Abstract
Objective: To examine amount of CD4+CXCR5+Tfh cells and B cells subsets in salivary gland and peripheral blood from patients with primary Sjogren’s syndrome (pSS) and to analyze whether the frequency of CD4+CXCR5+Tfh cells is associated with pSS pathologic process. Methods: The percentages of CD4+CXCR5+Tfh cells and B cell subsets were examined by flow cytometry. B-lymphocyte chemoattraetant (BLC; also called CXCL13), IL-21, IL-6 from the serum of pSS patients was assessed by polymerase chain reaction–enzyme-linked immunosorbent assay (ELISA). Results: The percentages of CD4+CXCR5+Tfh cells in peripheral blood were increased in pSS patients, but decreased after treatment with glucocorticoid and/or immunosuppressive drugs. Abnormal B cell subsets appeared in salivary and peripheral blood of pSS patients. The frequency of salivary CD4+CXCR5+Tfh cells was positively correlated with CD19+CD27+ memory B cells and CD19+CD27high plasma cells. Also increase of salivary CD19+CD27high plasma cells was positively associated with serum ANA titer of pSS patients. Conclusions: CD4+CXCR5+Tfh cells are significantly increased in salivary and peripheral blood in pSS patients with aberrant CD19+CD27+ memory B cells and CD19+CD27high plasma cells, suggesting that CD4+CXCR5+Tfh cells may contribute to the pathogenesis of pSS by promoting the maturation of B cells.
Keywords: Sjogren’s syndrome, follicular helper T cell, B cell, germinal center
Introduction
Follicular helper T (Tfh) cells have been recently identified as a separate CD4+T helper lineage, which are the specialized providers of help to B cells. It is one of the most studied subsets of CD4+T cells in the last decade, which is primarily described in 2000, when numbers of groups reported a large proportion of CD4+T cells in tonsils expressed chemokine receptor CXCR5 highly [1-3]. CXCR5 direct Tfh cell migration into B cell follicles in response to the specific ligand CXCL13 (B lymphocyte chemoattractant, BLC, B-cell–attracting chemokine 1, BCA-1) expressed by follicular dendritic cells (DCs) within the B-cell follicle [4,5]. This colocalization of CD4+T cell with B cells is pivotal to T-B interactions. Tfh cells also express inducible co-stimulator (ICOS), programmed death-1 (PD-1), and interleukin-21 (IL-21), which provide excellent markers for identification of Tfh cells and also serve important functions in their interactions with B cells [6,7]. Interestingly, dysregulated function of Tfh cell has been reported in patients with autoimmune diseases, such as systemic lupus erythematosus (SLE), autoimmune thyroid disease (AITD) and systemic sclerosis, and some chronic disease such as chronic hepatitis B (CHB) [8-10]. However, little is known in patients with primary Sjogren’s syndrome (pSS).
pSS is a chronic inflammatory autoimmune disease that affects the exocrine glands, mainly the salivary and lacrimal glands, but also frequently involve other exocrine glands, such as respiratory tract, gastrointestinal tract and skin, which are frequently accompanied by systemic symptoms [11-14]. The characteristic pSS histology is that T and B lymphocytes, dendritic cells (DCs) and macrophages infiltrate exocrine glands which impair the salivary and lacrimal glandular function and result clinically in xerostomia and keratoconjunctivitis sicca as well as high-titer serum autoantibodies and hypergammaglobulinemia. Nicholas Simpson et al have demonstrated increased circulating Tfh cells which were defined as ICOShigh CXCR5+CD4+ or PD-1high CD4+ in SS patients [9]. Ectopic germinalcenterlike structure found in one fifth of patients with SS represents the histologic hallmark of this abnormal B cell proliferation [14].
In this study, we examined the frequency of Tfh cells and B cell subsets in peripheral blood and/or salivary gland from pSS patients, and explored possible correlation between abnormality of Tfh cells and pathogenesis of pSS.
Materials and methods
Study subjects
A total of 24 patients diagnosed as pSS were referred to the Department of Rheumatology and Immunology in Anhui Provincial Hospital from February 2011 to December 2011 and fulfilled the 2002 revised criteria established by the American-European Consensus Group [15] (Table 1) . Disease activity was evaluated using the EULAR Sjogren’s Syndrome Disease Activity Index (ESSDAI). None of the patients enrolled were treated with glucocorticoid and/or immunosuppressive drugs. All patients of peripheral blood were collected. 20 healthy controls enrolled from Health Screening Center in Anhui Provincial Hospital. Salivary gland biopsies were obtained from 24 patients and controls (4 patients with xerostomia and/or eye drying who did not fulfill the 2002 revised criteria). The clinical laboratory data such as erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), immunoglobulin and clinical characteristics are determined. The research protocol was reviewed and approved by the Hospital Ethics Committee. Informed consent was obtained from all patients and controls.
Table 1.
Patient Profiles
| No. patient | Sex | Age (years) | Clinical and Biological Features | ESSDAI | Treatment | Steroid Dose (mg) |
|---|---|---|---|---|---|---|
| 1 | F | 53 | keratoconjunctivitis sicca, xerostomia, leucopenia, anemia hypergammaglobulinemia | 6 | HCQ, | 10 |
| 2 | F | 61 | keratoconjunctivitis sicca, xerostomia, leucopenia, arthralgia, decayed tooth | 6 | MTX | 10 |
| 3 | F | 50 | keratoconjunctivitis sicca, xerostomia, RTA, hypergammaglobulinemia | 5 | HCQ | 15 |
| 4 | F | 45 | keratoconjunctivitis sicca, xerostomia, arthralgia, decayed tooth, hypergammaglobulinemia | 5 | HCQ | 10 |
| 5 | F | 58 | keratoconjunctivitis sicca, xerostomia, decayed tooth, hypergammaglobulinemia | 5 | / | 15 |
| 6 | M | 31 | xerostomia, leucopenia, anemia, hypergammaglobulinemia | 5 | / | 10 |
| 7 | F | 41 | keratoconjunctivitis sicca, xerostomia, RTA, hypergammaglobulinemia | 7 | HCQ | 15 |
| 8 | F | 59 | keratoconjunctivitis sicca, xerostomia, hypergammaglobulinemia | 4 | TGP | / |
| 9 | M | 75 | keratoconjunctivitis sicca, xerostomia, hypergammaglobulinemia | 5 | TGP | / |
| 10 | F | 25 | xerostomia, leucopenia, anemia hypergammaglobulinemia | 6 | HCQ | 10 |
| 11 | F | 27 | keratoconjunctivitis sicca, leucopenia, RTA, hypergammaglobulinemia | 8 | HCQ | 15 |
| 12 | F | 53 | keratoconjunctivitis sicca, xerostomia, hypergammaglobulinemia | 5 | / | 10 |
| 13 | F | 40 | xerostomia, hypergammaglobulinemia | 5 | / | 10 |
| 14 | F | 30 | keratoconjunctivitis sicca, xerostomia, hypergammaglobulinemia | 4 | TGP | / |
| 15 | F | 37 | keratoconjunctivitis sicca, xerostomia, hypergammaglobulinemia | 4 | TGP | / |
| 16 | F | 41 | keratoconjunctivitis sicca, xerostomia, leucopenia, hypergammaglobulinemia | 5 | HCQ | 10 |
| 17 | F | 47 | keratoconjunctivitis sicca, xerostomia, leucopenia, anemia hypergammaglobulinemia | 5 | HCQ | 10 |
| 18 | F | 23 | keratoconjunctivitis sicca, xerostomia, leucopenia, anemia hypergammaglobulinemia | 5 | HCQ | 10 |
| 19 | F | 28 | keratoconjunctivitis sicca, leucopenia, anemia hypergammaglobulinemia | 6 | HCQ | 10 |
| 20 | F | 71 | arthralgia, decayed tooth xerostomia, leucopenia, anemia hypergammaglobulinemia | 6 | HCQ | 10 |
| 21 | F | 78 | keratoconjunctivitis sicca, xerostomia, leucopenia, hypergammaglobulinemia, decayed tooth | 6 | / | 10 |
| 22 | F | 60 | keratoconjunctivitis sicca, xerostomia, decayed tooth hypergammaglobulinemia | 4 | TGP | / |
| 23 | F | 60 | keratoconjunctivitis sicca, xerostomia, leucopenia, anemia hypergammaglobulinemia | 7 | HCQ | 15 |
| 24 | F | 39 | leucopenia, anemia, RTA hypergammaglobulinemia | 8 | HCQ | 15 |
F, Female; M, Male; ESSDAI, EULAR Sjogren’s Syndrome Disease Activity Index; HCQ, hydroxychloroquine; MTX, Methotrexate and TGP, total glucosides of paeony.
Cell isolation
Peripheral blood mononuclear cells (PBMCs) from the patients and healthy controls were purified by density-gradient centrifugation on Ficoll-Hypaque centrifugation (1.077 density).
The fresh salivary gland samples were obtained from patients with pSS and controls. Single cells were collected by mechanical disruption, then digested with 0.5% collagenase II (Sigma) at 37°C for 1.5 hours, and mashed gently using a plunger through a 70 um strainer to make single-cell suspensions. A fraction of single-cell suspensions was filtered through a 40 um strainer to make mononuclear cells. After washing, cells were centrifuged at 1800 rpm for 10 min and re-suspended. All cell collections were stained for flow cytometry.
Flow cytometry
Human PBMCs and salivary single cell at 106/tube were stained in duplicate with PerCP-anti-CXCR5 (Biolegend, San Diego, USA), FITC-anti-CD4, and APC-anti-CD27, Pecy5.5-anti CD19, or isotype-matched control IgG (Beckton Dickinson, San Jose, USA) at 4°C for 30 minutes, respectively. After being washed with PBS, the cells were acquired by FACSCalibur (Beckon Dickinson) and data were analyzed using FlowJo software (v5.7.2).
Enzyme-linked immunosorbent assay (ELISA)
Level of plasma BLC-1, IL-6 and IL-21 was done using ELISA for human BLC-1, IL-6, and IL-21 (Cusabio). All samples were analyzed in duplicate using the average of the optical density (OD) values to calculate concentrations.
Laboratory assessment
Serum antinuclear antibodies (ANA) were analyzed by indirect immunofluorescence assay on HEp-2 cell slides. Antibodies against Ro/SSA and La/SSB were detected in serum by immunoblotting. IgG, IgA, and IgM serum levels were measured by immunoturbidimetry assay.
Statistical analysis
Data were analyzed with SPSS version 13.0 software. The data were presented as the mean values ± standard deviations. The significance of the difference between groups was analyzed using independent T testing with Bonferroni correction. For correlation analyses, either Spearman’s r or Pearson’s r was calculated. A two-sided P < 0.05 was considered statistically significant.
Results
Expression of CD4+CXCR5+Tfh cells in the peripheral blood and salivary glands of pSS patients
In our study, we enrolled 24 pSS patients without treatment and 20 healthy controls to determine the percentage of CD4+CXCR5+Tfh cell (Figure 1A) in CD4+T cells in peripheral blood. The frequency of CD4+CXCR5+T cell in pSS patients without treatment was significantly higher than that in healthy controls (17.90 ± 4.40% versus 14.45 ± 3.54%, P = 0.019, Figure 1B). At the same time, we detected the percentage of CD4+CXCR5+Tfh cells in CD4+T cells in the salivary glands, which was 11.47 ± 6.23%. However, CD4+CXCR5+Tfh cells were rarely detected in salivary glands of controls.
Figure 1.
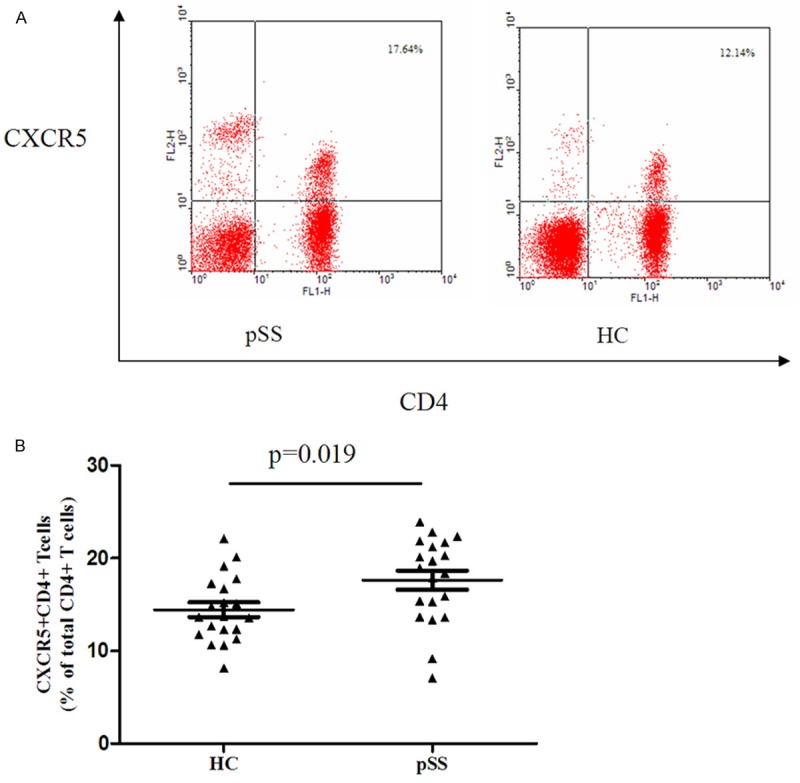
CD4+CXCR5+Tfh cells are increased in pSS patients compared with healthy control (HC) in peripheral blood. A. Representative cytofluorometric analysis of PBMCs from pSS patients (left panels) and healthy controls (right panels). B. Statistical dot plot of the percentage of CD4+CXCR5+Tfh cells.
Abnormal peripheral blood B cell subsets in pSS patients
We next examined the frequency of B cell subsets in peripheral blood from pSS patients and healthy controls. A significant reduction in the number of peripheral CD27+ memory B cells and a increase in frequency of peripheral CD27- naive B cells were found in pSS patients [16,17], CD27+ memory B cells were decreased in pSS patients without treatment compared with healthy controls (26.49 ± 9.26% versus 34.90 ± 8.94%, P = 0.018, Figure 2A). The percentage of CD27- naïve B cells were higher than that in healthy controls (72.50 ± 12.80% versus 63.51 ± 8.95%, P = 0.013, Figure 2B). On the other side, we also found the decreased number of peripheral CD27high plasma cells in pSS patients without treatment compared with healthy controls (1.12 ± 0.60% versus 1.58 ± 0.59%, P = 0.017, Figure 2C).
Figure 2.
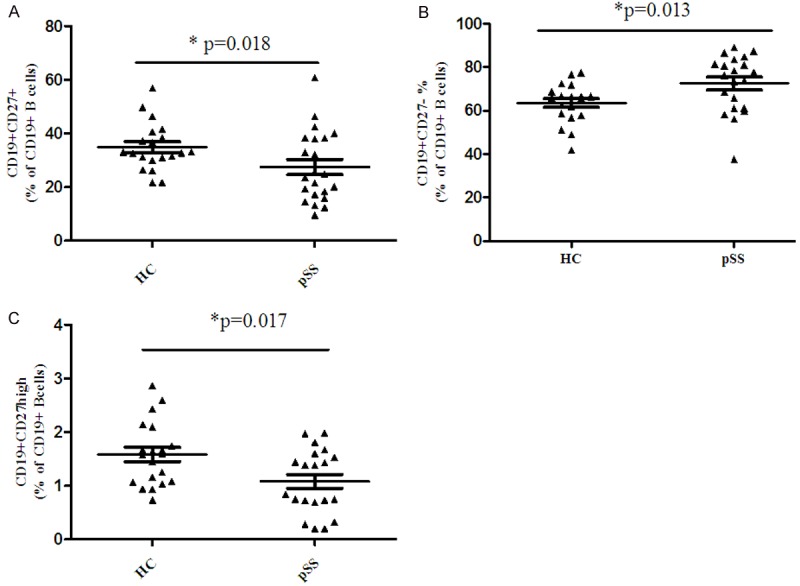
Abnormal B cell subsets in pSS patients. (A) An increased percentage of CD19+CD27- naïve B cells (A) and CD19+CD27+ memoryB cells (B) and decreased percentage of CD19+CD27high plasma cells (C) in the total CD19+ B cells in the peripheral blood were found in pSS patients.
Correlation between CD4+CXCR5+Tfh cells and B cell subsets in pSS patients
The frequency of CD4+CXCR5+Tfh cells and B cell subsets were also examined both in the peripheral blood and salivary gland in pSS patients without treatment. However, no CD4+CXCR5+Tfh cells, CD27+ memory B cells and CD27high plasma cells were found in salivary glands of controls. We found that there was no correlation between CD4+CXCR5+Tfh cells with B cell subsets in peripheral blood. However, the frequency of CD4+CXCR5+T cells in salivary glands was positively correlated with the percentage of CD27+ memory B cells (r = 0.619, P = 0.011, Figure 3A) and CD27high plasma cells in peripheral blood (r = 0.733, P = 0.001, Figure 3B).
Figure 3.
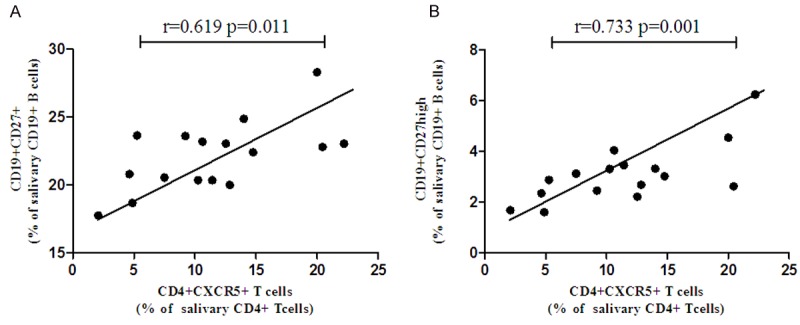
CD4+CXCR5+Tfh cells in salivary gland are positively correlated with memory B cells and plasma cells in untreated pSS patients.
Change of the expression of CD4+CXCR5+Tfh cells and B cells subsets in pSS patients by treatment
The percentage of CD4+CXCR5+Tfh cells in peripheral blood was obviously reduced in pSS patients after treatment with glucocorticoid and/or immunosuppressive drugs (15.09 ± 2.88% versus 17.90 ± 4.40%, P = 0.037, Figure 4A), along with the increased frequency of CD27high plasma (2.04 ± 0.92% versus 1.12 ± 0.60%, P = 0.001, Figure 4B). Whereas, there was no apparent influence to the frequency of CD27- naive B cells or CD27+ memory B cells by treatment.
Figure 4.

The influence of treatment to Tfh cells and B cell subsets in peripheral blood. A. Decreased percentage of CD4+CXCR5+Tfh cells in the peripheral blood after treatment for 4 weeks. B. Increased percentage of CD19+CD27high plasma cells in the peripheral blood after treatment for 4 weeks.
Relevance of CD4+CXCR5+Tfh cells and B cells subsets with laboratory parameter
We also explored the relevance of the frequency of CD4+CXCR5+Tfh cells and B cells subsets in the peripheral blood and salivary glands with clinical parameters. The frequency of CD19+CD27high plasma cells in salivary gland are significantly correlated with serum ANA titers (Figure 5). However, no correlation has been found among CD4+CXCR5+Tfh cells, B cells subsets in the peripheral blood and clinical parameters including ESR, Ro/SSA and La/SSB, ANA titers.
Figure 5.
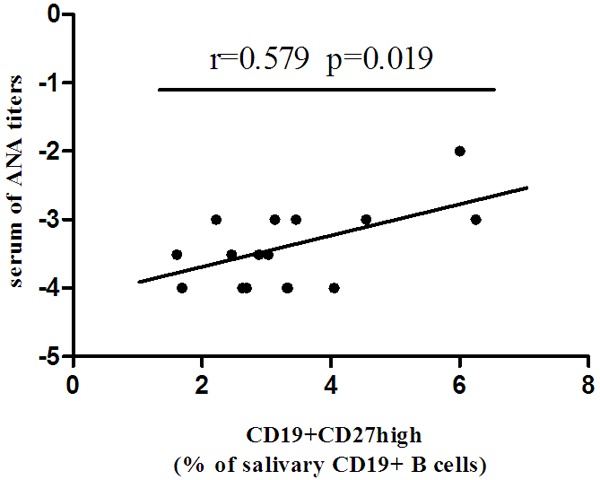
CD19+CD27high plasma cells in salivary gland are positively correlated with serum ANA titers in untreated pSS patients.
Serum levels of related cytokines in pSS patients
Finally the serum cytokines in pSS patients without treatment were measured by ELISA. Serum IL-21 levels in pSS without treatment and healthy controls have shown a significant increase in pSS compared to healthy controls (2.86 ± 0.47, and 1.68 ± 0.42, P < 0.05 (Figure 6). However, the levels of serum BLC1, IL-6 in pSS patients have no significant change compared with healthy controls.
Figure 6.
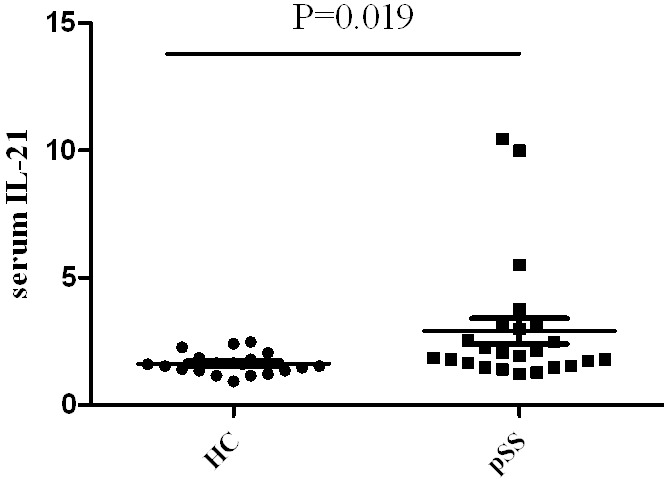
Serum IL-21 levels are increased in pSS patients compared with healthy control (HC).
Dsicussion
Tfh cells are a key regulator in humoral immunity. The crucial role of these Tfh cells is helping B cell maturation and the production of antibodies in response to foreign antigens [18]. Previous studies have shown that characterized the frequency of peripheral Tfh cells in several autoimmune diseases [10,19]. However, cellular and humoral mechanisms underlying pathogenesis of Sjögren’s syndrome are still not fully understood. In this study, we demonstrated that salivary gland CD4+CXCR5+Tfh cells and abnormal B cells subsets significantly increased in pSS patients. The findings also clearly indicated that the percentage of CD4+CXCR5+Tfh cells in the salivary glands from pSS patients were correlated positively with CD19+CD27+ memory B cells and CD19+CD27high plasma cells, yet the frequency of CD19+CD27high plasma cells in salivary gland are significantly correlated with serum ANA titers.
Although the clinical symptoms of pSS are varied among patients and the pathogenetic mechanisms of this autoimmune disease have not been exactly elucidated. The characteristic histological feature of pSS is peri-ductal focal lymphocytic aggregates such as CD4+T cells, B cells, plasma cells et al. Follicular dendritic cells (DCs) and proliferating B cells consist of germinal centerlike structures replace the secretory units [16,20]. However, subsets of T cells participate in pathogenesis of pSS is still unknown.
Tfh cells are newly discovered CD4+ effector helper T cell subsets, which have unique functions. According to the transcription factor and production of different cytokines, CD4+ effector helper T cells can be divided into several subsets. Tfh cells expressed transcription factor Bcl-6, and produced IL-21, for the sake of help B cells produce antibodies in humoral immune response, which are mostly located in the follicular area of lymphoid organs [21,22]. Tfh cells were guided by CXCR5, a chemokine receptor, and localized to the germinal center. Tfh cells are the helper T cells for helping B cells to produce antibodies [23]. The basic function of Tfh cells involve B cell selection and differentiate into memory cells and plasma cells in the process of antibody affinity maturation [24]. These studies demonstrated that ectopic germinal center of non lymphoid tissues in many autoimmune diseases may be closely related to the pathogenesis [25]. The importance of function of Tfh cells in autoimmune diseases have been gradually recognized in recent years [26]. abnormal Tfh related molecules expression were found in several murine models of autoimmune diseases [27]. In 2010, Nicholas Simpson et al. found that elevated CD4+CXCR5+T cells accounted for the proportion of CD4+T cells in the peripheral blood of SLE patients [9]. However, studies related to the role of Tfh cells are limited. We, for first time, reported salivary Tfh cells are increase abnormally and correlated to clinical parameter and treatment.
Results from our study showed that the frequency of CD4+CXCR5+T cells in CD4+T cells in peripheral blood from untreated pSS patients were higher than that of healthy controls and decreased after therapy. This result is lined partly with the report by Zhu et al [28]. However, Zhu et al showed that the percentage of CD4+CXCR5+T cells was correlated to the titer of ANA in pSS patients, suggesting that CD4+CXCR5+T cells in peripheral blood may be participated in the occurrence of disease. Our results, displayed no correlation between the percentage of peripheral blood of CD4+CXCR5+Tfh cells and the titer of ANA and Ig. However, in the salivary glands, the percentage of CD4+CXCR5+Tfh cells was significantly correlated with CD19+CD27+ memory B cells and CD19+CD27high plasma cells. Schaerli et al [2] have found that the majority of CD4+CXCR5+T cells in peripheral blood are CD45RO+ memory T cells, with no expression of cell activation and costimulatory molecules (CD40, OX40, ICOS), in which way they do not participate in the instant immune response. Experiments in vitro showed unstimulated peripheral blood CXCR5+T cells cannot help co-cultured B cells produce antibodies. Therefore, they indicated CD4+CXCR5+ cells in the peripheral blood are in quiescent condition [29]. Due to CD4+CXCR5+T cells are not the true type Tfh cells same as that in salivary, it may explain why salivary CD4+CXCR5+ cells, but not peripheral CD4+CXCR5+ cells, have positive correlation with levels of ANA.
B cell hyper-activation is a predominant feature of pSS related to hyper-gammaglobulinemia and to the production of autoantibodies. The frequency of B cell subsets are varied among patients with pSS. A significant reduction in the peripheral CD27+ memory B cells and increased frequency of peripheral CD27- naive B cells were found in pSS patients that is similar to results from other reports. We found that the frequency of CD19+CD27high plasma cells in salivary gland are significantly correlated with serum ANA titers. Increase of salivary Tfh cells and B cell subsets contributed to the formation of the germinal centers, which is critical to pathogens of pSS.
In conclusion, we found a significant increase in frequency of peripheral blood CD4+CXCR5+Tfh cells and a decrease of B cell subsets in peripheral blood of pSS patients, which do not correlated to serum ANA titers. However, abnormal increase of CD4+CXCR5+Tfh cells and B cell subsets in salivary gland were significantly correlate to serum ANA titers, suggesting that salivary CD4+CXCR5+Tfh cells are the effector cells and may participate in pathogenesis of pSS.
Acknowledgements
This work was funded by a grant from the National Nature Science Foundation of China (No. 81373187), the National Nature Science Foundation of Anhui Province (No. 1308085MH162) and the Clinical Research Special Foundation of Chinese Medical Association (12040730373).
Disclosure of conflict of interest
None.
References
- 1.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Förster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim CH, Rott LS, Clark-Lewis I, Campbell DJ, Wu L, Butcher EC. Subspecialization of Cxcr5+ T Cells B Helper Activity Is Focused in a Germinal Center-Localized Subset of Cxcr5+ T Cells. J Exp Med. 2001;193:1373–1382. doi: 10.1084/jem.193.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JG, Williams LT. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt’s lymphoma receptor-1. Nature. 1998;391:799–803. doi: 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- 5.Hardtke S, Ohl L, Förster R. Balanced expression of CXCR5 and CCR7 on follicular T helper cells determines their transient positioning to lymph node follicles and is essential for efficient B-cell help. Blood. 2005;106:1924–1931. doi: 10.1182/blood-2004-11-4494. [DOI] [PubMed] [Google Scholar]
- 6.Linterman MA, Vinuesa CG. Signals that influence T follicular helper cell differentiation and function. Sem Immunopathol. 2010;32:183–196. doi: 10.1007/s00281-009-0194-z. [DOI] [PubMed] [Google Scholar]
- 7.Laurent C, Fazilleau N, Brousset P. A novel subset of T-helper cells: follicular T-helper cells and their markers. Haematologica. 2010;95:356–358. doi: 10.3324/haematol.2009.019133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng J, Lu L, Hua C, Qin L, Zhao P, Wang J, Wang Y, Li W, Shi X, Jiang Y. High frequency of CD4+ CXCR5+ TFH cells in patients with immune-active chronic hepatitis B. PLoS One. 2011;6:e21698. doi: 10.1371/journal.pone.0021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simpson N, Gatenby PA, Wilson A, Malik S, Fulcher DA, Tangye SG, Manku H, Vyse TJ, Roncador G, Huttley GA. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010;62:234–244. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- 10.Zhu C, Ma J, Liu Y, Tong J, Tian J, Chen J, Tang X, Xu H, Lu L, Wang S. Increased frequency of follicular helper T cells in patients with autoimmune thyroid disease. J Clin Endocrinol Metab. 2011;97:943–950. doi: 10.1210/jc.2011-2003. [DOI] [PubMed] [Google Scholar]
- 11.Mitsias D, Kapsogeorgou E, Moutsopoulos H. The role of epithelial cells in the initiation and perpetuation of autoimmune lesions: lessons from Sjögren’s syndrome (autoimmune epithelitis) Lupus. 2006;15:255–261. doi: 10.1191/0961203306lu2290rr. [DOI] [PubMed] [Google Scholar]
- 12.Kassan SS, Moutsopoulos HM. Clinical manifestations and early diagnosis of Sjögren syndrome. Arch Intern Med. 2004;164:1275–1284. doi: 10.1001/archinte.164.12.1275. [DOI] [PubMed] [Google Scholar]
- 13.Hansen A, Lipsky PE, Dörner T. Immunopathogenesis of primary Sjögren’s syndrome: implications for disease management and therapy. Curr Opin Rheumatol. 2005;17:558–565. doi: 10.1097/01.bor.0000172801.56744.c3. [DOI] [PubMed] [Google Scholar]
- 14.Fox RI. Sjögren’s syndrome. Lancet. 2005;366:321–331. doi: 10.1016/S0140-6736(05)66990-5. [DOI] [PubMed] [Google Scholar]
- 15.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos H, Alexander E, Carsons S, Daniels T, Fox P, Fox R, Kassan S. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katsifis GE, Moutsopoulos NM, Wahl SM. T lymphocytes in Sjögren’s syndrome: contributors to and regulators of pathophysiology. Clin Rev Allergy Immunol. 2007;32:252–264. doi: 10.1007/s12016-007-8011-8. [DOI] [PubMed] [Google Scholar]
- 17.Hansen A, Odendahl M, Reiter K, Jacobi AM, Feist E, Scholze J, Burmester GR, Lipsky PE, Dörner T. Diminished peripheral blood memory B cells and accumulation of memory B cells in the salivary glands of patients with Sjögren‘s syndrome. Arthritis Rheum. 2002;46:2160–2171. doi: 10.1002/art.10445. [DOI] [PubMed] [Google Scholar]
- 18.Moon JJ, Suh H, Li AV, Ockenhouse CF, Yadava A, Irvine DJ. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc Natl Acad Sci U S A. 2012;109:1080–1085. doi: 10.1073/pnas.1112648109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma J, Zhu C, Ma B, Tian J, Baidoo SE, Mao C, Wu W, Chen J, Tong J, Yang M. Increased frequency of circulating follicular helper T cells in patients with rheumatoid arthritis. Clin Dev Immunol. 2012;2012:827480. doi: 10.1155/2012/827480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manoussakis M, Boiu S, Korkolopoulou P, Kapsogeorgou E, Kavantzas N, Ziakas P, Patsouris E, Moutsopoulos H. Rates of infiltration by macrophages and dendritic cells and expression of interleukin-18 and interleukin-12 in the chronic inflammatory lesions of Sjögren’s syndrome: Correlation with certain features of immune hyperactivity and factors associated with high risk of lymphoma development. Arthritis Rheum. 2007;56:3977–3988. doi: 10.1002/art.23073. [DOI] [PubMed] [Google Scholar]
- 21.MacLennan IC. Germinal centers. Ann Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 22.Fazilleau N, McHeyzer-Williams LJ, Rosen H, McHeyzer-Williams MG. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nature Immunol. 2009;10:375–384. doi: 10.1038/ni.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang YH, Dong C. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 25.King C. New insights into the differentiation and function of T follicular helper cells. Nature Rev Immunol. 2009;9:757–766. doi: 10.1038/nri2644. [DOI] [PubMed] [Google Scholar]
- 26.Veillette A. Immune regulation by SLAM family receptors and SAP-related adaptors. Nature Rev Immunol. 2006;6:56–66. doi: 10.1038/nri1761. [DOI] [PubMed] [Google Scholar]
- 27.Eddahri F, Denanglaire S, Bureau F, Spolski R, Leonard WJ, Leo O, Andris F. Interleukin-6/STAT3 signaling regulates the ability of naive T cells to acquire B-cell help capacities. Blood. 2009;113:2426–2433. doi: 10.1182/blood-2008-04-154682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li XY, Wu ZB, Ding J, Zheng ZH, Li XY, Chen LN, Zhu P. Role of the frequency of blood CD4+ CXCR5+ CCR6+ T cells in autoimmunity in patients with Sjögren’s syndrome. Biochem Biophys Res Comm. 2012;422:238–244. doi: 10.1016/j.bbrc.2012.04.133. [DOI] [PubMed] [Google Scholar]
- 29.Jang E, Cho SH, Park H, Paik DJ, Kim JM, Youn J. A positive feedback loop of IL-21 signaling provoked by homeostatic CD4+ CD25− T cell expansion is essential for the development of arthritis in autoimmune K/BxN mice. J Immunol. 2009;182:4649–4656. doi: 10.4049/jimmunol.0804350. [DOI] [PubMed] [Google Scholar]


