Introduction
Neuroendocrine tumor of the lung is classified into four subtypes according to the recent World Health Organization Classification: typical carcinoid, atypical carcinoid, small cell carcinoma, and large cell neuroendocrine carcinoma (LCNEC) [1]. LCNEC is a relatively rare histopathological subtype of lung carcinoma, which accounts for approximately 3% of all lung carcinomas [1]. It has been well recognized that this type of carcinoma as well as small cell carcinoma of the lung show an aggressive clinical course [2].
The frequency of cutaneous metastasis from visceral malignancies is relatively rare. A recent analysis has shown that the overall incidence of cutaneous metastasis is 5.3% of all internal malignancies [3], and it has been estimated to occur in 0.7 to 9% of patients with internal cancers [4-8]. Hu et al. analyzed 124 cases of cutaneous metastases from internal malignancies [4]. They found that the rate of cutaneous metastases was 1.02%, and the most common primary site was the breast, followed by the lung, oral mucosa, colorectum, stomach, and esophagus [4]. In their series, 23 cases of lung carcinoma among 1,292 primary tumors (1.78%) showed cutaneous metastasis, and the most common histopathological subtype was adenocarcinoma (16/23 cases), followed by squamous cell carcinoma (5 cases) and small cell carcinoma (1 case), while no large cell carcinoma cases showing cutaneous metastasis were documented [4]. Only two cases of cutaneous metastasis of LCNEC (one case was from the urinary bladder and the other was from the rectum) have been reported in the English-language literature [9,10]. Herein, we report the first documented case of metastatic pulmonary LCNEC in the skin.
Case report
A 55-year-old Japanese male with a 40-year history of smoking (40 cigarettes /day) presented with persistent left chest pain at an outpatient clinic. Computed tomography revealed a tumorous lesion in the left upper lung. He was referred to our hospital for evaluation of the lung tumor. Laboratory examinations showed a mild elevated level of carcinoembryonic antigen (10.9 mg/mL (range < 5)), however, other tumor markers were within normal ranges (SCC1.0 ng/mL (< 1.5), proGRP 38.0 pg/mL (< 80.9), neuron specific enolase 8.8 mg/mL (< 16.3), and CYFRA 1.2 ng/mL (< 3.5)). A bronchoscopic examination failed to detect any carcinomas, and subsequently, lobectomy of the left upper lung with lymph node dissection was performed.
His post-operative course was uneventful, however, he presented with left lower back pain 3 months after the surgery. Computed tomography revealed a metastatic carcinoma in the left iliac bone, and then radiation therapy (total 30 Gy) was performed. Five months after the surgery, a nodular lesion was detected in the scalp. Physical examination revealed a relatively well-circumscribed subcutaneous nodule in the scalp. Biopsy of the nodule was performed. Computed tomography demonstrated multiple metastatic lesions in the bilateral lungs and liver, and subsequently underwent chemotherapy (CDDP and CPT11).
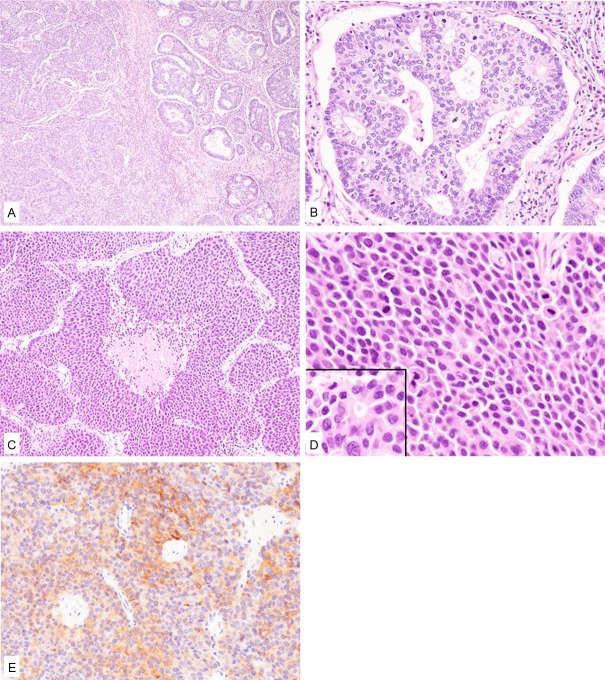
Histopathological study of the resected lung specimen demonstrated two different carcinoma components within the lesion (Figure 1A). The major component, which accounted for approximately 60% of the tumor, was an adenocarcinoma (Figure 1A and 1B). This component was composed of cribriform or tubular glands, and the atypical glandular cells had large round to oval nuclei containing small nucleoli (Figure 1A and 1B). No lepidic component was noted. Mitotic figures were frequently observed (48/10 high-power fields). The other component was composed of proliferation of variably-sized nests with or without central necrosis (Figure 1A, 1C). These neoplastic cells had relatively rich eosinophilic cytoplasm and large round to irregular-shaped nuclei (Figure 1D). Rosette formation was frequently observed (Figure 1D, inset). Mitotic figures were frequently noted (76/10 high-power fields). Both vascular and lymphatic invasions by the tumor cells were observed. This component was suspected as LCNEC. Pleural invasion was noted, however, no lymph node metastasis was present.
Figure 1.
Histopathological and immunohistochemical features of the lung tumor. A: The tumor is comprised of adenocarcinoma (right) and large cell neuroendocrine carcinoma (left) components. HE, x 40. B: The adenocarcinoma component is composed of cribriform glands consisting of atypical glandular cells containing large round to oval nuclei. HE, x 200. C: The large cell neuroendocrine carcinoma component is comprised of variably-sized nests with or without central necrosis. HE, x 100. D: Tumor cells of the large cell neuroendocrine carcinoma have relatively rich eosinophilic cytoplasm and large nuclei. Rosette formation is noted (inset). HE, x 400. E: Synaptophysin is expressed in the large cell neuroendocrine carcinoma component. x 200.
Immunohistochemical studies were performed using an autostainer (Ventana) by the same method as previously reported [11-15]. Synaptophysin and CD56 were diffusely positive in the LCNEC component (Figure 1E), but negative in the adenocarcinoma component. A few chromogranin A-positive neoplastic cells were present in the LCNEC component, but absent in the adenocarcinoma component. TTF-1 was negative in both components.
Accordingly, an ultimate diagnosis of combined LCNEC (LCNEC and adenocarcinoma) (pT3N0M0) was made.
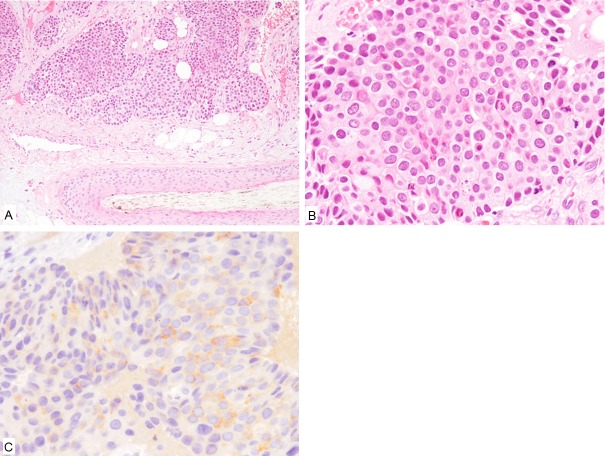
Histopathological study of the scalp nodule revealed proliferation of atypical epithelial cells that formed variably-sized nests in the subcutis (Figure 2A). The neoplastic cells had relatively rich eosinophilic cytoplasm and large round to oval nuclei (Figure 2B). Mitotic figures were frequently seen (23/5 high-power fields). No adenocarcinoma component was present.
Figure 2.
Histopathological and immunohistochemical features of the scalp nodule. A: Proliferation of variably-sized epithelial nests in the subcutis around the hair follicle. HE, x 100. B: The tumor cells have relatively rich eosinophilic cytoplasm and large round to oval nuclei. HE, x 400. C: Synaptophysin is expressed. x 400.
Immunohistochemical study revealed that these tumor cells were positive for synaptophysin and CD56 (Figure 2C), but negative for chromogranin-A and TTF-1.
Accordingly, an ultimate diagnosis of metastatic LCNEC of the lung in the scalp was made.
Discussion
In this report, we describe the first documented case of metastatic LCNEC of the lung in the skin. The lung carcinoma of the present case was combined LCNEC (LCNEC and adenocarcinoma), which is defined as a tumor that contains an LCNEC component and another non-small cell carcinoma component [1]. The incidence of combined LCNEC is very low. Ruffini et al. reported that only 0.43% of surgically resected primary lung carcinomas (5/1158 cases) were combined LCNEC (three of them had adenocarcinoma component, and the remaining two had squamous cell carcinoma component), while the incidence of combined small cell carcinoma was 1.2% [16]. Recently, Yamada et al. analyzed the clinicopathological features of combined LCNEC, and they reported that the incidence of combined LCNEC was 0.6% of all surgically resected cases [17]. The most common carcinoma component that co-existed with LCNEC was adenocarcinoma (16/25 cases), followed by squamous cell carcinoma (8 cases) [17]. In the present case, metastatic skin lesion had only a LCNEC component. This may be due to the higher malignant potential of LCNEC than adenocarcinoma of the lung.
To best of our knowledge, only two cases of cutaneous metastasis of LCNEC have been reported [9,10], although one case of LCNEC presenting with skin metastases of unknown origin has also been documented [18]. Table 1 summarizes the clinicopathological features of these three cases of metastatic LCNEC in the skin as well as the present one [9,10,18]. All patients were male, and this condition mainly affected the middle-aged, however, a case of urinary bladder LCNEC occurring in a 20-year-old male has been documented [9]. The primary site was variable, including urinary bladder and rectum, and this is the first documented case of primary lung LCNEC metastasizing to the skin. The scalp was the most common site of metastasis (3/4 cases), and the remaining one case had multiple metastases in the head, neck, trunk, and leg [18]. All cases showed positive immunoreactivity for neuroendocrine markers. TTF-1, a transcription factor that is expressed in normal thyroid and lung tissues, was expressed in one case [9], but negative in two cases including the present one [18]. Approximately 50% of lung LCNEC demonstrates TTF-1 expression [17,19,20], and it has been recognized that LCNEC arising in non-pulmonary sites sometimes shows positive immunoreactivity for TTF-1 [20]. TTF-1 expression does not necessarily indicate lung origin in LCNEC cases, therefore, clinical history and surveillance of primary site is needed.
Table 1.
Clinicopathological features of metastatic large cell neuroendocrine carcinoma in the skin
| Case No. | Age/Gender | Primary organ | Metastatic site | Immunohistochemical characteristics | Reference |
|---|---|---|---|---|---|
| 1 | 20/Male | Urinary bladder | Scalp | Synaptophysin (+), CD56 (+), TTF-1 (+) | [9] |
| 2 | 58/Male | Rectum | Scalp | CD56 (+) | [10] |
| 3 | 65/Male | Unknown | Head, neck, trunk, and leg | Synaptophysin (+), chromogranin A (+), CD56 (+), TTF-1 (-) | [18] |
| Present Case | 55/Male | Lung | Scalp | Synaptophysin (+), chromogranin A (-), CD56 (+), TTF-1 (-) |
Metastatic LCNEC in the skin must be differentiated from Merkel cell carcinoma and primary LCNEC of the skin [9,18,21]. Merkel cell carcinoma is a rare primary cutaneous carcinoma. This type of tumor is thought to originate from (or differentiate toward) Merkel cells, which are mechanoreceptors present in the epidermis and skin appendages. The typical histopathological feature of Merkel cell carcinoma is proliferation of small round cells containing vesicular chromatin and inconspicuous nucleoli, however, Merkel cell carcinomas with large cell features have been reported [22]. Therefore, differentiation from Merkel cell carcinoma is necessary, and immunohistochemical analyses are usually useful. The characteristic immunohistochemical characteristics of Merkel cell carcinoma include dot-like expression of cytokeratin 20 (86.7%) and a lack of expression of TTF-1 (3.3%), although cases of cytokeratin 20-negative/TTF-1-positive Merkel cell carcinoma have been documented [23]. Recently, it has been documented that approximately 80% of cases of Merkel cell carcinoma harbor a novel polyomavirus, referred to as Merkel cell polyomavirus (MCPyV), and immunodetection with monoclonal antibody against MCPyV large T antigen has been recognized as a useful tool in the evaluation of Merkel cell carcinoma [24]. However, MCPyV-negative Merkel cell carcinoma does exist, and interestingly, MCPyV-negative Merkel cell carcinomas morphologically have more abundant cytoplasm and more irregularly shaped nuclei than MCPyV-positive cases [24]. Moreover, only a few cases of primary cutaneous LCNEC have been reported, which shows positive immunoreactivity for synaptophysin, and lacks chromogranin A and dot-like cytokeratin 20 expression [21]. Histopathological and immunohistochemical characteristics cannot differentiate primary cutaneous LCNEC from metastatic LCNEC. Therefore, a combination of clinical history, and analyses of histopathological and immunohistochemical features is needed for correct diagnosis.
Disclosure of conflict of interest
None.
References
- 1.Brambilla E, Pugatch B, Geisinger K, Gal A, Sheppard MN, Guinee DG, Jiang SX, Lantuejoul S, Chang YL, Petersen I, Meyerson M, Hanash SM, Noguchi M. Large cell carcinoma. In: Travis WD, Bramblilla E, Muller-Hermelink HK, Harris CC, editors. World Health Organization Classification of Tumours.Pathology and Genetics of Tumours of the Lung, Pleura, Thymus, and Heart. Lyon: IARC Press; 2004. pp. 45–50. [Google Scholar]
- 2.Isaka M, Nakagawa K, Ohde Y, Okumura T, Watanabe R, Ito I, Nakajima T, Kondo H. A clinicopathological study of peripheral, small-sized high-grade neuroendocrine tumours of the lung: differences between small-cell lung carcinoma and large-cell neuroendocrine carcinoma. Eur J Cardiothorac Surg. 2012;41:841–846. doi: 10.1093/ejcts/ezr132. [DOI] [PubMed] [Google Scholar]
- 3.Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a meta-analysis of data. South Med J. 2003;96:164–167. doi: 10.1097/01.SMJ.0000053676.73249.E5. [DOI] [PubMed] [Google Scholar]
- 4.Hu SCS, Chen GS, Wu CS, Chai CY, Chen WT, Lan CCE. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009;60:379–387. doi: 10.1016/j.jaad.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228–236. doi: 10.1016/0190-9622(93)70173-q. [DOI] [PubMed] [Google Scholar]
- 6.Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma: A retrospective study of 7316 cancer patients. J Am Acad Dermatol. 1990;22:19–26. doi: 10.1016/0190-9622(90)70002-y. [DOI] [PubMed] [Google Scholar]
- 7.Spencer PS, Helm TN. Skin metastases in cancer patients. Cutis. 1987;39:119–121. [PubMed] [Google Scholar]
- 8.Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer. 1950;3:74–85. doi: 10.1002/1097-0142(1950)3:1<74::aid-cncr2820030111>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Lee WJ, Kim CH, Chang SE, Lee MW, Choi JH, Moon KC, Koh JK. Cutaneous metastasis from large-cell neuroendocrine carcinoma of the urinary bladder expressing CK20 and TTF-1. Am J Dermatopathol. 2009;31:166–169. doi: 10.1097/DAD.0b013e31818eba4c. [DOI] [PubMed] [Google Scholar]
- 10.Lee WJ, Oh SH, Chang SE, Lee MW, Choi JH, Moon KC, Koh JK. Skin metastasis of neuroendocrine carcinoma arising in the rectum. Ann Dermatol. 2007;19:163–165. [Google Scholar]
- 11.Ishida M, Iwai M, Yoshida K, Kagotani A, Okabe H. Sebaceous carcinoma associated with Bowen’s disease: a case report with emphasis on the pathogenesis of sebaceous carcinoma. Int J Clin Exp Pathol. 2013;6:3029–3032. [PMC free article] [PubMed] [Google Scholar]
- 12.Toriyama A, Ishida M, Amano T, Nakagawa T, Kaku S, Iwai M, Yoshida K, Kagotani A, Takahashi K, Murakami T, Okabe H. Leiomyomatosis peritonealis disseminata coexisting with endometriosis within the same lesions: a case report with review of the literature. Int J Clin Exp Pathol. 2013;6:2949–2954. [PMC free article] [PubMed] [Google Scholar]
- 13.Ishida M, Hodohara K, Yoshida K, Kagotani A, Iwai M, Yoshii M, Okuno K, Horinouchi A, Nakanishi R, Harada A, Yoshida T, Okabe H. Occurence of anaplastic large cell lymphoma following IgG4-related autoimmune pancreatitis and cholecystitis and diffuse large B-cell lymphoma. Int J Clin Exp Pathol. 2013;6:2560–2568. [PMC free article] [PubMed] [Google Scholar]
- 14.Ishida M, Yoshida K, Kagotani A, Iwai M, Yoshii M, Okuno K, Horinouchi A, Nakanishi R, Harada A, Yoshida T, Okuno T, Hodohara K, Okabe H. Anaplastic lymphoma kinase-positive large B-cell lymphoma: A case report with emphasis on the cytological features of the pleural effusion. Int J Clin Exp Pathol. 2013;6:2631–2635. [PMC free article] [PubMed] [Google Scholar]
- 15.Ishida M, Hodohara K, Yoshii M, Okuno H, Nakanishi R, Horinouchi A, Nakanishi R, Harada A, Iwai M, Yoshida K, Kagotani A, Yoshida T, Okabe H. Methotrexate-related Epstein-Barr virus-associated lymphoproliferative disorder occurring in the gingiva of a patient with rheumatoid arthritis. Int J Clin Exp Pathol. 2013;6:2237–2241. [PMC free article] [PubMed] [Google Scholar]
- 16.Ruffini E, Rena O, Oliaro A, Filosso PL, Bongiovanni M, Arslanian A, Papalia E, Maggi G. Lung tumors with mixed histologic pattern. Clinico-pathologic characteristics and prognostic significance. Eur J Cardiothorac Surg. 2002;22:701–707. doi: 10.1016/s1010-7940(02)00481-5. [DOI] [PubMed] [Google Scholar]
- 17.Yamada K, Miyagi Maeshima A, Tsuta K, Tsuda H. Combined high-grade neuroendocrine carcinoma of the lung: clinicopathological and immunohistochemical study of 34 surgically resected cases. Pathol Int. 2014;64:28–33. doi: 10.1111/pin.12127. [DOI] [PubMed] [Google Scholar]
- 18.Shin MK, Choi CM, Oh YJ, Kim NI. CK20 positive large-cell neuroendocrine carcinoma presenting with skin metastases. Ann Dermatol. 2011;23:S20–S24. doi: 10.5021/ad.2011.23.S1.S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sturm N, Rossi G, Lantuejoul S, Papotti M, Frachon S, Claraz C, Brichon PY, Brambilla C, Brambilla E. Expression of thyroid transcription factor-1 in the spectrum of neuroendocrine cell lung proliferations with special interest in carcinoids. Hum Pathol. 2002;33:175–182. doi: 10.1053/hupa.2002.31299. [DOI] [PubMed] [Google Scholar]
- 20.Kaufmann O, Dietel M. Expression of thyroid transcription factor-1 in pulmonary and extrapulmonary small cell carcinomas and other neuroendocrine carcinomas of various primary sites. Histopathology. 2000;36:415–420. doi: 10.1046/j.1365-2559.2000.00890.x. [DOI] [PubMed] [Google Scholar]
- 21.Kasami M, Muramatsu K, Kawahata K, Yoshikawa S, Kiyohara Y. Large-cell neuroendocrine carcinoma of the skin, with lymphoid stroma. Am J Dermatopathol. 2007;29:578–580. doi: 10.1097/DAD.0b013e318159b45e. [DOI] [PubMed] [Google Scholar]
- 22.Skelton HG, Smith KJ, Hitchcock CL, McCarthy WF, Lupton GP, Graham JH. Merkel cell carcinoma: analysis of clinical, histologic, and immunohistologic features of 132 cases with relation to survival. J Am Acad Dermatol. 1997;37:734–739. doi: 10.1016/s0190-9622(97)70110-5. [DOI] [PubMed] [Google Scholar]
- 23.Ishida M, Okabe H. Merkel cell carcinoma concurrent with Bowen’s disease: two cases, one with an unusual immunophenotype. J Cutan Pathol. 2013;40:839–843. doi: 10.1111/cup.12176. [DOI] [PubMed] [Google Scholar]
- 24.Kuwamoto S. Recent advances in the biology of Merkel cell carcinoma. Hum Pathol. 2011;42:1063–1077. doi: 10.1016/j.humpath.2011.01.020. [DOI] [PubMed] [Google Scholar]