Abstract
The significance of human epidermal growth factor receptor 2 (HER2) overexpression in breast cancer is well established, and these patients are subsequently treated with Trastuzumab. Although HER2 expression in urothelial carcinoma of the urinary bladder has also been recently characterized, it has not been well studied in urothelial carcinoma of the renal pelvis. We investigated the relationship between HER2 overexpression in urothelial carcinoma of the renal pelvis and clinicopathologic parameters. Forty six cases were identified. HER2 overexpression was present in 34/46 (74%) cases. Mean patient age with HER2 overexpression was 68 years (range: 42-87 years). There was a male predominance with 28/34 (82%) patients. High grade urothelial carcinoma was present in 32/34 (94%) cases and 2/34 (6%) cases had low grade urothelial carcinoma. Pathologic staging was as follows; 9/34 (26%) cases were pTa, 10/34 (29%) cases were pT1, 2/34 (6%) cases were pT2, 12/34 (35%) cases were pT3, and 1/34 (3%) cases was pT4. An inverted growth pattern was present in 23/46 (50%) cases. HER2 overexpression was present in 15/23 (65%) cases of urothelial carcinoma with an inverted growth pattern. Our study showed that HER2 overexpression is more common in male patients with high grade urothelial carcinoma, especially those with an inverted growth pattern. It is highly conceivable that patients with urothelial carcinoma of the renal pelvis may be further stratified based on HER2 overexpression, and may also be potential candidates for Trastuzumab therapy in the neoadjuvant or adjuvant setting.
Keywords: Urothelial carcinoma, renal pelvis, immunohistochemistry, HER2, targeted therapy
Introduction
Upper urinary tract urothelial carcinoma is defined as a tumor that arises from the urothelium that lines the calyx, renal pelvis or ureter [1]. Urothelial carcinoma of the renal pelvis is relatively uncommon and accounts for 5-7% of urothelial carcinomas with an estimated incidence of 1-4 cases per 100 000 individuals per year [2-4]. The overall incidence has increased over the past several decades due to the increased use of ureteroscopy [5]. Urothelial carcinoma of the renal pelvis occurs twice as frequently as ureteral tumors [4]. Compared to bladder cancers, upper urinary tract urothelial carcinoma are usually more invasive tumors at diagnosis and are associated with a worse prognosis [6]. Potential prognostic roles of tumor stage, tumor grade, lymphovascular invasion, and lymph node involvement are well established in this setting [7-9].
Despite advances in endoscopic and minimally invasive treatments, the gold standard procedure remains radical nephroureterectomy with excision of a bladder cuff and retroperitoneal lymph node dissection [10,11]. However, upper urinary tract urothelial carcinoma is known to have a high recurrence rate including intravesical recurrence, even after radical surgery [12]. Patient survival is mainly influenced by stage, and 5-year survival rates range from 90% in early pathologic stages (pTa/pT1) to less than 5% in stages with lymph node involvement or metastatic disease [13]. The high recurrence and mortality rates in high-risk patients indicate the essential role of choosing the effective additional adjuvant treatment. Cisplatin-based chemotherapy in various combinations was the most common regimen, depending on the patients’ eligibility and renal function [13]. Even some studies have shown that adjuvant chemotherapy in patients with upper urinary tract urothelial carcinoma may not prolong survival [13]. Therefore, we need better prognostic markers, which ideally could also be used for targeted therapy. One of the markers currently under investigation is HER2.
The HER2 proto-oncogene which was previously called HER2/neu or (C-)ErbB-2, is located on chromosome 17q21 and encodes the 185 kDa transmembrane tyrosine kinase receptor HER2. The HER2 receptor is part of the EGF receptor (EGFR) family, which is important in several biochemical pathways including activation of signal transduction pathways controlling epithelial cell growth and differentiation, and possibly angiogenesis [14,15]. Overexpression of HER2 protein products is observed in approximately 20% of human breast cancers [16]. It leads to an increase in HER2 messenger RNA levels and a concomitant overexpression of the HER2 receptor on the tumor cell surface [17]. In breast cancer it is crucial for both prognosis and prediction of the response to targeted therapies, and HER2 testing is recommended in all newly diagnosed cases of invasive breast cancer [18,19]. The introduction of trastuzumab (Herceptin®), a recombinant humanized monoclonal Ab to the extracellular domain of HER2, has dramatically changed the treatment of HER2-amplified breast tumors in the adjuvant and metastatic setting [20-22]. HER2 is also overexpressed in some patients with bladder cancer [23].
Unlike breast cancer, where the role of HER2-targeting agents has been well established in both metastatic and adjuvant settings, no strategies of this type have yet been approved for use in urothelial carcinoma of the bladder and urothelial carcinoma of the renal pelvis.
In this study we investigated the relationship between HER2 overexpression in urothelial carcinoma of the renal pelvis and clinicopathologic parameters.
Material and methods
Case selection
A search was made through the surgical pathology and consultation files of our institution for radical nephroureterectomy cases with urothelial carcinoma of the renal pelvis from 2008-2012. Only cases with available tissue blocks were selected for the study. The hospital records of each patient were retrospectively reviewed. Clinicopathologic parameters including: sex, age, grade, stage, and inverted growth pattern were documented.
Immunohistochemistry
Immunohistochemistry was performed on 5 micron sections cut from routinely processed formalin-fixed, Paraffin-embedded tissue blocks. The tissue sections were deparaffinized and rehydrated, pretreated with 0.01 M citrate buffer (pH 6), and then stained for HER2 (Dako, Monoclonal Mouse Anti-Humman, Carpinteria, Ca; RTU). Appropriate positive and negative controls were employed throughout. HER2 positivity was assessed using the ASCO scoring system, evaluating only membranous staining.[18] The level of HER2 protein expression was assessed semiquantitatively by the intensity and percentage of staining and scored on a scale of 0 to 3+. Scores of 0 and 1+ are categorized as negative, 2+ as equivocal, and 3+ as positive. A score of 1+ was defined as barely perceptible membrane staining in >10% of cells, a score of 2+ was defined as weak-to-moderate complete membrane staining prespresent in >10% of tumor cells, and a score of 3+ was defined as strong complete membrane staining in >30% of tumor cells. A cytoplasmic staining was considered nonspecific. We consider only 3+ staining as a HER2 overexpression. This study was completed following the guidelines of and with approval from our institutional review board.
Results
Forty six cases were identified. HER2 overexpression was identified in 34/46 (74%) cases (Figure 1A and 1B). Mean patient age with HER2 overexpression was 68 years (range: 42-87 years). There was a male predominance with 28/34 (82%) patients and 6/34 (18%) patients were female. High grade urothelial carcinoma was present in 32/34 (94%) cases and 2/34 (6%) cases had low grade urothelial carcinoma (Figure 2A and 2B). Pathologic staging was as follows; 9/34 (26%) cases were pTa, 10/34 (29%) cases were pT1, 2/34 (6%) cases were pT2, 12/34 (35%) cases were pT3, and 1/34 (3%) cases was pT4. (Table 1) An inverted growth pattern was present in 23/46 (50%) cases. HER2 overexpression was present in 15/23 (65%) cases of UCA with an inverted growth pattern (Figure 2A and 2B).
Figure 1.
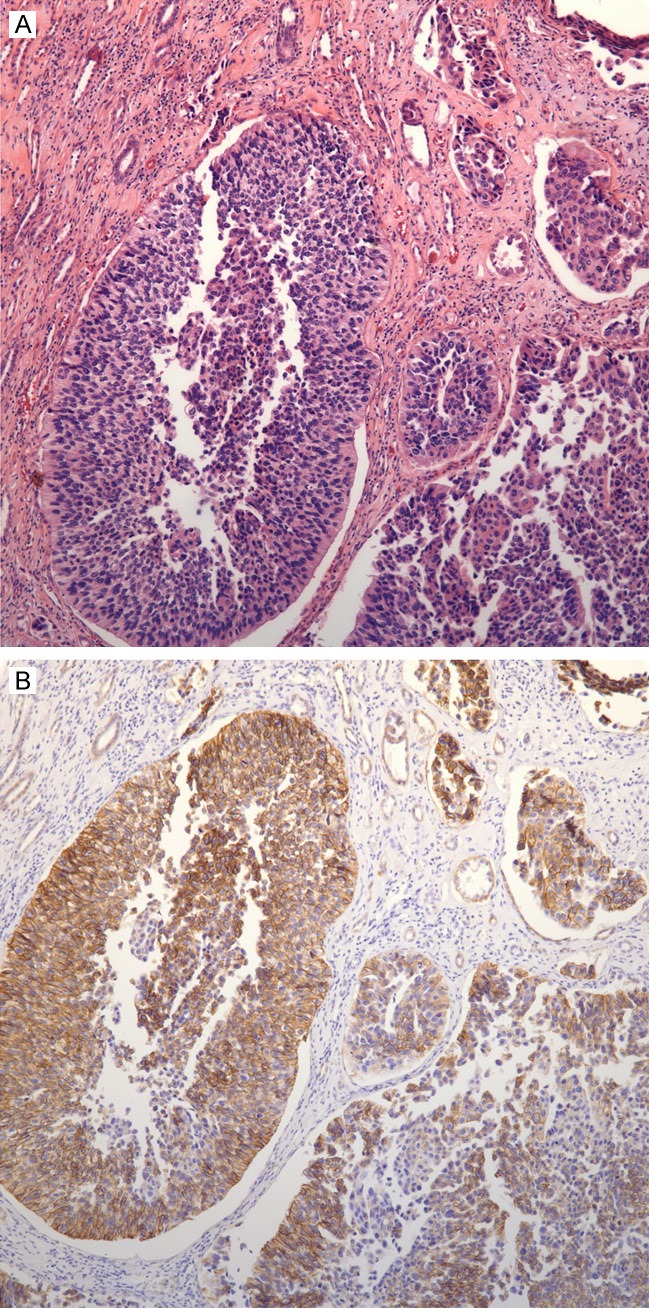
A: Invasive high grade papillary urothelial carcinoma with an inverted growth Pattern; B: HER2 overexpression (3+) in invasive high grade papillary urothelial carcinoma with an inverted growth pattern (corresponds to A).
Figure 2.
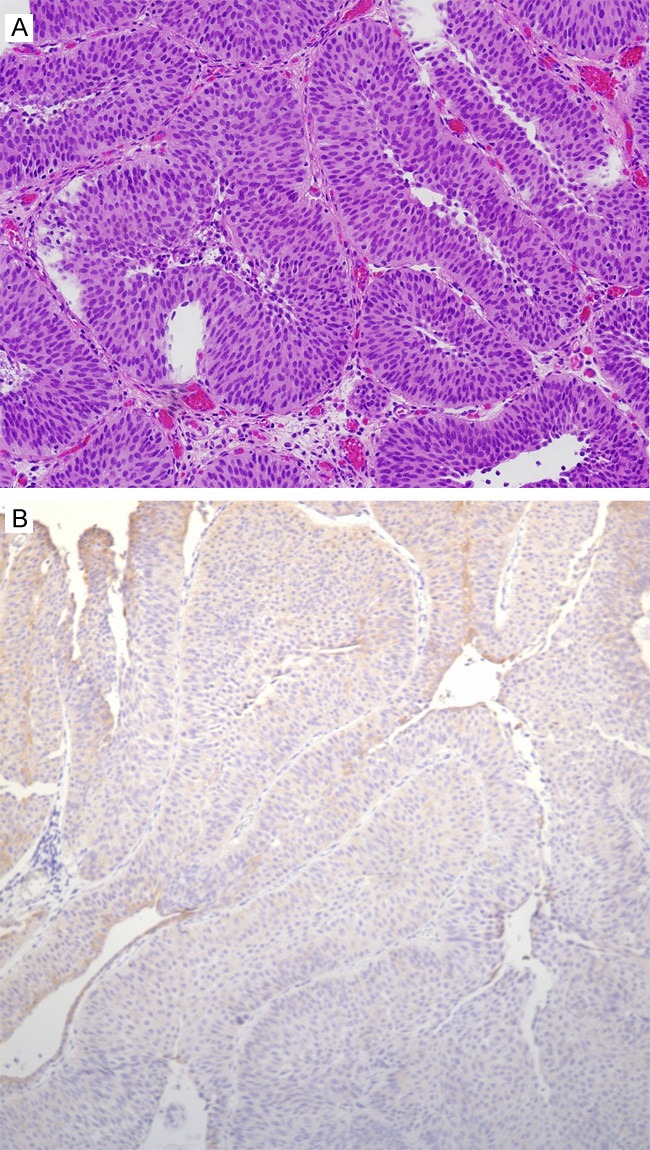
A: Non-invasive low grade papillary urothelial carcinoma with an inverted growth pattern; B: Negative HER2 expression in non-invasive low grade papillary urothelial carcinoma with an inverted growth pattern (corresponds to A).
Table 1.
Correlation of clinicopathologic parameters with overexpression of HER2 (3+ staining)
| Clinicopathologic data | Breakdown of cases with HER2 | Total number of cases with HER2 overexpression (%) |
|---|---|---|
| Male | 28 | 34a (82%) |
| Female | 6 | 34 (18%) |
| High grade UCA | 32 | 34 (94%) |
| Low grade UCA | 2 | 34 (6%) |
| pTa | 9 | 34 (26%) |
| pT1 | 10 | 34 (29%) |
| pT2 | 2 | 34 (6%) |
| pT3 | 12 | 34 (35%) |
| pT4 | 1 | 34 (3%) |
| Inverted growth pattern | 15 | 23b (65%) |
34/46 (74%) cases had HER2 overexpression (3+ staining);
23/46 (50%) cases had an inverted growth pattern;
UCA: urothelial carcinoma.
Discussion
Due to relatively low incidence of urothelial carcinoma of the renal pelvis compared to urothelial carcinoma of the bladder, more studies regarding HER2 amplification and HER2 overexpression have been published on the latter. However these studies have yielded conflicting results, with extensive variability in the incidence rates of HER2 gene amplification ranging from 0% to 59% and HER2 receptor protein overexpression ranging from 21% to 89% [28]. The variability in immunohistochemical assays, were likely related to the heterogeneity between kits, antibodies, protocols, interpretations or cut-off values. Additionally, variability in gene amplification has been related to differences in the evaluation criteria and laboratory methods, since various target molecules related to HER2 amplification/overexpression, including DNA, mRNA, and receptor protein, have been used in different assays. Among the diagnostic techniques used, immunohistochemistry and fluorescence in situ hybridization (FISH) are both useful and practical. Immunohistochemistry is a simple and rapid procedure. However, the intensity of the staining can affect by tissue fixation, tissue processing, and antigen retrieval. Furthermore, the sensitivity and specificity of immunohistochemical assays can vary considerably depending on the antibody used [29].
Four studies have investigated HER2 expression in upper urinary tract urothelial carcinoma [24-27]. Two of the four studies evaluated HER2 overexpression by immunohistochemistry. In the study by Bjerkehagen et al, they did not did not document HER2 expression in 20 cases of urothelial carcinoma of the renal pelvis, but in a study by Imai et al., HER2 expression was present in 11/20 (37%) cases of urothelial carcinoma of the renal pelvis and ureteral tumors [24,25]. In the study by Imai et al. it was suggested that the immunohistochemical detection of the expression of EGFR and c-erbB-2 in upper urinary tract urothelial carcinoma may be a useful in predicting the likelihood of secondary bladder cancer recurrences [25]. Langner et al. performed a systematic analysis of HER2 status in upper urinary tract urothelial carcinoma using both IHC and FISH with respect to the associations with tumor stage and grade, as well as prognostic significance [26]. They found HER2 expression in about 50% of tumors with weak expression (HercepTest score 2+) and also an amplification of HER2 in 9% of the cases (4/53) but with a low amplification ratio in all cases. In addition, they noted a correlation between HER2 expression with tumor stage and grade, comparable to results obtained in bladder urothelial carcinoma, but other investigators have failed to detect these associations [30-35]. Due to low rate of HER2 overexpression and HER2 gene amplification in their studies, they concluded that only a small number of patients with upper urinary tract urothelial carcinoma might benefit from HER2-targeted (Herceptin) therapy [26]. Vershasselt-Crinquette et al. investigated the frequency of HER2 overexpression and amplification in upper urinary tract urothelial carcinoma using dual-color in situ hybridization (ISH) and immunohistochemistry. In their study, all tumors with HER2 gene amplification were high grade and high stage with a HER2 overexpression 2+/3+ score [27]. They did not observe any prognostic value for HER2 overexpression or amplification, but found a correlation between HER2 amplification and lymph node invasion. They concluded that HER2 gene amplification is a rare event in upper urinary tract urothelial carcinoma and is correlated with high-grade tumors and lymph node invasion, thus selected patients with aggressive tumors might benefit from adjuvant anti-HER2 therapies [27]. Our study showed HER2 overexpression in 34/46 (74%) cases of urothelial carcinoma of the renal pelvis with high frequency in high grade urothelial carcinoma 32/34 (94%).
Urothelial carcinoma of the renal pelvis and ureter may develop as a manifestation of hereditary nonpolyposis colorectal cancer syndrome, a disorder characterized by mutation or inactivation of a number of DNA mismatch repair genes and detectable as microsatellite instability. Hartmann et al. showed that inverted growth in urothelial carcinoma of the upper urinary tract may serve as a marker lesion for microsatellite instability (with a sensitivity and specificity of .82) and may help identify patients who should be offered testing for hereditary nonpolyposis colorectal cancer syndrome [36].
Our study, the first to investigate the correlation between HER2 overexpression with inverted growth pattern, showed that HER2 overexpression is more common in male patients with high grade urothelial carcinoma, especially those with an inverted growth pattern. It is highly conceivable that a select group of patients may benefit from HER2-targeted (Herceptin) therapy. Additional multi-institutional studies are needed to confirm the potential diagnostic and prognostic utility of HER2 expression in upper urinary tract urothelial carcinoma.
Disclosure of conflict of interest
None.
References
- 1.Ouzzane A, Colin P, Xylinas E, Pignot G, Ariane MM, Saint F, Hoarau N, Adam E, Azemar MD, Bensadoun H, Cormier L, Cussenot O, Houlgatte A, Karsenty G, Bruyère F, Maurin C, Nouhaud FX, Phe V, Polguer T, Roumiguié M, Ruffion A, Rouprêt M French Collaborative National Database on UUT-UC. Ureteral and multifocal tumours have worse prognosis than renal pelvic tumours in urothelial carcinoma of the upper urinary tract treated by nephroureterectomy. Eur Urol. 2011;60:1258–65. doi: 10.1016/j.eururo.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 2.Hall MC, Womack S, Sagalowsky AI, Carmody T, Erickstad MD, Roehrborn CG. Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: a 30-year experience in 252 patients. Urology. 1998;52:594–601. doi: 10.1016/s0090-4295(98)00295-7. [DOI] [PubMed] [Google Scholar]
- 3.Olgac S, Mazumdar M, Dalbagni G, Reuter VE. Urothelial carcinoma of the renal pelvis: a clinicopathologic study of 130 cases. Am J Surg Pathol. 2004;28:1545–52. doi: 10.1097/00000478-200412000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Colin P, Koenig P, Ouzzane A, Berthon N, Villers A, Biserte J, Rouprêt M. Environmental factors involved in carcinogenesis of urothelial cell carcinomas of the upper urinary tract. BJU Int. 2009;104:1436–40. doi: 10.1111/j.1464-410X.2009.08838.x. [DOI] [PubMed] [Google Scholar]
- 5.Kirkali Z, Tuzel E. Transitional cell carcinoma of the ureter and renal pelvis. Crit Rev Oncol Hematol. 2003;47:155–69. doi: 10.1016/s1040-8428(03)00079-9. [DOI] [PubMed] [Google Scholar]
- 6.Catto JW, Yates DR, Rehman I, Azzouzi AR, Patterson J, Sibony M, Cussenot O, Hamdy FC. Behavior of urothelial carcinoma with respect to anatomical location. J Urol. 2007;177:1715–20. doi: 10.1016/j.juro.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 7.Bolenz C, Shariat SF, Fernandez MI, Margulis V, Lotan Y, Karakiewicz P, Remzi M, Kikuchi E, Zigeuner R, Weizer A, Montorsi F, Bensalah K, Wood CG, Roscigno M, Langner C, Koppie TM, Raman JD, Mikami S, Michel MS, Ströbel P. Risk stratification of patients with nodal involvement in upper tract urothelial carcinoma: value of lymph-node density. BJU Int. 2009;103:302–6. doi: 10.1111/j.1464-410X.2008.07988.x. [DOI] [PubMed] [Google Scholar]
- 8.Novara G, De Marco V, Gottardo F, Dalpiaz O, Bouygues V, Galfano A, Martignoni G, Patard JJ, Artibani W, Ficarra V. Independent predictors of cancer-specific survival in transitional cell carcinoma of the upper urinary tract: multi-institutional dataset from 3 European centers. Cancer. 2007;110:1715–22. doi: 10.1002/cncr.22970. [DOI] [PubMed] [Google Scholar]
- 9.Langner C, Hutterer G, Chromecki T, Winkelmayer I, Rehak P, Zigeuner R. pT classification, grade, and vascular invasion as prognostic indicators in urothelial carcinoma of the upper urinary tract. Mod Pathol. 2006;19:272–9. doi: 10.1038/modpathol.3800529. [DOI] [PubMed] [Google Scholar]
- 10.Roupret M, Zigeuner R, Palou J, Boehle A, Kaasinen E, Sylvester R, Babjuk M, Oosterlinck W. European guidelines for the diagnosis and management of upper urinary tract urothelial cell carcinomas: 2011 update. Eur Urol. 2011;59:584–94. doi: 10.1016/j.eururo.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 11.Margulis V, Shariat SF, Matin SF, Kamat AM, Zigeuner R, Kikuchi E, Lotan Y, Weizer A, Raman JD, Wood CG Upper Tract Urothelial Carcinoma Collaboration The Upper Tract Urothelial Carcinoma Collaboration. Outcomes of Radical Nephroureterectomy: A Series From the Upper Tract Urothelial Carcinoma Collaboration. Cancer. 2009;115:1224–33. doi: 10.1002/cncr.24135. [DOI] [PubMed] [Google Scholar]
- 12.Cozad SC, Smalley SR, Austenfeld M, Noble M, Jennings S, Raymond R. Transitional cell carcinoma of the renal pelvis or ureter: patterns of failure. Urology. 1995;46:796–800. doi: 10.1016/S0090-4295(99)80346-X. [DOI] [PubMed] [Google Scholar]
- 13.Vassilakopoulou M, de la Motte Rouge T, Colin P, Ouzzane A, Khayat D, Dimopoulos MA, Papadimitriou CA, Bamias A, Pignot G, Nouhaud FX, Hurel S, Guy L, Bigot P, Roumiguié M, Rouprêt M French Collaborative National Database on UUT-UCC. Outcomes after adjuvant chemotherapy in the treatment of high-risk urothelial carcinoma of the upper urinary tract (UUT-UC): results from a large multicenter collaborative study. Cancer. 2011;117:5500–8. doi: 10.1002/cncr.26172. [DOI] [PubMed] [Google Scholar]
- 14.Petit AM, Rak J, Hung MC, Rockwell P, Goldstein N, Fendly B, Kerbel RS. Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol. 1997;151:1523–1530. [PMC free article] [PubMed] [Google Scholar]
- 15.Klapper LN, Glathe S, Vaisman N, Hynes NE, Andrews GC, Sela M, Yarden Y. The ErbB-2/HER2 oncoprotein of human carcinomas may function solely as a shared coreceptor for multiple stroma-derived growth factors. Proc Natl Acad Sci U S A. 1999;96:4995–5000. doi: 10.1073/pnas.96.9.4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer. 2004;5:63–69. doi: 10.3816/cbc.2004.n.011. [DOI] [PubMed] [Google Scholar]
- 17.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 18.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF American Society of Clinical Oncology/College of American Pathologists. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J. Clin. Oncol. 2007;25:118–45. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 19.Goldhirsch A, Wood WC, Gelber RD, Coates AS, Thürlimann B, Senn HJ 10th St. Gallen conference. Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol. 2007;18:1133–1144. doi: 10.1093/annonc/mdm271. [DOI] [PubMed] [Google Scholar]
- 20.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 21.Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, Goldhirsch A, Untch M, Mariani G, Baselga J, Kaufmann M, Cameron D, Bell R, Bergh J, Coleman R, Wardley A, Harbeck N, Lopez RI, Mallmann P, Gelmon K, Wilcken N, Wist E, Sánchez Rovira P, Piccart-Gebhart MJ HERA study team. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 22.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 23.Laé M, Couturier J, Oudard S, Radvanyi F, Beuzeboc P, Vieillefond A. Assessing HER2 gene amplification as a potential target for therapy in invasive urothelial bladder cancer with a standardized methodology: results in 1005 patients. Ann Oncol. 2010;21:815–9. doi: 10.1093/annonc/mdp488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bjerkehagen B, Fossa SD, Raabe N, Holm R, Nesland JM. Transitional cell carcinoma of the renal pelvis and its expression of p53 protein, c-erbB-2 protein, neuron-specific enolase, Phe5, chromogranin, laminin and collagen type IV. Eur Urol. 1994;26:334–339. doi: 10.1159/000475410. [DOI] [PubMed] [Google Scholar]
- 25.Imai T, Kimura M, Takeda M, Tomita Y. Significance of epidermal growth factor receptor and c-erbB-2 protein expression in transitional cell carcinoma of the upper urinary tract for tumour recurrence at the urinary bladder. Br J Cancer. 1995;71:69–72. doi: 10.1038/bjc.1995.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langner C, Gross C, Rehak P, Ratschek M, Rüschoff J. HER2 protein overexpression and gene amplification in upper urinary tract transitional cell carcinoma: systematic analysis applying tissue microarray technique. Urology. 2005;65:176–80. doi: 10.1016/j.urology.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 27.Vershasselt-Crinquette M, Colin P, Ouzzane A, Gnemmi V, Robin YM, Aubert S, Villers A, Leroy X. Assessment of human epidermal growth factor receptor 2 status in urothelial carcinoma of the upper urinary tract: a study using dual color in situ hybridization and immunohistochemistry. Appl Immunohistochem Mol Morphol. 2012;20:363–6. doi: 10.1097/PAI.0b013e318241cab9. [DOI] [PubMed] [Google Scholar]
- 28.Latif Z, Watters AD, Dunn I, Grigor K, Underwood MA, Bartlett JM. HER2/neu gene amplification and protein overexpression in G3 pT2 transitional cell carcinoma of the bladder: a role for anti-HER2 therapy? Eur J Cancer. 2004;40:56–63. doi: 10.1016/j.ejca.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 29.Press MF, Hung G, Godolphin W, Slamon DJ. Sensitivity of HER-2/neu antibodies in archival tissue samples: potential source of error in immunohistochemical studies of oncogene expression. Cancer Res. 1994;54:2771–2777. [PubMed] [Google Scholar]
- 30.Krüger S, Weitsch G, Büttner H, Matthiensen A, Böhmer T, Marquardt T, Sayk F, Feller AC, Böhle A. Overexpression of c-erbB-2 oncoprotein in muscle-invasive bladder carcinoma: relationship with gene amplification, clinicopathological parameters and prognostic outcome. Int J Oncol. 2002;21:981–987. [PubMed] [Google Scholar]
- 31.Gorgoulis VG, Barbatis C, Poulias I, Karameris AM. Molecular and immunohistochemical evaluation of epidermal growth factor receptor and c-erb-B-2 gene product in transitional cell carcinomas of the urinary bladder: a study in Greek patients. Mod Pathol. 1995;8:758–764. [PubMed] [Google Scholar]
- 32.Moch H, Sauter G, Mihatsch MJ, Gudat F, Epper R, Waldman FM. p53 but not erbB-2 expression is associated with rapid tumor proliferation in urinary bladder cancer. Hum Pathol. 1994;25:1346–1351. doi: 10.1016/0046-8177(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 33.Underwood M, Bartlett J, Reeves J, Gardiner DS, Scott R, Cooke T. C-erbB-2 gene amplification: a molecular marker in recurrent bladder tumors? Cancer Res. 1995;55:2422–30. [PubMed] [Google Scholar]
- 34.Mellon JK, Lunec J, Wright C, Horne CH, Kelly P, Neal DE. C-erbB-2 in bladder cancer: molecular biology, correlation with epidermal growth factor receptors and prognostic value. J Urol. 1996;155:321–326. doi: 10.1016/s0022-5347(01)66653-9. [DOI] [PubMed] [Google Scholar]
- 35.Chow NH, Chan SH, Tzai TS, Ho CL, Liu HS. Expression profiles of ErbB family receptors and prognosis in primary transitional cell carcinoma of the urinary bladder. Clin Cancer Res. 2001;7:1957–1962. [PubMed] [Google Scholar]
- 36.Hartmann A, Dietmaier W, Hofstädter F, Dietmaier W, Zaak D, Hofstaedter F, Knuechel R. Urothelial carcinoma of the upper urinary tract: inverted growth pattern is predictive ofmicrosatellite instability. Hum Pathol. 2003;34:222–7. doi: 10.1053/hupa.2003.22. [DOI] [PubMed] [Google Scholar]


