Abstract
The aim of the present study is to confirm the value of electronic bronchoscopy-aided catheter aspiration technique with liquid-based cytological test in the diagnosis of bronchogenic carcinoma. A total of 815 patients of lung cancer were evaluated by bronchoscopy between February 2011 and June 2012. Catheter aspiration technique and forceps biopsy during bronchoscopy were employed to obtain adequate tissue specimens. Liquid-based cytological test and conventional smears for catheter aspiration were used for cytological detection of the tumors. For all cytological specimens, slide preparations with LCT and CS were reviewed by two senior pathologists, who were blinded to patient medical history. Complications related to electronic bronchoscopy, such as bleeding, were clinically judged as light, moderate or severe by the needs for clinical interventions. The diagnostic yield of catheter aspiration in endobronchial visible lesions (tumor, infiltrative and necrotic lesions) was 94.6% (success rates concerning malignancy), which was slightly higher than that of the forceps biopsy (91.4%, P < 0.05). The diagnostic yield of catheter aspiration in endobronchial invisible lesions (normal, compressive and nonspecific lesions) was 82.8%, which was significantly higher than that of the forceps biopsy (51.4%, P < 0.01). The combination of the forceps biopsy with the cytological analysis of the catheter aspiration increased the diagnostic sensitivity in both lesion types (P < 0.05). For catheter aspiration, the positive rate of lung cancer by liquid-based cytological test was superior to that by conventional smears (P < 0.05). The catheter aspiration is a sampling technique that produces higher diagnostic rate for lung cancers compared with forceps biopsy. Liquid-based cytological test is routinely applicable for the diagnosis of lung cancer using samples collected through electronic bronchoscopy.
Keywords: Electronic bronchoscope, lung cancer, catheter aspiration, sampling technique, liquid-based cytology test
Introduction
Electronic bronchoscopy is the most commonly used technique for diagnosis of lung cancer [1-3]. Bronchial washing, brushing plus forceps biopsy via flexible bronchoscopy have been routinely used to obtain adequate specimens from the endoscopically visible or invisible lung cancers [4]. Forceps biopsy provides the best diagnosis for patients with endoscopically visible lung cancer. However, the positive rate of biopsy is low in the absence of visible endobronchial abnormalities. Additional cell brushings and washings generally result in low diagnosis sensitivity for lung cancer, and some researchers suggest that these procedures are not carried out routinely for the suspected lung cancer [5].
Another valuable and minimally invasive cytological technique, transbronchial catheter aspiration, is underutilized, although it is continued in modernized techniques [6]. Despite its simplicity and low cost [7], the catheter aspiration technique is not well known. There are only a few articles discussing its diagnostic efficiency in peripheral pulmonary lesions [6-8]. A study showed that transbonchial catheter aspiration as well as needle aspiration was the most utilized cytological technique by only 28% physicians [9]. In our study, we aim to evaluate the diagnostic efficiency of catheter aspiration cytological analysis and to determine whether the sensitivity of the diagnosis depends on the bronchoscopic appearance and histological type of the lesions. In addition to conventional smears (CS), the use of liquid-based cytology preparations has been introduced recently [10-12]. Another aim of our study is to determine the impact of the liquid-based cytological test (LCT) on the diagnosis by catheter aspiration.
Materials and methods
Subjects
From February 2011 to June 2012, 1365 patients with pulmonary nodules and masses underwent a flexible electronic bronchoscopy at the First Affiliated Hospital, Chongqing Medical University, China. Written informed consent was obtained from all participants. If the patient was not older than 18 years, the written informed consent was obtained from his/her parents. Patients who had contraindications or could not provide informed consents were excluded from the examination. In addition, patients were not enrolled if they presented benign lung diseases (such as granuloma and pulmonary infectious diseases) according to electronic bronchoscopy or other examinations. In total, 815 patients with definitive diagnosis of lung cancer were included in this study. The study was approved by the local ethics committee of the First Affiliated Hospital of Chongqing Medical University, China.
Electronic bronchoscopy
Before bronchoscopy, every patient underwent detailed historical and physical examinations including chest and systematic clinical examinations and routine laboratory tests, particularly a hemogram with coagulation tests. The patients were given local anesthesia with lidocaine (2% solution), and dolantin was used in minimal amount. Bronchoscopy was performed under direct visualization using an electronic bronchoscope (PENTAX FB-1970Ks, Japan) for all diagnostic procedures. Specimens were obtained via extended working channel by catheter suction (Olympus PR-2B, 2.0 mm in diameter) and biopsy forceps (Olympus FB21C-1, 120 cm in length). The forceps biopsy technique was performed in the standard fashion after catheter suction. All lesions were sampled five times by forceps afterwards unless technical or patient limitations precluded diagnostic tissue biopsies. In this study, forceps biopsy did not obtain tissue specimens in 5 cases due to complications. Then, all biopsy specimens were fixed with 10% buffered formalin before being sent to pathology laboratory.
Catheter aspiration technique for cytological specimen
In every case, catheter aspiration technique (Figure 1) was performed. The technique included moving the catheter back and forth in a definitive lesion or aspirating blindly from segments or bronchial openings corresponding to radiological opacities if there were no endobronchial abnormalities. The continued suction was applied with a 20 ml syringe until a satisfactory macroscopic specimen was obtained, which was decided by the bronchoscopist of each case. For each patient, at least one cytological specimen was obtained. One part of the material from catheter aspiration sampling technique was smeared immediately on two glass slides, namely conventional smear method, and the remaining was collected into a small bottle containing liquid-based cytology reagents (An Bi Ping, Guangzhou, China) for liquid-based cytological preparation. For all cytological specimens, slides were prepared for each patient by both liquid-based and conventional preparations. All histological and cytological specimens were stained with hematoxylin and eosin (HE) using standard procedure.
Figure 1.
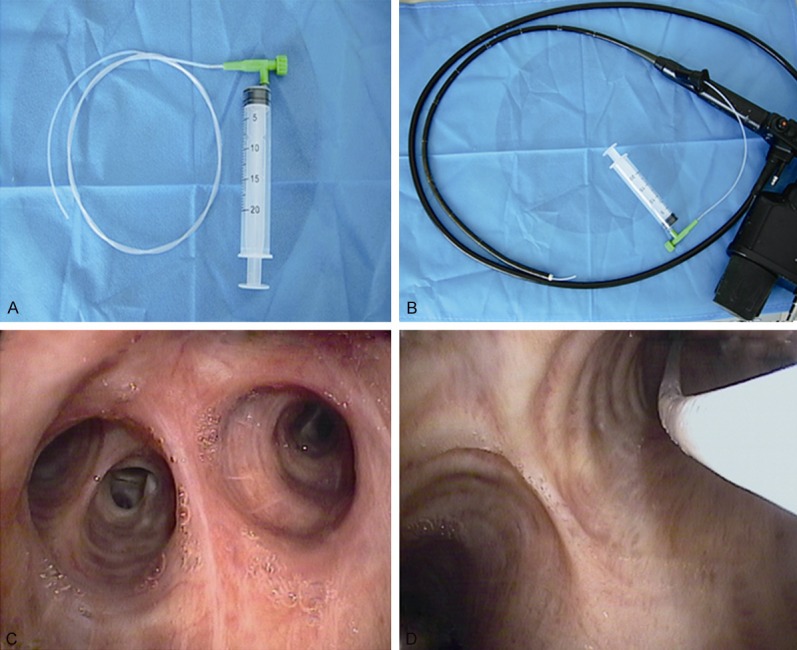
Catheter aspiration technique. A. A hard suction catheter with a diameter of 2 mm, one end of which was connected to a 20-ml syringe. B. A hard suction catheter that was connected to a syringe was threaded through bronchofiberscope biopsy hole. C. A lesion site. D. A hard suction catheter that was threaded through the bronchofiberscope biopsy hole was inserted into the lesion site to draw suction specimens under negative pressure. Continued suction including back-and-forth movement of the catheter was applied with the syringe, until satisfactory macroscopic specimens were collected via the hard suction catheter.
Conventional method
Two glass slides overlapping each other on the side of the sample were pushed together, and then fixed with 95% ethanol immediately for at least 15 minutes. The standard procedure of usual staining of the glass slides with spread smear was used.
Liquid-based cytological method
The material collected in the liquid fixative was further processed after a minimum duration of 24 hours. The sample was mixed properly before being transferred to a clean test tube and centrifuged at 1,000 rpm for 5 minutes. After removing the supernatant, Tris buffer solution (2 ml, pH = 7.2) was added to the deposit. The mixture was further centrifuged at 2,000 rpm (600-800 g) for 5-10 minutes. Again, the supernatant was discarded and the deposit was mixed with Tris buffer solution (2 ml, pH = 7.2). A thin layer of the cells was deposited naturally onto a special slide carrying the mixture, followed by HE staining.
HE staining
For conventional smear preparations, conventional smear glass slides were fixed with 95% ethanol for at least 15 minutes, and then treated with water for 1 minute, hematoxylin for 10 minutes, running water for 15 minutes, eosin for 30 seconds, 95% ethanol for 1 minute, and 100% ethanol for 2 minutes. Stained slides were cover-slipped with Permount. Finally, the entire HE stained cells were examined under a bright light microscope using 100-400× magnification.
For liquid-based cytological preparations, a thin layer of the cells was deposited naturally onto a special slide for 10-15 minutes, and the supernatant was discarded. The slides were then washed with 95% ethanol for three times, soaked with hematoxylin for 5 minutes and water for 10 minutes, and washed with eosin for 15 seconds, 95% ethanol for 3 times, and 100% ethanol for 1 time. Stained slides were cover-slipped with Permount. Finally, the entire HE stained cells were examined under a bright light microscope using 100-400× magnification.
Complications related to electronic bronchoscopy
In this study, patients’ symptoms were observed and recorded in detail during and after the bronchoscopy. Complications were classified as minor or major according to guidelines by the British Thoracic Society [13]. Bleeding was clinically judged as light, moderate or severe by the needs for clinical interventions [14]. After obtaining samples, we analyzed possible related complications such as bleeding and pneumothorax.
Pathological diagnosis standards
For all cytological specimens, slide preparations with LCT and CS were reviewed by two senior pathologists, who were blinded to patient medical history. Discrepancies were discussed to reach a consensus diagnosis. Immunohistochemistry staining was performed when it was difficult to differentiate some histological types of the lung cancer. Diagnostic sensitivity (concerning malignancy) was defined as the number of true positive results/(true positive results + false negative results) × 100%, in which true or false represented match or mismatch between the histological and the cytological results, respectively.
Histological and cytological specimens were only classified as positive when cellular morphological features showed obvious malignant characteristics. All the other interpretations including the marked atypia were pooled together as the negative data for statistical purposes. If some patients were not given a diagnosis with the results of their first bronchoscopic examination, they were followed up until either a definitive diagnosis was obtained or the diagnosis was verified by other standard techniques, such as post-bronchoscopy sputum cytology, re-bronchoscopy, pleural effusion cytology, computed tomography-guided fine needle aspiration, and surgery, etc.
Statistical analysis
The diagnosis of these sampling techniques in bronchoscopy for lung cancer was compared by the McNemar’s test and stratified based on bronchoscopic appearance. P < 0.05 was considered statistically significant.
Results
The diagnostic modality and histological type can be determined by electronic bronchoscopy
To investigate the diagnostic modality and histological type, electronic bronchoscopy was performed on 1365 patients. Electronic bronchoscopic images showed that lung cancer occurred in 815 cases (Table 1). The combination of the catheter aspiration and the forceps biopsy during bronchoscopy in the 815 patients confirmed the diagnosis of lung cancer in 756 (92.8%) cases, in which 734 cases were diagnosed by catheter aspiration alone, and 619 cases (75.9%) were diagnosed by forceps biopsy alone. Fifty-nine patients (7.2%) were diagnosed by other procedures, such as post-bronchoscopy sputum cytology, re-bronchoscopy, pleural effusion cytology, computed tomography-guided fine needle aspiration, or surgery, etc. Histological investigations showed that 689 patients (84.6%) had non-small-cell carcinoma, 110 (13.5%) had small-cell carcinoma, and 16 (2.0%) had unclassified cell types. The bronchoscopic appearance of the lesions was classified as the following: tumor (443 cases, 61.4%), infiltration (126 cases, 17.5%), compression (69 cases, 9.6%) and normal (84 cases, 11.6%) (Table 2). These data indicated that the diagnostic modality and histological type could be determined by electronic bronchoscopy.
Table 1.
Different bronchoscopic sampling techniques for the confirmation of pulmonary malignant tumors
| Pulmonary malignant tumors | 815 |
| With bronchoscopy (catheter aspiration and or forceps biopsy) | 756 (92.8) |
| With catheter aspiration alone | 734 (90.1) |
| With forceps biopsy alone* | 619 (80.0) |
| With other sampling techniques# | 59 (7.2) |
Note: Data are presented as n (%).
failure to obtain specimens from 5 cases due to complications.
post-bronchoscopy sputum cytology, re-bronchoscopy, pleural effusion cytology, computed tomography-guided fine needle aspiration, or surgery, etc.
Table 2.
Characteristics of patients included
| No. of patients | 815 |
| Age (range) | 66 (16-85) |
| Gender (male %) | 475 (76.8) |
| Site of bronchoscopic sampling (%) | |
| Trachea | 3 (0.36) |
| Main bronchus | 56 (6.9) |
| Lobar bronchus | 443 (54.4) |
| Segmental bronchus | 187 (22.9) |
| Peripheral lesion | 126 (15.5) |
| Bronchoscopic appearance (%) | |
| Tumor | 296 (36.3) |
| Infiltrative | 155 (19.0) |
| Necrotic | 49 (6.0) |
| Compressive | 101 (12.4) |
| Nonspecific | 79 (9.6) |
| Normal | 135 (16.5) |
| Histological type (%) | |
| Non-small-cell carcinoma | 689 (84.6%) |
| Squamous cell carcinoma | 286 (35.1%) |
| Adenocarcinoma | 352 (43.2%) |
| Other Non-small-cellcarcinoma | 51 (6.3%) |
| Small-cell carcinoma | 110 (13.5%) |
| Unclassified carcinoma | 16 (1.9%) |
Diagnostic sensitivity of catheter aspiration is higher than that of forceps biopsy, but lower than the combination of both of them
To compare the diagnosis between forceps biopsy and catheter aspiration, the bronchoscopic appearance of the lesions was investigated. Tissue pathology and suction-catheter aspiration cytology analysis was outlined in Table 3. Catheter-aspiration cytology alone resulted in significantly increased diagnosis rate (90.1%) when compared to separate forceps biopsy (75.9%) (P < 0.01). When forceps biopsy method was evaluated in combination with catheter aspiration, the total diagnostic sensitivity for the detection of lung cancer was 92.8%, which was significantly higher than that of the forceps biopsy alone (75.9%, P < 0.01). When endobronchial lesions (tumor, infiltrative, and necrotic) were compared with peribronchial lesions (compressive, nonspecific, and normal) regarding bronchial findings, the diagnostic sensitivity of catheter aspiration was significantly higher than that of forceps biopsy for both of the lesion types (P < 0.05). Similarly, when patients were statistically stratified based on central and peripheral lesions, the diagnostic sensitivity of catheter aspiration was increased compared to that of forceps biopsy in both lesion types (P < 0.01). When combining catheter aspiration and forceps biopsies for the diagnosis and typing of centrally located, endobronchial, primary lung cancers, the diagnostic sensitivity of electronic bronchoscopy reached 98.6%, which was close to 100% (Table 3).
Table 3.
Diagnosis by forceps biopsy and catheter aspiration cytology
| Forceps biopsy alone | Catheter aspiration alone | Combination of forceps biopsy and catheter aspiration | P value | |
|---|---|---|---|---|
| Overall | 75.9 (619/815) | 90.1 (734/815) | 92.8 (756/815) | < 0.001 |
| Endobronchoscopic appearance | ||||
| Tumor | 94.9 (281/296) | 94.2 (279/296) | 99.7 (295/296) | 0.001 |
| Infiltration | 85.2 (132/155) | 96.1 (146/155) | 96.1 (149/155) | 0.001 |
| Necrotic | 89.8 (44/49) | 98.0 (48/49) | 100.0 (49/49) | 0.030 |
| Compression | 69.2 (69/101) | 88.1 (89/101) | 90.1 (91/101) | < 0.001 |
| Nonspecific | 52.1 (41/79) | 87.3 (69/79) | 87.3 (69/79) | < 0.001 |
| Normal | 38.5 (52/135) | 76.3 (103/135) | 76.3 (103/135) | < 0.001 |
| Bronchoscopic morphology | ||||
| Endobronchial lesions | 91.4 (457/500) | 94.6 (473/500) | 98.6 (493/500) | < 0.001 |
| Peribronchial lesions | 51.4 (162/315) | 82.8 (261/315) | 83.5 (263/315) | < 0.001 |
| Sampling site | ||||
| Centrally located | 80.8 (557/689) | 92.5 (637/689) | 95.9 (660/689) | < 0.001 |
| Peripherally located | 49.2 (62/126) | 76.7 (97/126) | 76.7 (97/126) | < 0.001 |
Note: Data are presented as the number of positive samples/all samples (%).
In endobronchial lesions (tumor, infiltrative and necrotic), the combination of two techniques was diagnostic in 98.6% cases (493/500), which was slightly higher than catheter aspiration alone (473/500 cases, 94.6%), and forceps biopsy alone (457/500 cases, 91.4%). For peribronchial lesions (nonspecific, compressive, and normal), the total diagnostic sensitivity of these procedures was 83.5% (263/315), whereas that of catheter aspiration alone was 82.8% (261/315), which is obviously higher than that of the forceps biopsy (51.4%, 162/315) (P < 0.01) (Table 3). These results indicated that the diagnostic sensitivity of catheter aspiration was higher than that of forceps biopsy, but lower than the combination of both of them.
Diagnostic sensitivities of LCT alone and the combination of LCT and CS were higher than that of CS alone
To determine which one of LCT or CS had a higher diagnostic sensitivity, we compared LCT and CS on the diagnosis of SCC, ADC and SCLC. In general, LCT processing reduced the duration and increased the efficiency of screening, with cleaner backgrounds and fewer cell conglomerations (Figure 2). Of the 815 samples from patients with lung cancer examined by CS, 688 were found to have cancer cells, and the diagnostic sensitivity was 84.4% (688/815). LCT showed that a clearer background and well-preserved cell morphology could account for 89.6% (730/815) of the diagnostic sensitivity. The difference in diagnostic sensitivity between the two methods was significant (P < 0.05). When CS and LCT were evaluated in combination, the diagnostic sensitivity for detecting lung cancer was 90.1% which was significantly higher than that of the CS method alone (84.4%, P < 0.01) (Table 4).
Figure 2.
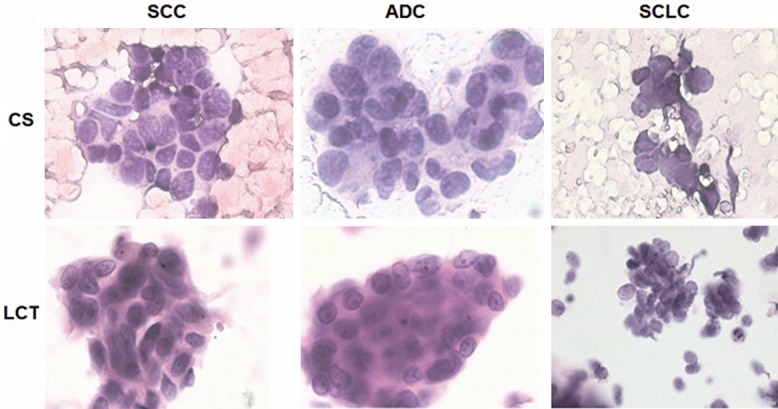
Microscopic investigation of slides of SCC, ADC and SCLC prepared for CS and LCT. CS, conventional smears; LCT, liquid-based cytological test; SCC, squamous cell carcinoma; ADC, adenocarcinoma; SCLC, small-cell lung carcinoma. For each tumor type, the samples were obtained from the same patient. The samples were stained with Hematoxylin & Eosin, and investigated under the microscope (400×).
Table 4.
Comparison of the liquid-based cytological test (LCT), conventional smear (CS) method, and the combination of LCT and the CS for the detection of lung cancer in 815 patients
| Pathology | N | LCT Positive (%) | CS Positive (%) | LCT and CS Positive (%) | P value |
|---|---|---|---|---|---|
| SCC | 286 | 273 (95.5) | 268 (93.7%) | 273 (95.5) | 0.600 |
| ADC | 352 | 293 (83.2) | 281 (79.8%) | 296 (84.1) | 0.291 |
| SCLC | 110 | 105 (95.4) | 83 (75.5%) | 106 (96.4) | < 0.001 |
| Other* | 67 | 59 (88.1) | 56 (83.6%) | 59 (88.1) | 0.680 |
| Total | 815 | 730 (89.6) | 688 (84.4%) | 734 (90.1) | 0.001 |
non-small-cell carcinoma and unclassified carcinoma were included.
SCC, squamous cell carcinoma; ADC, adenocarcinoma; SCLC, small-cell lung carcinoma.
Of the 273 positive SCC cases determined by LCT, 268 were confirmed positive by CS. When LCT and CS were combined, the diagnostic sensitivity for the detection of SCC was 95.5%, without significant difference compared with either method alone (P > 0.05) (Table 4).
Of the 293 positive ADC cases determined by LCT, 281 were confirmed positive by CS. There was significant difference in diagnostic sensitivity between the two methods (P < 0.05). When the LCT and CS method were combined, the diagnostic sensitivity for the ADC was 84.1%, which was significantly higher than that of the CS method alone (79.8%, P < 0.05) (Table 4).
Of the 105 positive SCLC cases determined by LCT, 83 were confirmed positive by CS. The diagnostic sensitivity of LCT to detect SCLC was 95.4%, which was significantly higher than that of CS method alone (75.5%, P < 0.05) (Table 4).
These results suggested that the diagnostic sensitivities of LCT alone and the combination of LCT and CS were higher than that of CS alone.
Catheter aspiration and forceps biopsy have little chance to lead to complications that are more severe than moderate
To evaluate the safety of catheter aspiration and forceps biopsy, we observed the occurrence of bleeding, the common complication for catheter aspiration and forceps biopsy. Mild bleeding was observed in 38 cases (4.7%) with catheter aspiration and 129 cases (15.8%) with forceps biopsy (P < 0.05), and moderate bleeding was observed in 1 case with forceps biopsy, but none required further action except for the administration of noradrenaline. Severe bleeding was not observed in any case and no pneumothorax or death occurred during the diagnostic procedure. The results suggested that catheter aspiration and forceps biopsy had little chance to lead to complications that were more severe than moderate.
Discussion
Forceps biopsy is a conventional bronchoscopy procedure used to obtain tissue biopsy from endobronchial tumors. However, small samples usually lead to variable and low diagnostic yield [15,16]. The total diagnostic yield of forceps biopsy in the present study was 75.9% (success rates concerning malignancy). This study showed that the diagnostic yield depended on the bronchoscopic appearance of the lesions. For lesions with endobronchial tumors such as infiltrative and necrotic lesions the diagnostic yield of forceps biopsy was high (91.4%), while for lesions without definite endobronchial appearance such as compression, nonspecific or normal lesion, the diagnostic yield of forceps biopsy was 51.4%, which was similar to the results of previous studies [17].
Unlike bronchial brushing, catheter aspiration and needle aspiration are underutilized for different reasons. Despite the fact, they are the only procedures that have significantly higher sensitivity than forceps biopsy. A few studies have shown that catheter aspiration appears to be efficient for diagnosis of peripheral lung carcinoma with a relatively good diagnostic yield independent of the lesion size [7,8,18]. Combining both the endobronchial ultrasound and the electromagnetic navigation bronchoscopy, the yield of catheter aspiration without fluoroscopic guidance was 90%. Interestingly, in lesions that were not seen via ultrasound, the diagnostic yield of catheter suction was 100% compared to 33% for forceps biopsy [6].
The use of catheter aspiration may retrieve larger sizes of samples with better quality. This study confirmed previous reports that cytological analysis by catheter aspiration had a better diagnostic yield than forceps biopsy alone in patients with peribronchial lung cancer [6-8,18]. In addition, the study also showed that catheter aspiration increased the diagnostic yield of centrally-located lung cancer, and the diagnostic yield was different depending on the bronchoscopic appearance of the lesion, which was determined by its endoscopic morphology. Generally speaking, endobronchial lesions classified as tumor or infiltration had higher diagnostic yields for catheter aspiration cytological analysis than peribronchial lesions such as the normal, nonspecific and compressive lesions. For lesions with endobronchial tumors, the diagnostic yield of catheter aspiration reached 94.6%, and there was statistical difference compared with forceps biopsy, which was higher than that of brushing and washing reported in other articles [19,20]. For lesions with no definite endobronchial appearance, the diagnostic yield of catheter aspiration was superior to forceps biopsy and other sampling procedures, such as brushing and washing [1,2]. A combination of catheter aspiration and forceps biopsy resulted in an increase in the total positive rate.
Higher diagnostic sensitivity of catheter aspiration for lung cancer was due to several reasons. First, catheter aspiration allows not only the collection of more sample material from local lesions, but also from a larger area of interest, such as distal bronchial materials. Second, opening of the forceps becomes more difficult as the airways become narrower, while the tip of the suction catheter functions like a curette when moving back and forth, and the volume of specimens is increased by continuous suction. Third, higher flexibility of catheter allows better access to strongly distorted subsegmental ostia, such as the apicoposterius segmentum of lobi superior and the dorsal segment of lobi inferior, into which biopsy forceps and cell brush are difficult to be placed. In addition, catheter aspiration can also be used instead of forceps or cell brushes to obtain materials from the sites of bleeding to avoid the risk of massive hemorrhage. Fourth, the mucin of cancer cells reduces or prevents cadherin synthesis, resulting in reduced adherence of cancer cells compared with homologous normal tissues [21]. Therefore, cancerous tissues around the suction catheter can exfoliate easily and enter the suction catheter under small negative pressure.
Flexible bronchoscopy is a minimally invasive procedure. In a large retrospective study, minor bronchoscopy-related complication rate was 0.8% and major life-threatening complication rate was 0.5% [22,23]. In our study, flexible bronchoscopy showed no major life-threatening complication, but had a higher diagnostic yield.
For the diagnosis of lung cancer, cytological pathology is an important supplement for histological pathology. Specially, cytopathology is the only means for the diagnosis of lung cancers [24-26] in samples that cannot be retrieved via flexible bronchoscopy from many peripheral lung cancers or in the presence of complications. For 5 cases in this study, no biopsy specimen was obtained and only cytological samples obtained by catheter aspiration technique were available.
Liquid-based thin layer cytological preparation produces a significant breakthrough. The LCT method can provide more information for lung cancer diagnosis and decrease false negative results [27,28]. Compared to CS, LCT processing kit can enrich tumor cells effectively to establish a confirmatory diagnosis through low-speed centrifugal filtration, which removes non-diagnostic materials, such as red blood cells and necrotic materials, mucus, etc.
In this study, LCT had significantly higher diagnostic sensitivity for lung cancer (89.6%) compared with CS (84.4%, P < 0.05). Especially, the present study showed that with LCT increased the diagnostic sensitivity for SCLC from 75.5% (CS) to 95.4%. The diagnostic sensitivity for SCLC was better than that for non-SCLC (P < 0.05).
To summarize, catheter aspiration under bronchoscopy is easy to perform and more successful than forceps for lung cancer. Therefore, the procedure should be performed during all bronchoscopy cases, even if the bronchoscopy reveals no definite endobronchial lesions or mucosal changes. The LCT processing kit can achieve better diagnostic yield for catheter aspiration technique than CS.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81101216), Chongqing Municipal Education Commission (KJ100310) and State Key Clinical Specialty Construction Project Funding ([2012] 949).
Disclosure of conflict of interest
None.
References
- 1.Baaklini WA, Reinoso MA, Gorin AB, Sharafkaneh A, Manian P. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest. 2000;117:1049–1054. doi: 10.1378/chest.117.4.1049. [DOI] [PubMed] [Google Scholar]
- 2.Mak VH, Johnston ID, Hetzel MR, Grubb C. Value of washings and brushings at fibreoptic bronchoscopy in the diagnosis of lung cancer. Thorax. 1990;45:373–376. doi: 10.1136/thx.45.5.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JH, Min D, Song SH, Lee JH, Jeong HC, Kim EK. Endobronchial metastases from extrathoracic malignancies: recent 10 years’ experience in a single university hospital. Tuberc Respir Dis (Seoul) 2013;74:169–176. doi: 10.4046/trd.2013.74.4.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chau CH, Yeu WW, Wong PC, Lee J, Wong CF. Usefulness of collecting routine cytologic specimens during fiberoptic bronchoscopy for endoscopically visible and nonvisible lung carcinoma. Chest. 1997;111:522–523. doi: 10.1378/chest.111.2.522. [DOI] [PubMed] [Google Scholar]
- 5.Griffin JP, Zaman MK, Niell HB, Tolley EA, Cole FH Jr, Weiman DS. Diagnosis of lung cancer: a bronchoscopist’s perspective. J Bronchology Interv Pulmonol. 2012;19:12–18. doi: 10.1097/LBR.0b013e3182425b5d. [DOI] [PubMed] [Google Scholar]
- 6.Eberhardt R, Morgan RK, Ernst A, Beyer T, Herth FJ. Comparison of suction catheter versus forceps biopsy for sampling of solitary pulmonary nodules guided by electromagnetic navigational bronchoscopy. Respiration. 2010;79:54–60. doi: 10.1159/000232394. [DOI] [PubMed] [Google Scholar]
- 7.Franke KJ, Nilius G, Ruhle KH. Transbronchial catheter aspiration compared to forceps biopsy in the diagnosis of peripheral lung cancer. Eur J Med Res. 2009;14:13–17. doi: 10.1186/2047-783X-14-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franke KJ, Nilius G, Rühle KH. Transbronchial biopsy in comparison with catheter aspiration in the diagnosis of peripheral pulmonary nodules. Pneumologie (Stuttgart, Germany) 2006;60:7–10. doi: 10.1055/s-2005-919107. [DOI] [PubMed] [Google Scholar]
- 9.Franke KJ, Nilius G, Ruhle KH. Frequency of cytological procedures in diagnostic bronchoscopy of peripheral pulmonary modules and masses. Pneumologie. 2006;60:663–666. doi: 10.1055/s-2006-944263. [DOI] [PubMed] [Google Scholar]
- 10.Gauchotte G, Vignaud JM, Ménard O, Wissler MP, Martinet Y, Siat J, Paris C, Clément-Duchêne C. A combination of smears and cell block preparations provides high diagnostic accuracy for endobronchial ultrasound-guided transbronchial needle aspiration. Virchows Arch. 2012;461:505–512. doi: 10.1007/s00428-012-1296-x. [DOI] [PubMed] [Google Scholar]
- 11.Li XJ, Zhao J, Wang P, Wu Q, Li JY, Wang J, Li XH. Comparative analysis of cytopathologic and histopathologic diagnosis in the transbronchial needle aspiration specimens. Zhonghua Bing Li Xue Za Zhi. 2012;41:400–404. doi: 10.3760/cma.j.issn.0529-5807.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi Y, Uehara T, Ota H. Liquid-based thin-layer cytology can be routinely used in samples obtained via fiberoptic bronchoscope. Acta Cytol. 2011;55:69–78. doi: 10.1159/000320872. [DOI] [PubMed] [Google Scholar]
- 13.British Thoracic Society Bronchoscopy Guidelines Committee aSoSoCCoBTS. British Thoracic Society guidelines on diagnostic flexible bronchoscopy. Thorax. 2001;56(Suppl 1):1–21. doi: 10.1136/thorax.56.suppl_1.i1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herth FJ, Becker HD, Ernst A. Aspirin does not increase bleeding complications after transbronchial biopsy. Chest. 2002;122:1461–1464. doi: 10.1378/chest.122.4.1461. [DOI] [PubMed] [Google Scholar]
- 15.Hetzel J, Eberhardt R, Herth FJ, Petermann C, Reichle G, Freitag L, Dobbertin I, Franke KJ, Stanzel F, Beyer T, Möller P, Fritz P, Ott G, Schnabel PA, Kastendieck H, Lang W, Morresi-Hauf AT, Szyrach MN, Muche R, Shah PL, Babiak A, Hetzel M. Cryobiopsy increases the diagnostic yield of endobronchial biopsy: a multicentre trial. Eur Respir J. 2012;39:685–690. doi: 10.1183/09031936.00033011. [DOI] [PubMed] [Google Scholar]
- 16.Rubio ER, le SR, Whatley RE, Boyd MB. Cryobiopsy: should this be used in place of endobronchial forceps biopsies. Biomed Res Int. 2013;2013:730574. doi: 10.1155/2013/730574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schreiber G, McCrory DC. Performance characteristics of different modalities for diagnosis of suspected lung cancer: summary of published evidence. Chest. 2003;123(Suppl 1):115S–128S. doi: 10.1378/chest.123.1_suppl.115s. [DOI] [PubMed] [Google Scholar]
- 18.Peschke A, Wiedemann B, Höffken G, Koschel D. Forceps biopsy and suction catheter for sampling in pulmonary nodules and infiltrates. Eur Respir J. 2012;39:1432–1436. doi: 10.1183/09031936.00024111. [DOI] [PubMed] [Google Scholar]
- 19.Karahalli E, Yilmaz A, Türker H, Ozvaran K. Usefulness of various diagnostic techniques during fiberoptic bronchoscopy for endoscopically visible lung cancer: should cytologic examinations be performed routinely? Respiration. 2001;68:611–614. doi: 10.1159/000050581. [DOI] [PubMed] [Google Scholar]
- 20.Lee HS, Kwon SY, Kim DK, Yoon HI, Lee SM, Lee JH, Lee CT, Chung HS, Han SK, Shim YS, Yim JJ. Bronchial washing yield before and after forceps biopsy in patients with endoscopically visible lung cancers. Respirology. 2007;12:277–282. doi: 10.1111/j.1440-1843.2006.01001.x. [DOI] [PubMed] [Google Scholar]
- 21.Branch KM, Hoshino D, Weaver AM. Adhesion rings surround invadopodia and promote maturation. Biol Open. 2012;1:711–722. doi: 10.1242/bio.20121867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pue CA, Pacht ER. Complications of fiberoptic bronchoscopy at a university hospital. Chest. 1995;107:430–432. doi: 10.1378/chest.107.2.430. [DOI] [PubMed] [Google Scholar]
- 23.Holty JJ, Kuschner W, Gould M. Accuracy of transbronchial needle aspiration for mediastinal staging of non-small cell lung cancer: a meta-analysis. Thorax. 2005;60:949–955. doi: 10.1136/thx.2005.041525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choudhury M, Singh S, Agarwal S. Efficacy of bronchial brush cytology and bronchial washings in diagnosis of non neoplastic and neoplastic bronchopulmonary lesions. Turk Patoloji Derg. 2012;28:142–146. doi: 10.5146/tjpath.2012.01113. [DOI] [PubMed] [Google Scholar]
- 25.Dobler CC, Crawford AB. Bronchoscopic diagnosis of endoscopically visible lung malignancies: should cytological examinations be carried out routinely. Intern Med J. 2009;39:806–811. doi: 10.1111/j.1445-5994.2008.01882.x. [DOI] [PubMed] [Google Scholar]
- 26.Govert JA, Kopita JM, Matchar D, Kussin PS, Samuelson WM. Cost-effectiveness of collecting routine cytologic specimens during fiberoptic bronchoscopy for endoscopically visible lung tumor. Chest. 1996;109:451–456. doi: 10.1378/chest.109.2.451. [DOI] [PubMed] [Google Scholar]
- 27.Kim S, Owens CL. Analysis of ThinPrep cytology in establishing the diagnosis of small cell carcinoma of lung. Cancer. 2009;117:51–56. doi: 10.1002/cncy.20007. [DOI] [PubMed] [Google Scholar]
- 28.Stone J, Boost T, Bushell A, Cooper V, Hall S. A quality improvement comparative study for respiratory specimen processing, screening and reporting. Pathology. 2014;46(Suppl 1):S58. [Google Scholar]


