669–676. doi: 10.1248/bpb.33.669. [DOI] [PubMed] [Google Scholar]
- 16.Isshiki M, Umezawa K, Tamura H. Coffee induces breast cancer resistance protein expression in Caco-2 cells. Biol Pharm Bull. 2011;34:1624–1627. doi: 10.1248/bpb.34.1624. [DOI] [PubMed] [Google Scholar]
- 17.Ma B, Sun GB, Xu HB, Li M, Yang ZH, Sun XB. Rhynchophylline in rat blood by high-performance liquid chromatography-coupled microdialysis. J Med Plants Res. 2012;6:2977–2984. [Google Scholar]
- 18.Uchida M, Fukazawa T, Yamazaki Y, Hashimoto H, Miyamoto Y. A modified fast (4 day) 96-well plate Caco-2 permeability assay. J Pharmacol Toxicol Methods. 2009;59:39–43. doi: 10.1016/j.vascn.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Yasuno T, Okamoto H, Nagai M, Kimura S, Yamamoto T, Nagano K, Furubayashi T, Yoshikawa Y, Yasui H, Katsumi H, Sakane T, Yamamoto A. In vitro study on the transport of zinc across intestinal epithelial cells using Caco-2 monolayers and isolated rat intestinal membranes. Biol Pharm Bull. 2012;35:588–593. doi: 10.1248/bpb.35.588. [DOI] [PubMed] [Google Scholar]
- 20.Wright JA, Haslam IS, Coleman T, Simmons NL. Breast cancer resistance protein BCRP (ABCG2)-mediated transepithelial nitrofurantoin secretion and its regulation in human intestinal epithelial (Caco-2) layers. Eur J Pharmacol. 2011;672:70–76. doi: 10.1016/j.ejphar.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Hirohashi T, Suzuki H, Chu XY, Tamai I, Tsuji A, Sugiyama Y. Function and expression of multidrug resistance-associated protein family in human colon adenocarcinoma cells (Caco-2) J Pharmacol Exp Ther. 2009;292:265–270. [PubMed] [Google Scholar]
- 22.Iwanaga K, Yoneda S, Hamahata Y, Miyazaki M, Shibano M, Taniguchi M, Baba K, Kakemi M. Inhibitory Effects of Furanocoumarin Derivatives in Kampo Extract Medicines on P-Glycoprotein at the Blood–Brain Barrier. Biol Pharm Bull. 2011;34:1246–1251. doi: 10.1248/bpb.34.1246. [DOI] [PubMed] [Google Scholar]
- 23.Ma B, Sun GB, Yang ZH, Li M, Sun XB. Comparative study on different culture conditions for caco-2 cell model. Chinese Journal of Experimental Traditional Medical Formulae. 2011;17:205–210. [Google Scholar]
- 24.Zhao SS, Yuan L, Wang JC, Zhang X, He ZG, Zhang Q. A novel and facile approach to imaging nanoparticles transport across transwell filter grown cell monolayer in real-time and in situ under confocal laser scanning microscopy. Biol Pharm Bull. 2012;35:335–345. doi: 10.1248/bpb.35.335. [DOI] [PubMed] [Google Scholar]
- 25.Hou XL, Takahashi K, Kinoshita N, Qiu F, Tanaka K, Komatsu K, Takahashi K, Azuma J. Possible inhibitory mechanism of curcuma drugs on CYP3A4 in 1,25 dihydroxyvitamin D3 treated Caco-2 cells. Int J Pharm. 2007;337:169–177. doi: 10.1016/j.ijpharm.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 26.Wahlang B, Pawar YB, Bansal AK. Identification of permeability-related hurdles in oral delivery of curcumin using the Caco-2 cell model. Eur J Pharm Biopharm. 2011;77:275–282. doi: 10.1016/j.ejpb.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Takizawa Y, Kishimoto H, Kitazato T, Tomita M, Hayashi M. Changes in the localization of lleal P-glycoprotein induced by intestinal ischemia/reperfusion. Biol Pharm Bull. 2011;34:408–414. doi: 10.1248/bpb.34.408. [DOI] [PubMed] [Google Scholar]
- 28.Ishibashi M, Arai M, Tanaka S, Onda K, Hirano T. Antiproliferative and apoptosis-inducing effects of lipophilic vitamins on human melanoma A375 cells in vitro. Biol Pharm Bull. 2012;35:10–17. doi: 10.1248/bpb.35.10. [DOI] [PubMed] [Google Scholar]
- 29.Bergstrom CAS, Bolin S, Artursson P, Ronn R, Sandstrom A. Hepatitis C virus NS3 protease inhibitors: large, flexible molecules of peptide origin show satisfactory permeability across Caco-2 cells. Eur J Pharm Sci. 2009;38:556–563. doi: 10.1016/j.ejps.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Tang FX, Ouyang H, Yang JZ, Borchardt RT. Bidirectional transport of rhodamine 123 and Hoechst 33342, fluorescence probes of the binding sites on P-glycoprotein, across MDCK–MDR1 cell monolayers. J Pharm Sci. 2004;93:1185–1194. doi: 10.1002/jps.20046. [DOI] [PubMed] [Google Scholar]
- 31.Granzotto M, Drigo I, Candussio L, Rosati A, Bartoli F, Giraldi T, Decorti G. Rifampicin and verapamil induce the expression of P-glycopretein in vivo in Ehrlich ascites tumor cells. Cancer Lett. 2004;205:107–115. doi: 10.1016/j.canlet.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 32.Yin J, Meng Q, Dong XM. Auto-inhibition of verapamil metabolism in rat hepatocytes of gel entrapment culture. Biomed Pharmacother. 2011;65:328–333. doi: 10.1016/j.biopha.2011.04.011. [DOI] [PubMed] [Google Scholar]