Abstract
Aim: Expression of the oncofetal protein insulin like growth factor II messenger ribonucleic acid binding protein 3 (IMP3) has been shown to differentiate between benign and malignant lesions in several tissues. Our aim was to assess the immunohistochemical expression of IMP3 in inflammatory and neoplastic lesions of the gastric mucosa and to determine whether IMP3, alone or in combination with p53, could be used for identifying neoplasia of the gastric mucosa. Methods: IMP3 and p53 immunohistochemistry was performed on 57 cases of gastritis, 28 cases of dysplasia of the gastric mucosa and 63 cases of gastric carcinomas. Focal IMP3 positivity was detected in 86% of non-neoplastic lesions of the gastric mucosa. Using a simple product score (PS), 96% of non-neoplastic lesions of the gastric mucosa were assessed as IMP3(PS) negative. None of the low-grade dysplasia but 83% of high-grade dysplasia were IMP3(PS) positive. Gastric carcinomas showed IMP3(PS) positivity in 65%. Adding p53 to the diagnostic panel increased sensitivity significantly. Conclusion: High-grade dysplasia and gastric carcinomas can be distinguished from low-grade dysplasia and inflammatory lesions of the gastric mucosa with a high specificity and good sensitivity using a combination of the immunohistochemical markers IMP3 and p53.
Keywords: IMP3, p53, immunohistochemistry, gastric mucosa, inflammatory, neoplastic
Introduction
Gastric cancer accounts for 7.8% of cancers worldwide. Although both the incidence and the mortality of gastric cancer have decreased in the last 15 years, it is still the fourth most common cancer and ranges in second place of the most common cancer related deaths [1,2]. The most important causative factor of gastric cancer is helicobacter pylori infection [3]. The stage of gastric cancer is still the most reliable prognostic indicator. It is therefore essential to make the diagnosis of gastric cancer at an early stage. Consequently, the accurate and safe diagnosis of gastric cancer and premalignant lesions of the gastric mucosa in biopsy material plays a vital role. However, problems often arise in routine pathologic workup of gastric biopsies, as it is often difficult to differentiate between neoplastic and reactive/regenerative changes of the gastric mucosa. The identification of sensitive and specific immunohistochemical markers for the detection of invasive and intraepithelial neoplasia of the gastric mucosa is therefore very desirable.
One potential candidate in this context is the human insulin-like growth factor (IGF) II mRNA binding protein (IMP3). IMP3, originally named “KH domain containing protein overexpressed in cancer (KOC)”, plays an important role in early human embryogenesis, but is commonly expressed only at low levels in adult tissues [4,5]. As of now, little is known about the function of IMP3. However, research has shown that IMP3 immunohistochemistry can be employed to differentiate between benign and malignant lesions in tissues as diverse as the skin [6,7], the uterine cervix [8,9], the pancreas [10,11] and the mesothelium [12,13].
So far, IMP3 has only been examined in two series of gastric carcinomas. In their studies, Okada et al and Wang et al demonstrated that IMP3 expression in gastric carcinomas correlates significantly with worse overall survival and recurrence free survival [14,15]. However, the setup of the studies was aimed at analyzing the prognostic relevance of IMP3 expression and its correlation with clinicopathological parameters, with little (Wang et al) or no data (Okada et al) being provided about IMP3 expression in inflammatory and reactive gastric lesions.
Another interesting marker in this context is the tumor suppressor gene p53. P53 is the most important negative regulator of the cell cycle [16]. Mutation of p53 can lead to a loss of its tumour suppressor activity, making mutated p53 an important factor in tumour genesis. It has been estimated that p53 is mutated in 50% of human cancers [17]. Several studies have analyzed the expression of p53 in gastric cancer, with the percentage of p53 positive cancers ranging between 27.4% and 50%, regardless of histological subtype [18-21]. Considering this data, p53 lacks adequate sensitivity to be the sole marker in the immunohistochemical detection of gastric neoplasia. However, it might be a helpful auxiliary marker.
In this study, we examined the expression of IMP3 and p53 in non-neoplastic, inflammatory lesions of the gastric mucosa and in intraepithelial and invasive neoplastic lesions of the gastric mucosa. The value of IMP3 as a marker for differentiating between reactive inflammatory lesions and neoplastic lesions of the gastric mucosa was assessed statistically; in doing so it was determined whether diagnostic sensitivity and specificity could be improved by considering both IMP3 and p53 expression.
Materials and methods
33 cases of gastric carcinoma, intestinal type (18 surgical resection specimens, 15 biopsies), 30 cases of gastric carcinoma, diffuse type (12 surgical resection specimens, 18 biopsies), 16 cases of low grade dysplasia of the gastric mucosa (all biopsies), 12 cases of high grade dysplasia of the gastric mucosa (6 surgical resection specimens, 6 biopsies), as well as 20 cases of autoimmune chronic gastritis (Type A, all biopsies), 20 cases of Helicobacter pylori induced gastritis (Type B, all biopsies) and 17 cases of reactive gastritis (Type C, all biopsies) were retrieved from the archives of the Institute of Pathology of the University Clinic Erlangen. The cases were selected from the time period between 2008 and 2011. Patients with prior radiotherapy or chemotherapy were excluded from the study. The study was covered by the ethical votum of the medical faculty of the University of Erlangen-Nuremberg. For routine histological workup, the tissue specimens had been fixed in 4% buffered formalin and embedded in paraffin. The Hematoxylin-Eosin (HE) -stains of the surgical specimen cases were screened and one representative paraffin block of the lesion was selected for the study in each case.
Immunohistochemistry with IMP3 (Dako, clone 69.1, dilution 1:100) and p53 (Dako, clone DO-7, dilution 1:50) was performed on 1 μm sections all of the selected paraffin blocks using the fully automated slide preparation system “Benchmark XT System” (Ventana Medical Systems Inc, 1910 Innovation Park Drive, Tucson, Arizona). Tonsil tissue and Barrett’s mucosa with high grade dysplasia were employed as positive controls for IMP3 and p53 immunohistochemistry respectively. Additionally, one 5 μm section per selected block was stained with HE.
All histochemical and immunohistochemical analyses were performed independently by two surgical pathologists (MOR and JDS). In cases of divergent diagnoses, the specimens were reassessed by the two pathologists until consensus was achieved.
The lesion type was determined in the standard HE stain. For the diagnosis of dysplasia and invasive neoplasia of the gastric mucosa, the diagnostic criteria as detailed by the WHO were employed [22]. In the categories of invasive lesions and dysplastic lesions, biopsy samples and surgical resection samples were first regarded separately and later pooled for statistical analysis as detailed in the results section.
IMP3 staining was assessed by determining the percentage of IMP3 positive epithelial cells and the intensity of the IMP3 stain within the lesion. The percentage of IMP3 positive epithelial cells in relation to the whole of the lesion was documented on a continuous scale.
The staining intensity was evaluated on an ordinal scale using a 4-tiered grading system, “0” being a negative result, “1” a weak staining evident only in high magnification (400x), “2” a moderate staining readily visible on low magnification (200x) and “3” a strong membranous and/or cytoplasmic staining readily visible on low magnification (200x) (Figure 1). A product score was then calculated using the percentage of IMP3 positive cells and the degree of staining intensity (Product score = percentage of IMP3 positive cells x staining intensity).
Figure 1.
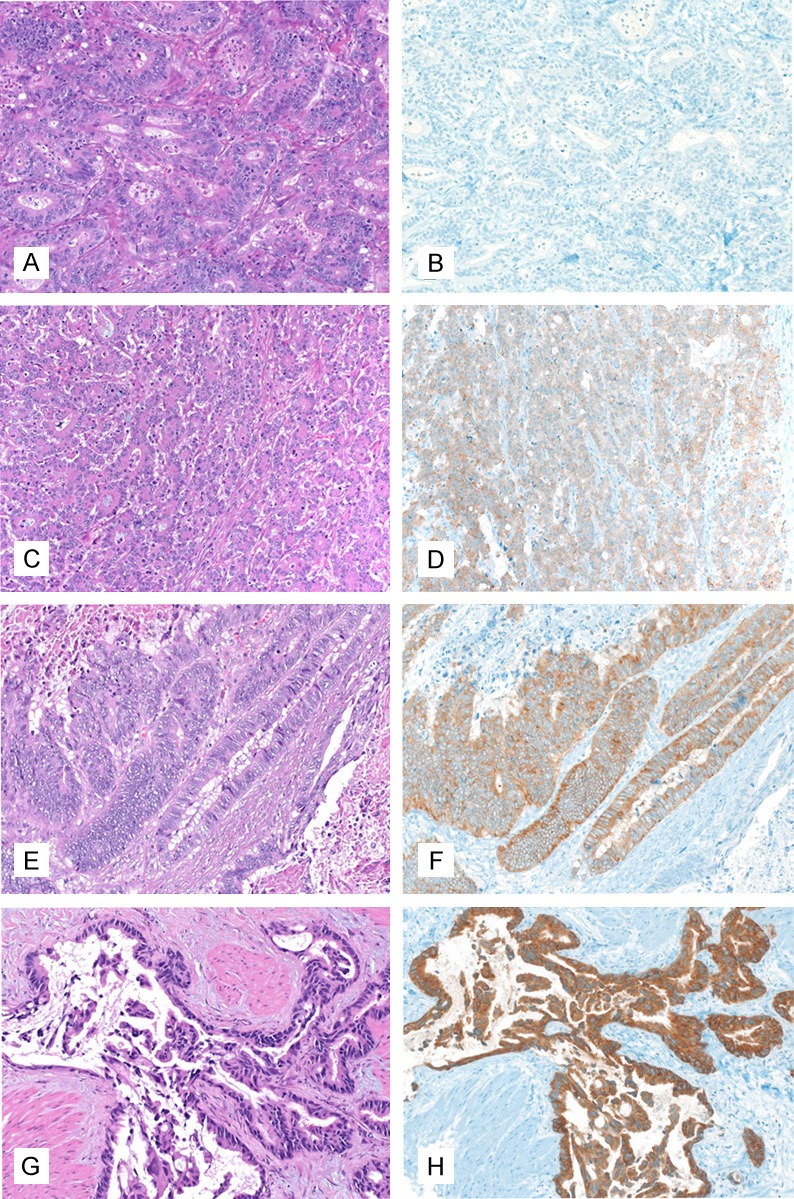
IMP3 staining intensity in gastric adenocarcinomas, 200x magnification. A, B: IMP3 staining intensity grade 0 (A: HE, B: Immunohistochemistry with IMP3); C, D: IMP3 staining intensity grade 1 (C: HE, D: Immunohistochemistry with IMP3); E, F: IMP3 staining intensity grade 2 (E: HE, F: Immunohistochemistry with IMP3); G, H: IMP3 staining intensity grade 3 (G: HE, H: Immunohistochemistry with IMP3).
P53 was also analyzed in all of the lesions. Only a strong nuclear positivity was accepted as a positive stain (Figure 2). The p53 stain was graded either as “positive” or as “negative”, with the values “1” or “0” being awarded accordingly. Since positive cases showed p53 positivity in at least 10% of tumor cells, the percentage of p53 positive cells was not taken into account in the assessment of the p53 immunostain.
Figure 2.
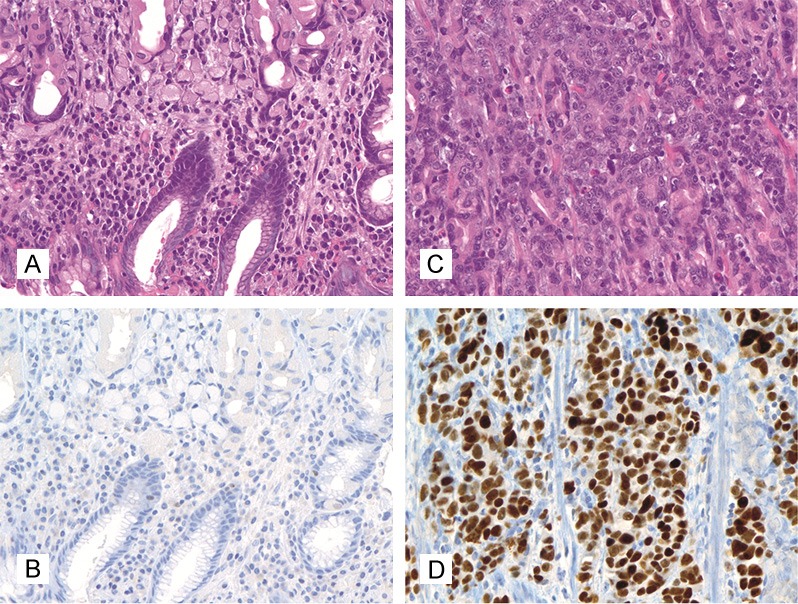
p53 staining score in gastric adenocarcinomas, 400x magnification. A, B: Score 0, negative (A: HE, B: Immunohistochemistry with p53); C, D: Score 1, positive (C: HE, D: Immunohistochemistry with p53).
Statistical analysis
In the categories “dysplasia” and “gastric carcinoma” multiple samples from individual patients were included (low grade dysplasia: 16 patients with 17 lesions; high grade dysplasia: 12 patients with 14 lesions; gastric carcinoma, intestinal type: 33 patients with 35 lesions, gastric carcinoma, diffuse type: 30 patients with 31 lesions). To maintain independence of observations, the arithmetic average of multiple samples was computed in these cases. In the categories high-grade dysplasia, gastric carcinoma, intestinal type and gastric carcinoma, diffuse type the two sample Wilcoxon test was used to compare biopsy samples and resection samples regarding IMP3 expression. With regard to p53 Fisher’s exact test was applied for the same question. Receiver operating characteristic (ROC) curve analysis was employed to identify the cutoff values for the product score which yielded the best values for specificity and sensitivity.
Generally, for comparing categories and category combinations, the unpaired or paired Wilcoxon test was utilized for quantitative traits and the chi2-test, Fisher’s exact test, or the Cochran-Armitage trend test for contingency tables, as appropriate.
Tests with p-values < 0.05 were termed statistically significant. In general, proportions given (%) refer to patients rather than specimens. The R language and statistical environment was used to perform statistical analysis of the data.
Results
IMP3 staining in resection specimens and biopsies
No numerically remarkable or statistically significant difference was found between biopsy specimens and resection specimens in the categories high-grade dysplasia, gastric carcinoma, intestinal type and gastric carcinoma, diffuse type (IMP3(PS) positivity high grade dysplasia: biopsies 4/6, resection specimens 6/6; gastric carcinoma intestinal type: biopsies 11/15, resection specimens 14/18; gastric carcinoma diffuse type: biopsies 10/17, resection specimens 6/13). The resection specimens and biopsy specimens of the respective categories were consequently pooled for further statistical analysis.
IMP3 staining pattern in non-neoplastic lesions of the gastric mucosa
Focal IMP3 positivity in patches of adjacent foveolar epithelial cells could be found in 86% of cases with manifest gastritis (all cases: 86%, type A gastritis: 95%, type B gastritis: 85%, type C gastritis: 76%).
The glandular epithelium of the antrum and corpus didn’t display significant IMP3 positivity. Intestinal metaplasia was found in 32 of the examined gastritis cases (type A: 13 cases, type B: 2 cases, type C: 17 cases). Mostly, the areas with intestinal metaplasia were negative for IMP3, with only 5 cases demonstrating IMP3 positivity within intestinal metaplasia.
The IMP3 positivity observed in non-neoplastic gastric mucosa showed several characteristics clearly divergent from the IMP3 positivity in dysplasia and gastric carcinoma. IMP3 staining in neoplastic lesions of the gastric mucosa is characterized by a diffuse, non-polarized cytoplasmic and/or membranous positivity.
In contrast, IMP3 positivity in normal and reactive gastric mucosa is of a linear, patchy nature, encompassing continuous stretches of up to several hundred epithelial cells (Figure 3). The IMP3 stain is usually moderate to strong in these cases and can be both cytoplasmic and membranous. Because of the structure of the normal, apically vacuolated foveolar cell, IMP3 positivity is located in the basal portion of the cell, leading to a “polarized” staining pattern.
Figure 3.
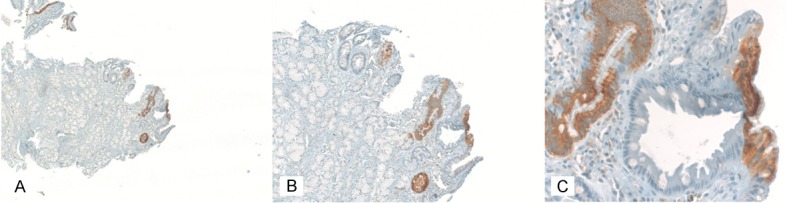
Typical IMP3 immunoreactivity in a case with type B gastritis. A: 50x magnification; B: 100x magnification; C: 200x magnification.
Apart from the columnar cells of the gastric mucosa, IMP3 could also be detected in neuroendocrine cells and in cells of follicular germ centres. IMP3 positivity in neuroendocrine cells was mostly membranous and significantly weaker than in the IMP3 positive patches of normal and reactive gastric epithelium.
This finding is in accordance with our previous observation of IMP3 positivity in neuroendocrine cells of the islet of Langerhans in the pancreas.
IMP3 staining in dysplasia and carcinomas of the gastric mucosa
63% of low grade dysplasia were completely negative for IMP3. Looking at the other individual staining score categories, 37% of low grade dysplasia showed IMP3 positivity up to score “1” or “2”, and 0% showed IMP3 staining intensity score “3”.
Amongst the cases of high grade dysplasia of the gastric mucosa, an IMP3 staining intensity score “1” and “2” was recorded in 17% respectively, with score “3” documented in 75%. There was only one case with completely negative IMP3 staining.
Gastric carcinoma, intestinal type showed an IMP3 staining score of “0” in 9%, “1” in 21%, “2” in 27% and “3” in 43% of all cases.
Gastric carcinoma, diffuse type displayed score “0” in 30%, score “1” in 20%, score “2” in 23% and score “3” in 27% of all cases.
All in all, of 63 examined invasive gastric carcinomas 26 cases (41%) showed a homogenous IMP3 staining pattern (completely IMP3 positive: 14 cases, completely IMP3 negative: 12 cases). 37 cases (59%) showed a heterogenous IMP3 staining pattern, with patches of IMP3 positive tumour cells being located next to patches with IMP3 negative tumour cells (17 cases (27%) with IMP3 positivity in more than 50% of the tumour cells and 20 cases (32%) with IMP3 positivity in less than 50% of the tumour cells). In the cases with a mixed staining pattern, different IMP3 staining intensities were also seen. Due to the diffuse nature of the mixed staining pattern and the intermingling of IMP3 positive and IMP3 negative tumour cells the IMP3 expression must be described as heterogenous in these cases.
Additionally, IMP3 expression was analyzed separately in the tumour centre and in the tumour invasion front in the resection specimens (30 cases). In 25 cases (83%) there was no discernible difference regarding the immunohistochemical IMP3 expression in these two tumour components. In only 5 cases (17%) slight differences were found between the tumor center and the invasion front. All in all, there is no significant difference between IMP3 expression in the tumor center and at the invasion front in our collective.
IMP3 product score and cutoff value
Due to the high percentage of non-neoplastic lesions of the gastric mucosa showing patchy IMP3 positivity, a product score (PS, as described in the Materials and Methods section) was employed in order to obtain a better differentiation between neoplastic and non-neoplastic lesions.
With the aid of ROC curves, a product score of 30 was determined as the cutoff value which yields the best trade-off between specificity and sensitivity when considering the sum of both (Figure 4). Using this score, focal IMP3 positivity translating into a score of 30 or less is classified as a negative result.
Figure 4.
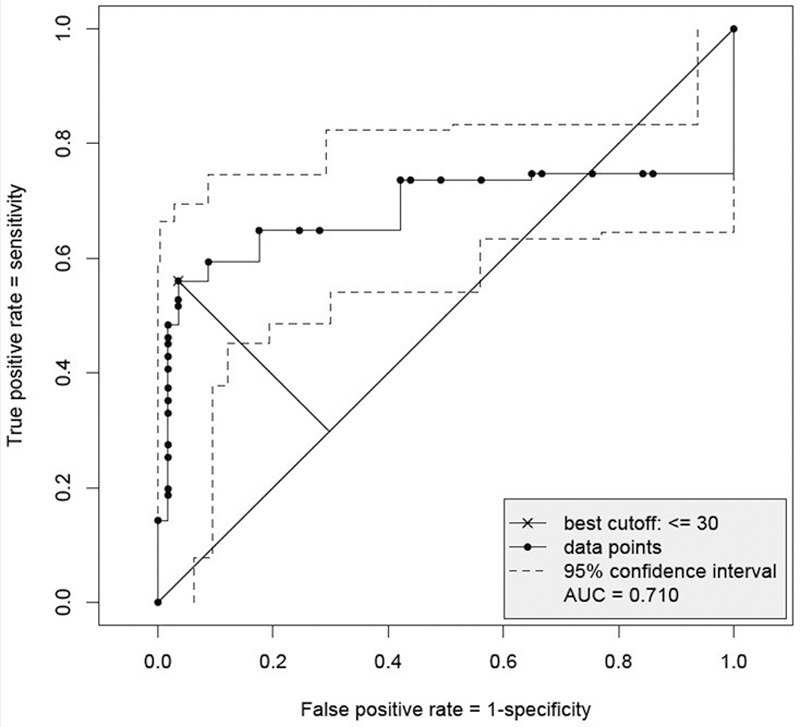
Receiver Operating Characteristic (ROC) curve with pointwise 95% confidence interval for gastritis A, B, C versus low grade dysplasia, high grade dysplasia and gastric carcinoma: best cutoff-value based on the largest distance of (1-specifity, sensitivity) from the angle bisector.
Applying the product score to the three categories of gastritis, only 4% of the cases showed IMP3(PS) positivity (2/57).
With regard to dysplasia of the gastric mucosa, none of the cases of low-grade dysplasia yielded a positive result with the product score (0/16).
In contrast, 83% of the cases of high grade dysplasia were positive for IMP3 as determined by the product score (10/12) (Table 1) (Figure 5).
Table 1.
Tumor grade in dysplasia and carcinoma of the gastric mucosa and IMP3/p53 immunohistochemistry
| Category | IMP3 product score | P53 | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| N | Positive (%) | Negative (%) | Positive (%) | Negative (%) | ||
| Low grade dysplasia of the gastric mucosa | 16 | 0 (0) | 16 (100) | 2 (13) | 14 (87) | |
| High grade dysplasia of the gastric mucosa | 12 | 10 (83) | 2 (17) | 7 (58) | 5 (42) | |
| Low grade vs. high grade dysplasia of the gastric mucosa | Fisher Test: p = 5.0-06 | Fisher Test: p = 0.017 | ||||
| Invasive gastric adenocarcinoma, both diffuse and intestinal type | All grades | 63 | 41 (65) | 22 (35) | 29 (46) | 35 (56) |
| G1 | 5 | 4 (80) | 1 (20) | 1 (20) | 4 (80) | |
| G2 | 16 | 13 (81) | 3 (19) | 10 (63) | 6 (37) | |
| G3 | 42 | 24 (57) | 18 (43) | 17 (40) | 25 (60) | |
| Armitage Trend Test: p = 0.10 | Armitage Trend Test: p = 0.93 | |||||
| Invasive gastric adenocarcinoma, intestinal type | All grades | 33 | 26 (77) | 8 (24) | 13 (40) | 20 (60) |
| G1 | 5 | 4 (80) | 1 (20) | 1 (20) | 4 (80) | |
| G2 | 16 | 13 (81) | 3 (19) | 10 (63) | 6 (37) | |
| G3 | 12 | 8 (67) | 4 (33) | 2 (17) | 10 (83) | |
| Armitage Trend Test: p = 0.52 | Armitage Trend Test: p = 0.52 | |||||
| Invasive gastric adenocarcinoma, diffuse type | All grades | 30 | 16 (53) | 14 (47) | 15 (50) | 15 (50) |
| G3 | 30 | 16 (53) | 14 (47) | 15 (50) | 15 (50) | |
Figure 5.
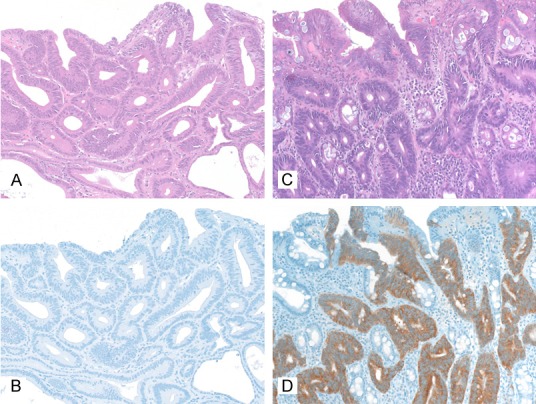
IMP3 immunohistochemistry in low-grade and high-grade intraepithelial neoplasia of the stomach, 200x. A, B: Low grade intraepithelial neoplasia (A: HE, B: Immunohistochemistry with IMP3); C, D: High grade intraepithelial neoplasia (C: HE, D: Immunohistochemistry with IMP3).
Mixed results were obtained for invasive gastric carcinomas using the product score. Gastric carcinoma intestinal type stained positively for IMP3(PS) in 77% of the cases (25/33). Gastric carcinoma diffuse type were IMP3(PS) positive in 53% of the cases (16/30).
Tumour grade and IMP3 positivity (PS)
Gastric carcinomas with good differentiation (G1) were IMP3(PS) positive in 80% (4/5), gastric carcinomas with moderate differentiation (G2) were IMP3(PS) positive in 81% (13/16) and poorly differentiated gastric carcinomas (G3) were IMP3(PS) positive in 57% of the cases (24/42) (Table 1). Regarding tumour type, grade G3 Gastric carcinoma intestinal type show IMP3 positivity in 67% of cases (8/12) whereas gastric carcinomas diffuse type, which are by definition grade G3, are IMP3(PS) positive in 53% of cases (16/30) (Table 1).
The data does not show a trend of more frequent IMP3 product score positivity with progressive dedifferentiation. On the contrary, there is a slight decrease in IMP3 positive cases when comparing grade G1 and G2 gastric carcinomas with grade G3 gastric carcinomas.
IMP3 sensitivity and specificity
IMP3(PS) sensitivity and specificity ranged at 0.56 and 0.96 respectively when comparing gastritis against neoplastic lesions and at 0.7 and 0.96 respectively when comparing gastritis and low grade dysplasia against high grade dysplasia, gastric carcinoma intestinal type and gastric carcinoma diffuse type. When comparing low grade dysplasia against high grade dysplasia, sensitivity is 0.83 and specificity is 1.0 (Table 2).
Table 2.
Specificity and sensitivity for IMP3(PS) and p53 in different group comparisons
| Category comparison with PS cutoff = 30 | Marker | Specifity | Conf. Interv. | Sensitivity | Conf. Interv. Mean | |
|---|---|---|---|---|---|---|
| Gastritis A, B, C Vs. Low grade and high grade dysplasia and invasive gastric adenocarcinoma (both intestinal and diffuse type) | IMP3 | 0.96 | [0.88, 1.00] | 0.56 | [0.45, 0.67] | 0.76 |
| IMP3 OR p53 | 0.96 | [0.88, 1.00] | 0.70 | [0.60, 0.80] | 0.83 | |
| IMP3 AND p53 | 1.00 | [0.94, 1.00] | 0.26 | [0.18, 0.37] | 0.63 | |
| p53 | 1.00 | [0.94, 1.00] | 0.41 | [0.31, 0.52] | 0.71 | |
| Gastritis A, B, C and low grade dysplasia Vs. High grade dysplasia and invasive gastric adenocarcinoma (both intestinal and diffuse type) | IMP3 | 0.97 | [0.91, 1.00] | 0.68 | [0.56, 0.78] | 0.83 |
| IMP3 OR p53 | 0.95 | [0.87, 0.99] | 0.83 | [0.72, 0.90] | 0.89 | |
| IMP3 AND p53 | 1.00 | [0.95, 1.00] | 0.32 | [0.22, 0.44] | 0.66 | |
| p53 | 0.97 | [0.91, 1.00] | 0.47 | [0.35, 0.59] | 0.72 | |
| Low grade and high grade dysplasia Vs. Invasive gastric adenocarcinoma (both intestinal and diffuse type) | IMP3 | 0.64 | [0.44, 0.81] | 0.65 | [0.52, 0.77] | 1.29 |
| IMP3 OR p53 | 0.54 | [0.34, 0.73] | 0.81 | [0.69, 0.90] | 0.68 | |
| IMP3 AND p53 | 0.79 | [0.59, 0.92] | 0.29 | [0.18, 0.41] | 1.08 | |
| p53 | 0.68 | [0.48, 0.84] | 0.44 | [0.32, 0.58] | 1.12 | |
| Low grade dysplasia Vs. High grade dysplasia | IMP3 | 1.00 | [0.79, 1.00] | 0.83 | [0.52, 0.98] | 0.92 |
| IMP3 OR p53 | 0.88 | [0.62, 0.98] | 0.92 | [0.62, 1.00] | 0.90 | |
| IMP3 AND p53 | 1.00 | [0.79, 1.00] | 0.50 | [0.21, 0.79] | 0.75 | |
| p53 | 0.88 | [0.62, 0.98] | 0.58 | [0.28, 0.85] | 0.73 |
Definition Boolean Operator “AND”: Both IMP3(PS) and p53 must be positive for a positive result; Definition Boolean Operator “OR”: Either IMP3(PS) or p53 or both markers must be positive for a positive result.
P53 staining in dysplasia and carcinomas of the gastric mucosa
Strong nuclear p53 positivity was detected in 40% (13/33) and 50% (15/30) of gastric carcinoma intestinal type and diffuse type respectively, amounting to p53 positivity in 44% (28/63) of all invasive gastric carcinomas.
Regarding the cases of dysplasia, 13% of low grade dysplasia (2/16) and 58% of high grade dysplasia (7/12) stained positive for p53. In the cases of gastritis no p53 positivity was detected (0/57).
P53 sensitivity and specificity range at 0.41 and 1.0 respectively when comparing gastritis against dysplastic and invasive lesions and at 0.47 and 0.97 respectively when comparing gastritis and low grade dysplasia against high grade dysplasia and gastric carcinoma. When comparing low grade dysplasia against high grade dysplasia, sensitivity is 0.58 and specificity is 0.88 (Table 2).
Combination of IMP3 and p53 in a diagnostic panel
When combining IMP3(PS) and p53 using the Boolean operator “OR”, the sensitivity in all of the constellations discussed above is improved considerably at the cost of little or no decrease in specificity. When gastritis is compared to dysplasia and gastric carcinoma, a sensitivity of 0.7 and a specificity of 0.96 are achieved. Comparing gastritis and low grade dysplasia to high grade dysplasia and gastric carcinoma, sensitivity and specificity amount to 0.83 and 0.95 respectively. For the comparison of low grade dysplasia with high grade dysplasia, sensitivity ranges at 0.92 and specificity at 0.88 (Table 2).
In contrast, combining IMP3(PS) and p53 using the Boolean operator “AND” results in a significant loss of sensitivity (Table 2).
Discussion
IMP3 was first cloned from pancreatic cancer tissue and cell lines [23]. It belongs to the Insulin-like growth factor II (IGF-II) mRNA binding protein (IMP) family, which is made up of IMP1, IMP2 and IMP3 [5]. The precise function of the IMP family is still unclear, but it has been hypothesized that IMPs move into the nucleus and bind to mRNA. After being exported to the cytoplasm, IMPs may influence the post-transcriptional expression of the mRNAs they are attached to [24]. Functional analyses indicate a role for IMP3 in tumor cell adhesion and cancer [25,26].
However, as of now, the actual function of IMP3 in the modulation of tumor cell function is still unclear [27].
In several studies, it has been demonstrated that IMP3 immunohistochemistry can be employed to differentiate between benign and malignant lesions in the skin [6,7], the uterine cervix [8,9], the pancreas [8-11] and the mesothelium [12,13].
IMP3 expression also seems to correlate with aggressive histological subtypes in urothelial carcinomas [28], breast carcinomas [29], endometrial carcinomas [30,31] and neuroendocrine tumors of the lung [32].
Furthermore, an association has been shown between IMP3 expression and poorer prognosis in gastric adenocarcinoma [14,15], urothelial carcinoma [33], renal cell carcinoma [34], bile duct carcinoma [35], hepatocellular carcinoma [36], colorectal carcinoma [37], meningioma [38], pediatric astrocytoma [39], neuroblastoma [40] and squamous cell carcinoma of the tongue [41].
Up to now, IMP3 expression has been analyzed immunohistochemically in two collectives of gastric carcinomas. The assessment of the IMP3 immunohistochemical stain was handled differently in these two studies, with Wang et al scoring by staining intensity only [15] and Okada et al scoring by percentage of IMP3 positive cells only [14]. In both studies, a correlation between IMP3 expression in gastric adenocarcinomas and poor overall survival was found. Additionally, Wang et al documented a weak IMP3 staining in 10 of 10 cases of dysplasia of the gastric mucosa adjacent to invasive carcinoma. However, no further information was provided regarding the degree of dysplasia and the percentage of IMP3 positive cells in the lesions [15].
Okada et al did not analyze dysplasias of the gastric mucosa but reported “nonspecific and weak staining” for IMP3 in the normal gastric mucosa [14]. All in all, data regarding IMP3 expression in reactive and dysplastic lesions of the gastric mucosa is scant in these two studies.
In our study, we showed that IMP3 is expressed in both reactive and neoplastic lesions of the gastric mucosa. We devised an easy to use product score based on the percentage of IMP3 positive cells and IMP3 staining intensity. Using the IMP3 product score, a good specificity and acceptable sensitivity are achieved regarding the differentiation between reactive/inflammatory and neoplastic lesions of the gastric mucosa. However, regarding our methodology, it is necessary to draw attention to the fact that no separate validation cohort was included in this study. Due to this limitation of our study, our product score must be regarded as only partly validated.
Our results show that low grade dysplasia of the gastric mucosa is usually negative for IMP3(PS) whilst high grade dysplasia of the gastric mucosa displays strong IMP3(PS) positivity in the majority of the cases. These results are comparable to the data presented by Lu et al who analyzed oesophagus specimens [42] and to our previous study on bile duct specimens [35] where strong IMP3 positivity was found in high grade dysplasia and IMP3 negativity in most cases of low grade dysplasia.
IMP3(PS) may therefore be a helpful auxiliary marker for distinguishing low grade dysplasia from high grade dysplasia of the gastric mucosa in routine pathological workup.
In invasive gastric carcinomas, mixed results are obtained with IMP3 immunohistochemistry. Our study demonstrated that IMP3 staining varies strongly between gastric carcinomas and sometimes also remarkably within the same lesion, underscoring the heterogeneous nature of IMP3 expression in gastric carcinomas. All in all, 65% of gastric carcinomas were IMP3(PS) positive in our study. Wang et al reported 75% IMP3 positive gastric carcinomas (92 cases) [15] and Okada et al reported 82% IMP3 positive gastric carcinomas (96 cases) [14]. Despite the differences in the IMP3 scoring systems and in the makeup of the three collectives, the results are comparable.
Regarding the tumour grade, no clear cut correlation between tumour grade and IMP3(PS) positivity could be established in our study. Our results are in accordance with the studies by Okada et al and Wang et al, in which the analysis of IMP3 expression yielded no significant difference between different tumour grades [14,15].
Statistical workup showed that adding p53 to the diagnostic panel significantly improves sensitivity in identifying high grade dysplasia and gastric carcinoma as well as in differentiating between low grade and high grade dysplasia of the gastric mucosa. However, p53 should only be employed as a facultative marker as the sensitivity is seriously compromised if a positive result is exclusively defined as concurrent IMP3 and p53 positivity. This is due both to the fact that high grade dysplasia and invasive neoplasia show no reliable co-expression of IMP3 and p53 and to the fact that only half of gastric cancers express p53, resulting in a greater number of false negative results.
Our study shows that the combination of IMP3 and p53 immunohistochemistry, with IMP3 immunohistochemistry assessed by a simple product score, may be a helpful diagnostic tool in the often difficult differential diagnosis of reactive and neoplastic lesions of the gastric mucosa. However, to completely validate our product score for the assessment of IMP3 immunohistochemistry the analysis of an adequately sized validation cohort is required. Further studies are therefore needed before the combination of IMP3 and p53 immunohistochemistry can be safely incorporated into the routine pathologic workup of lesions of the gastric mucosa.
Acknowledgements
We acknowledge support by Deutsche Forschungsgemeinschaft and Friedrich-Alexander-Universität Erlangen-Nürnberg within the funding programme Open Access Publishing.
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V WHO International Agency for Research on Cancer Monograph Working Group. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10:321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 4.Mueller-Pillasch F, Pohl B, Wilda M, Lacher U, Beil M, Wallrapp C, Hameister H, Knochel W, Adler G, Gress TM. Expression of the highly conserved RNA binding protein KOC in embryogenesis. Mech Dev. 1999;88:95–99. doi: 10.1016/s0925-4773(99)00160-4. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen FC, Nielsen J, Christiansen J. A family of IGF-II mRNA binding proteins (IMP) involved in RNA trafficking. Scand J Clin Lab Invest Suppl. 2001;234:93–99. [PubMed] [Google Scholar]
- 6.Pryor JG, Bourne PA, Yang Q, Spaulding BO, Scott GA, Xu H. IMP-3 is a novel progression marker in malignant melanoma. Mod Pathol. 2008;21:431–437. doi: 10.1038/modpathol.3801016. [DOI] [PubMed] [Google Scholar]
- 7.Soddu S, Di Felice E, Cabras S, Castellanos ME, Atzori L, Faa G, Pilloni L. IMP-3 expression in keratoacanthomas and squamous cell carcinomas of the skin: an immunohistochemical study. Eur J Histochem. 2013;57:e6. doi: 10.4081/ejh.2013.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danialan R, Assaad M, Burghardt J, Newcomb P, Cartun RW, Mandavilli S. The utility of PAX8 and IMP3 immunohistochemical stains in the differential diagnosis of benign, premalignant, and malignant endocervical glandular lesions. Gynecol Oncol. 2013;130:383–8. doi: 10.1016/j.ygyno.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 9.Li C, Rock KL, Woda BA, Jiang Z, Fraire AE, Dresser K. IMP3 is a novel biomarker for adenocarcinoma in situ of the uterine cervix: an immunohistochemical study in comparison with p16(INK4a) expression. Mod Pathol. 2007;20:242–247. doi: 10.1038/modpathol.3800735. [DOI] [PubMed] [Google Scholar]
- 10.Morimatsu K, Aishima S, Yamamoto H, Hayashi A, Nakata K, Oda Y, Shindo K, Fujino M, Tanaka M, Oda Y. Insulin-like growth factor II messenger RNA-binding protein-3 is a valuable diagnostic and prognostic marker of intraductal papillary mucinous neoplasm. Hum Pathol. 2013;44:1714–21. doi: 10.1016/j.humpath.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 11.Wachter DL, Schlabrakowski A, Hoegel J, Kristiansen G, Hartmann A, Riener MO. Diagnostic value of immunohistochemical IMP3 expression in core needle biopsies of pancreatic ductal adenocarcinoma. Am J Surg Pathol. 2011;35:873–877. doi: 10.1097/PAS.0b013e3182189223. [DOI] [PubMed] [Google Scholar]
- 12.Lee AF, Gown AM, Churg A. IMP3 and GLUT-1 immunohistochemistry for distinguishing benign from malignant mesothelial proliferations. Am J Surg Pathol. 2013;37:421–426. doi: 10.1097/PAS.0b013e31826ab1c0. [DOI] [PubMed] [Google Scholar]
- 13.Shi M, Fraire AE, Chu P, Cornejo K, Woda BA, Dresser K, Rock KL, Jiang Z. Oncofetal protein IMP3, a new diagnostic biomarker to distinguish malignant mesothelioma from reactive mesothelial proliferation. Am J Surg Pathol. 2011;35:878–882. doi: 10.1097/PAS.0b013e318218985b. [DOI] [PubMed] [Google Scholar]
- 14.Okada K, Fujiwara Y, Nakamura Y, Takiguchi S, Nakajima K, Miyata H, Yamasaki M, Kurokawa Y, Takahashi T, Mori M, Doki Y. Oncofetal protein, IMP-3, a potential marker for prediction of postoperative peritoneal dissemination in gastric adenocarcinoma. J Surg Oncol. 2012;105:780–785. doi: 10.1002/jso.22108. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Li HG, Xia ZS, Lu J, Peng TS. IMP3 is a novel biomarker to predict metastasis and prognosis of gastric adenocarcinoma: a retrospective study. Chin Med J (Engl) 2010;123:3554–3558. [PubMed] [Google Scholar]
- 16.Levine AJ, Momand J, Finlay CA. The p53 tumour suppressor gene. Nature. 1991;351:453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- 17.Soussi T, Beroud C. Assessing TP53 status in human tumours to evaluate clinical outcome. Nat Rev Cancer. 2001;1:233–240. doi: 10.1038/35106009. [DOI] [PubMed] [Google Scholar]
- 18.Karim S, Ali A. Correlation of p53 over-expression and alteration in p53 gene detected by polymerase chain reaction-single strand conformation polymorphism in adenocarcinoma of gastric cancer patients from India. World J Gastroenterol. 2009;15:1381–1387. doi: 10.3748/wjg.15.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lazar D, Taban S, Sporea I, Dema A, Cornianu M, Lazar E, Goldis A, Ratiu I, Vernic C. The immunohistochemical expression of the p53-protein in gastric carcinomas. Correlation with clinicopathological factors and survival of patients. Rom J Morphol Embryol. 2010;51:249–257. [PubMed] [Google Scholar]
- 20.Muhlmann G, Ofner D, Zitt M, Muller HM, Maier H, Moser P, Schmid KW, Zitt M, Amberger A. 14-3-3 sigma and p53 expression in gastric cancer and its clinical applications. Dis Markers. 2010;29:21–29. doi: 10.3233/DMA-2010-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nabizadeh Marvast M, Sima HR, Ghaffarzadehgan K, Taghizadeh Kermani A, Norouzi N. Clinicopathological significance of macrophage migration inhibitory factor and its relation with p53 in gastric cancer. J Gastrointest Cancer. 2011;42:5–10. doi: 10.1007/s12029-010-9215-3. [DOI] [PubMed] [Google Scholar]
- 22.Bosman F, Carneiro F, Hruban R, Theise N. WHO Classification of Tumors of the Digestive System. Lyon, France: IARC; 2010. [Google Scholar]
- 23.Mueller-Pillasch F, Lacher U, Wallrapp C, Micha A, Zimmerhackl F, Hameister H, Varga G, Friess H, Buchler M, Beger HG, Vila MR, Adler G, Gress TM. Cloning of a gene highly overexpressed in cancer coding for a novel KH-domain containing protein. Oncogene. 1997;14:2729–2733. doi: 10.1038/sj.onc.1201110. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen J, Adolph SK, Rajpert-De Meyts E, Lykke-Andersen J, Koch G, Christiansen J, Nielsen FC. Nuclear transit of human zipcode-binding protein IMP1. Biochem J. 2003;376:383–391. doi: 10.1042/BJ20030943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao B, Hu Y, Herrick DJ, Brewer G. The RNA-binding protein IMP-3 is a translational activator of insulin-like growth factor II leader-3 mRNA during proliferation of human K562 leukemia cells. J Biol Chem. 2005;280:18517–18524. doi: 10.1074/jbc.M500270200. [DOI] [PubMed] [Google Scholar]
- 26.Vikesaa J, Hansen TV, Jonson L, Borup R, Wewer UM, Christiansen J, Nielsen FC. RNA-binding IMPs promote cell adhesion and invadopodia formation. EMBO J. 2006;25:1456–1468. doi: 10.1038/sj.emboj.7601039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bell JL, Wachter K, Muhleck B, Pazaitis N, Kohn M, Lederer M, Huttelmaier S. Insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs): post-transcriptional drivers of cancer progression? Cell Mol Life Sci. 2013;70:2657–75. doi: 10.1007/s00018-012-1186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sitnikova L, Mendese G, Liu Q, Woda BA, Lu D, Dresser K, Mohanty S, Rock KL, Jiang Z. IMP3 predicts aggressive superficial urothelial carcinoma of the bladder. Clin Cancer Res. 2008;14:1701–1706. doi: 10.1158/1078-0432.CCR-07-2039. [DOI] [PubMed] [Google Scholar]
- 29.Walter O, Prasad M, Lu S, Quinlan RM, Edmiston KL, Khan A. IMP3 is a novel biomarker for triple negative invasive mammary carcinoma associated with a more aggressive phenotype. Hum Pathol. 2009;40:1528–1533. doi: 10.1016/j.humpath.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Li C, Zota V, Woda BA, Rock KL, Fraire AE, Jiang Z, Lu D, Xu B, Dresser K, Lutman CV, Fischer AH. Expression of a novel oncofetal mRNA-binding protein IMP3 in endometrial carcinomas: diagnostic significance and clinicopa-thologic correlations. Mod Pathol. 2007;20:1263–1268. doi: 10.1038/modpathol.3800960. [DOI] [PubMed] [Google Scholar]
- 31.Mhawech-Fauceglia P, Yan L, Liu S, Pejovic T. ER+/PR+/TFF3+/IMP3-immunoprofile distinguishes endometrioid from serous and clear cell carcinomas of the endometrium: a study of 401 cases. Histopathology. 2013;62:976–985. doi: 10.1111/his.12096. [DOI] [PubMed] [Google Scholar]
- 32.Xu H, Bourne PA, Spaulding BO, Wang HL. High-grade neuroendocrine carcinomas of the lung express K homology domain containing protein overexpressed in cancer but carcinoid tumors do not. Hum Pathol. 2007;38:555–563. doi: 10.1016/j.humpath.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Szarvas T, vom Dorp F, Niedworok C, Melchior-Becker A, Fischer JW, Singer BB, Reis H, Bankfalvi A, Schmid KW, Romics I, Ergun S, Rubben H. High insulin-like growth factor mRNA-binding protein 3 (IMP3) protein expression is associated with poor survival in muscle-invasive bladder cancer. BJU Int. 2012;110:E308–317. doi: 10.1111/j.1464-410X.2012.11149.x. [DOI] [PubMed] [Google Scholar]
- 34.Hoffmann NE, Sheinin Y, Lohse CM, Parker AS, Leibovich BC, Jiang Z, Kwon ED. External validation of IMP3 expression as an independent prognostic marker for metastatic progression and death for patients with clear cell renal cell carcinoma. Cancer. 2008;112:1471–1479. doi: 10.1002/cncr.23296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riener MO, Fritzsche FR, Clavien PA, Pestalozzi BC, Probst-Hensch N, Jochum W, Kristiansen G. IMP3 expression in lesions of the biliary tract: a marker for high-grade dysplasia and an independent prognostic factor in bile duct carcinomas. Hum Pathol. 2009;40:1377–1383. doi: 10.1016/j.humpath.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 36.Wachter DL, Kristiansen G, Soll C, Hellerbrand C, Breuhahn K, Fritzsche F, Agaimy A, Hartmann A, Riener MO. Insulin-like growth factor II mRNA-binding protein 3 (IMP3) expression in hepatocellular carcinoma. A clinicopa-thological analysis with emphasis on diagnostic value. Histopathology. 2012;60:278–286. doi: 10.1111/j.1365-2559.2011.04091.x. [DOI] [PubMed] [Google Scholar]
- 37.Lochhead P, Imamura Y, Morikawa T, Kuchiba A, Yamauchi M, Liao X, Qian ZR, Nishihara R, Wu K, Meyerhardt JA, Fuchs CS, Ogino S. Insulin-like growth factor 2 messenger RNA binding protein 3 (IGF2BP3) is a marker of unfavourable prognosis in colorectal cancer. Eur J Cancer. 2012;48:3405–3413. doi: 10.1016/j.ejca.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hao S, Smith TW, Chu PG, Liu Q, Ok CY, Woda BA, Lu D, Lin P, Wang SA, Dresser K, Rock KL, Jiang Z. The oncofetal protein IMP3: a novel molecular marker to predict aggressive meningioma. Arch Pathol Lab Med. 2011;135:1032–1036. doi: 10.5858/2009-0652-OAR2. [DOI] [PubMed] [Google Scholar]
- 39.Barton VN, Donson AM, Birks DK, Kleinschmidt-DeMasters BK, Handler MH, Foreman NK, Rush SZ. Insulin-like growth factor 2 mRNA binding protein 3 expression is an independent prognostic factor in pediatric pilocytic and pilomyxoid astrocytoma. J Neuropathol Exp Neurol. 2013;72:442–449. doi: 10.1097/NEN.0b013e31829023dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen ST, Jeng YM, Chang CC, Chang HH, Huang MC, Juan HF, Hsu CH, Lee H, Liao YF, Lee YL, Hsu WM, Lai HS. Insulin-like growth factor II mRNA-binding protein 3 expression predicts unfavorable prognosis in patients with neuroblastoma. Cancer Sci. 2011;102:2191–2198. doi: 10.1111/j.1349-7006.2011.02100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li HG, Han JJ, Huang ZQ, Wang L, Chen WL, Shen XM. IMP3 is a novel biomarker to predict metastasis and prognosis of tongue squamous cell carcinoma. J Craniofac Surg. 2011;22:2022–2025. doi: 10.1097/SCS.0b013e3182319750. [DOI] [PubMed] [Google Scholar]
- 42.Lu D, Vohra P, Chu PG, Woda B, Rock KL, Jiang Z. An oncofetal protein IMP3: a new molecular marker for the detection of esophageal adenocarcinoma and high-grade dysplasia. Am J Surg Pathol. 2009;33:521–525. doi: 10.1097/PAS.0b013e31818aada9. [DOI] [PubMed] [Google Scholar]


