Abstract
The adequate treatment for stage IB endometrial cancer (EC) with G1-G2 grading (intermediate risk patients) is still debated. FIGO guidelines recommend adjuvant radio-therapy in order to avoid recurrences, despite it has been demonstrated that this does not improve the overall survival. Recently, other than the conventional risk-factor (histology, stage and grading), lymph-vascular involvement, tumor size and neoplasia molecular patterns has been proposed with intent to establish the most appropriated EC oncologic treatment and prognosis. We report an interesting case of a patient affected by an early stage EC (estimated intermediate low risk), treated by the adequate surgical staging and subsequent adjuvant radio-therapy that showed, in a follow up period, a very poor prognosis, similarly to patients affected by high risk cancer. Even if the classical validated risk factors remain the “cornerstone” in risk assessment, adjuvant treatments and follow up planning after surgery, the molecular investigation of estimated intermediate risk EC could represent a “keystone” to solve and avoid the “oncologic dilemma” of cases in which the observed prognosis results very different from the expected one. Only a detailed molecular evaluation of these cases could allow a more specific treatment targeting, leading to an individualized therapy and low recurrence-risk. The importance of recurrence-risk reduction is linked to difficulties in both their early detection and appropriate management. The delay in diagnosis as well as the performance of not adequate treatment can potentially make the prognosis of these cases worst that the one detected in case of uterine sarcoma or mixed müllerian tumors.
Keywords: Endometrial cancer, intermediate risk, cancer recurrence, poor prognosis, microsatellites instability, molecular biomarkers, post-transcriptional factors, therapy individualization
Introduction
Endometrial cancer (EC) is the most common gynaecological malignancy in developed Countries. It is usually detected at an early stage, presents low grading and manifests with abnormal uterine bleeding (AUB) [1,2].
Hysteroscopy represents the gold standard technique for endometrial evaluation when AUB or endometrial thickening occur since it can represent a therapeutic approach in case of pre-neoplastic lesions or selected early stage EC (micro-invasive small lesions or dysplastic cancerous endometrial polyp) [2-5].
In early stages EC, the standard treatment requires total hysterectomy, bilateral adnexectomy with or without pelvic lymphadenectomy performed by laparotomic, laparoscopic or robotic approach, preferring endoscopic approach even in clinical conditions such as obesity [6,7].
According to the last FIGO guidelines surgery alone represents the standard of care in case of stage IA with grading 1 and 2 (G1-G2), followed by clinical observation [8,9].
The adequate treatment for stage IB EC with G1-G2 grading (intermediate risk patients) is still debated. FIGO guidelines recommend adjuvant radio-therapy in order to avoid recurrences, despite it has been demonstrated that this does not improve the overall survival [10,11].
Recently, other than the conventional risk-factor (histology, stage and grading), lymph-vascular involvement, tumor size and neoplasia molecular patterns has been proposed with intent to establish the most appropriated EC oncologic treatment and prognosis [12-14].
We report an interesting case of a patient affected by an early stage EC (estimated intermediate low risk), treated by the adequate surgical staging and subsequent adjuvant radio-therapy that showed, in a follow up period, a very poor prognosis, similarly to patients affected by high risk cancer.
Case presentation
We reported a case of 63 years old woman (BMI 24.5) with a history of hypertension, dyslipidaemia and AUB affected by endometrioid G2 EC and addressed to our Units in order to receive an adequate endoscopic surgical treatment.
At laparoscopic examination, both pelvis and abdomen resulted free of macroscopic disease. Patient underwent laparoscopic class A Querleu-Morrow hysterectomy, bilateral adnexectomy and systematic pelvic lymphadenectomy (29 lymph nodes removed), strictly applying all the usual criteria of surgical oncology (Figure 1).
Figure 1.
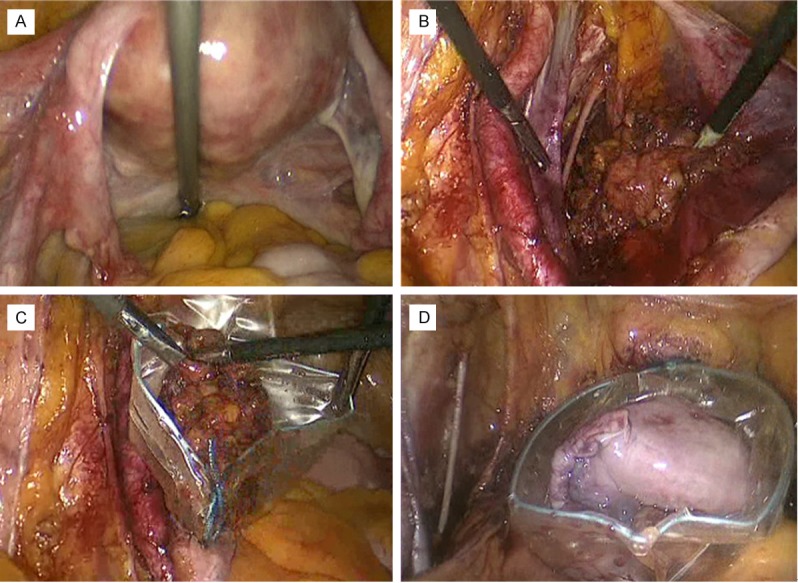
Laparoscopic features of different steps of surgical staging. A: View of pelvis without macroscopic signs of extra-uterine disease; B: Systematic pelvic lymph nodes removal; C: Abdomen extraction of removed lymph nodes through the ancillary trocar (using the endobag device); D: Abdomen extraction of removed uterus and adnexa through vaginal route (using the endobag device).
The surgical/pathological staging permitted to classify the neoplasia at FIGO IB stage, grading G2, with a primary tumour size of 5 cm and positivity for lymph vascular space invasion. None of the removed lymph nodes resulted positive for metastatic involvement.
One month after surgery, the patient was addressed to Radiotherapy Unit and treated by both adjuvant brachytherapy (12 Gy) end external radiotherapy (38 Gy) without complications. The patient resulted asymptomatic for the subsequent five months after the surgery but, unfortunately, during the sixth months of follow up the Computerized Tomography showed extensive peritoneal carcinomatosis, liver and spleen metastasis (Figure 2). General conditions deteriorated rapidly and the patient died 1 month later.
Figure 2.
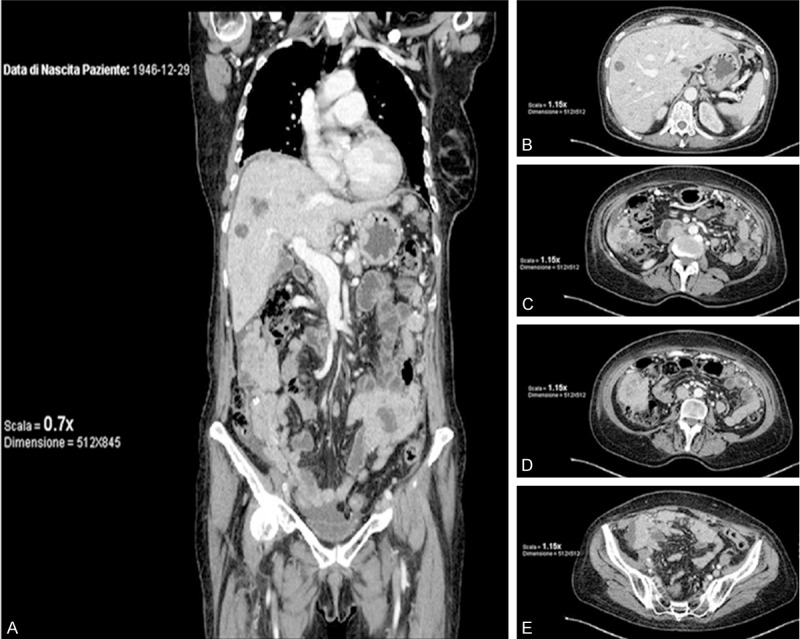
Computerized Tomography features six month after surgery: nodular multiple signs of lung metastatic lesions, diffuse abdominal carcinomatosis, ascites and multiple metastasis (liver, spleen bowel). A: Intermediate dorsal-ventral coronal imaging. B-E: Progressive cranial-caudal axial imaging of abdomen and pelvis.
Discussion
The unexpected and apparently unexplained adverse prognosis of this case focused our attention on risk factors which could allow to further differentiate the prognosis of estimated intermediate risk patients in high or low intermediate risk. Recently, the prognostic role of molecular biomarkers in endometrial cancer has been reviewed by Matias Guiu et al. [15].
Authors analysed the role of EC microsatellites, a non-codifying region of the genome composed by in tandem repeated nucleotides that participate to cellular DNA replication.
Even if microsatellites instability (MI) is associated with errors in the DNA replication (mismatch repair) its role in oncological prognosis estimation is controversial and few data have been collected in case of EC recurrence. MI was firstly documented in cases of EC detected in patients affected by Lynch Syndrome, but more recent studies reported that more than 25-30% of sporadic endometrial cancer showed positivity for MI [15].
Anyway, despite this findings, only Mackay et al. demonstrated the utility of MI evaluation, proposing it in prognosis assessment of early stage EC [16].
The same Authors debated about the role of PTEN, which results both the most frequent mutated gene (25-83%) detected during molecular investigation of EC and often associated with MI.
PTEN is an onco-suppressor gene and the alteration in their physiological pathway seems to be involved in the first steps of carcinogenesis through pathological deregulation of the PI3k/Akt/mTor cascade. This pathway is involved in angiogenesis, protein translation and cell cycle regulation, so its alteration ensures an increased survival to cancer cells [16].
In the last years new therapies targeting these factors are under investigation. There are 20 clinical trials on analogs of rapamycin (Rapalogs), panPI3K inhibitors, dual PI3K/mTOR inhibitors and AKT inhibitors, but nowadays no conclusive results have been reached [17].
Recently, a great interest is created by MicroRNAs expression analysis in different cancers. The relationship between MicroRNA expression and EC prognosis has been analysed in some studies: the MicroRNA205 over-expression is associated to a poor prognosis while high levels of MicroRNA194 are considered a good prognostic factor [15,18].
Certainly evidences on genetic field require further validation in EC prognosis estimation. According to these evidences and in agreement with our hypothesis, the estimated intermediate risk patients could potentially benefit from the molecular profile investigations.
This molecular investigations may improve the management of this cohort of EC (detecting the sub-cohort of patients at “high” intermediate risk) and subsequently may improve their prognosis that usually appears very different from the one estimated at “low” intermediate risk.
Interestingly, research has recently focused on post-transcriptional molecular markers involved in oncological prognosis of patients affected by EC. FGFRs (fibroblast growth factor receptors) are implicated in tumour cell proliferation, migration ad survival. New therapeutic agents were proposed with the aim to inhibit FGFR in order to reduce the aggressiveness of endometrial cancer [19].
Similarly, treatments using antagonists of vascular endothelial growth factor (VEGF) have been proposed with intent to reduce neo-angiogeneses and recurrence [20].
Post-transcriptional abnormalities are probably linked also to the different tumour responses to adjuvant treatment. The over-expression of progesterone receptors, b-catenin and hypoxia inducible factor 1 alpha (HIF 1α) are more common in EC showing radio-resistance and poor prognosis due to disease progression despite adjuvant treatment [15,21].
The mechanism trough these post-transcriptional changes reduce the radio-sensitivity of neoplastic EC cells are linked to the ability to produce tissue hypoxia, reducing in this way the radio-induced oxidative cells damage [21].
On this basis, post-transcriptional investigation could represent a possible detection of patients that may benefit from chemotherapy more than radiotherapy.
A possible chemo-radio association in performing adjuvant treatment has already been proposed by GOG and PORTEC studies for intermediate risk patients without any information about cancer molecular pattern.
In GOG study LVSI is reported to be the most significant risk factor to individuate high risk patients while PORTEC considers “high” intermediate risk patients presenting at least 2 risk factors: age above 60, outer half myometrium invasion and grade 3 neoplasm [22-24].
Even if the classical validated risk factors remain the “cornerstone” in risk assessment, adjuvant treatments and follow up planning after surgery, the molecular investigation of estimated intermediate risk EC could represent a “keystone” to solve and avoid the “oncologic dilemma” of cases in which the observed prognosis results very different from the expected one.
Only a detailed molecular evaluation of these cases could allow a more specific treatment targeting, leading to an individualized therapy and low recurrence risk. The importance of recurrence risk reduction is linked to difficulties in both their early detection and appropriate management. The delay in diagnosis as well as the performance of not adequate treatment can potentially make the prognosis of these cases worst that the one detected in case of uterine sarcoma or mixed müllerian tumors [25,26].
Conclusions
Estimated intermediate risk endometrial cancer still represents a challenge for gynaecologic oncologists. Its behaviour can be very aggressive and depend upon clinical, molecular and pathological features that are not fully understood. A better understanding of prognostic factors could influence the choice between lymphadenectomy, adjuvant and complementary therapies.
Adequate definition of sub-class of risk and “individualized treatment” are fundamental in order to avoid under-treatment and, consequently, poor prognosis.
Disclosure of conflict of interest
All Authors declare no conflicts of interest.
References
- 1.American Joint Committee on Cancer. AJCC Staging manual. 7th Edition. New York: Springer; 2010. Corpus Uteri. [Google Scholar]
- 2.Saccardi C, Gizzo S, Patrelli TS, Ancona E, Anis O, Di Gangi S, Vacilotto A, D’Antona D, Nardelli GB. Endometrial surveillance in tamoxifen users: role, timing and accuracy of hysteroscopic investigation: observational longitudinal cohort study. Endocr Relat Cancer. 2013;20:455–62. doi: 10.1530/ERC-13-0020. [DOI] [PubMed] [Google Scholar]
- 3.Litta P, Merlin F, Saccardi C, Pozzan C, Sacco G, Fracas M, Capobianco G, Dessole S. Role of hysteroscopy with endometrial biopsy to rule out endometrial cancer in postmenopausal women with abnormal uterine bleeding. Maturitas. 2005;50:117–23. doi: 10.1016/j.maturitas.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Litta P, Bartolucci C, Saccardi C, Codroma A, Fabris A, Borgato S, Conte L. Atypical endometrial lesions: hysteroscopic resection as an alternative to hysterectomy. Eur J Gynaecol Oncol. 2013;34:51–3. [PubMed] [Google Scholar]
- 5.Litta P, Codroma A, D’Agostino G, Breda E. Morular endometrial metaplasia: review of the literature and proposal of the management. Eur J Gynaecol Oncol. 2013;34:243–7. [PubMed] [Google Scholar]
- 6.Cardenas-Goicoechea J, Shepherd A, Momeni M, Mandeli J, Chuang L, Gretz H, Fishman D, Rahaman J, Randall T. Survival analysis of robotic versus traditional laparoscopic surgical staging for endometrial cancer. Am J Obstet Gynecol. 2014;210:160, e1–e11. doi: 10.1016/j.ajog.2013.10.871. [DOI] [PubMed] [Google Scholar]
- 7.Tinelli R, Litta P, Meir Y, Surico D, Leo L, Fusco A, Angioni S, Cicinelli E. Advantages of Laparoscopy Versus Laparotomy in Extremely Obese Women (BMI>35) with Early-stage Endometrial Cancer: A Multicenter Study. Anticancer Res. 2014 May;34:2497–502. [PubMed] [Google Scholar]
- 8.Rungruang B, Olawaiye AB. Comprehensive surgical staging for endometrial cancer. Rev Obstet Gynecol. 2012;5:28–34. [PMC free article] [PubMed] [Google Scholar]
- 9.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–4. doi: 10.1016/j.ijgo.2009.02.012. Erratum in: Int J Gynaecol Obstet 2010; 108: 176. [DOI] [PubMed] [Google Scholar]
- 10.Kong A, Johnson N, Kitchener HC, Lawrie TA. Adjuvant radiotherapy for stage I endometrial cancer. Cochrane Database Syst Rev. 2012;4:CD003916. doi: 10.1002/14651858.CD003916.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong A, Johnson N, Kitchener HC, Lawrie TA. Adjuvant radiotherapy for stage I endometrial cancer: an updated Cochrane systematic review and meta-analysis. J Natl Cancer Inst. 2012;104:1625–34. doi: 10.1093/jnci/djs374. [DOI] [PubMed] [Google Scholar]
- 12.Todo Y, Choi HJ, Kang S, Kim JW, Nam JH, Watari H, Tamakoshi A, Sakuragi N. Clinical significance of tumor volume in endometrial cancer: a Japan-Korea cooperative study. Gynecol Oncol. 2013;131:294–8. doi: 10.1016/j.ygyno.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Laufer J, Scasso S, Papadia A, Sosa C, Cirillo F, Raspagliesi F. Association between tumor diameter and lymphovascular space invasion among women with early-stage endometrial cancer. Int J Gynaecol Obstet. 2013;123:142–5. doi: 10.1016/j.ijgo.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Salvesen HB, Haldorsen IS, Trovik J. Markers for individualised therapy in endometrial carcinoma. Lancet Oncol. 2012;13:e353–61. doi: 10.1016/S1470-2045(12)70213-9. [DOI] [PubMed] [Google Scholar]
- 15.Matias-Guiu X, Prat J. Molecular pathology of endometrial carcinoma. Histopathology. 2013;62:111–23. doi: 10.1111/his.12053. [DOI] [PubMed] [Google Scholar]
- 16.Mackay HJ, Gallinger S, Tsao MS, McLachlin CM, Tu D, Keiser K, Eisenhauer EA, Oza AM. Prognostic value of microsatellite instability (MSI) and PTEN expression in women with endometrial cancer: results from studies of the NCIC Clinical Trials Group (NCIC CTG) Eur J Cancer. 2010;46:1365–73. doi: 10.1016/j.ejca.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 17.Myers AP. New strategies in endometrial cancer: targeting the PI3K/mTOR pathway--the devil is in the details. Clin Cancer Res. 2013;19:5264–74. doi: 10.1158/1078-0432.CCR-13-0615. [DOI] [PubMed] [Google Scholar]
- 18.Zhai H, Karaayvaz M, Dong P, Sakuragi N, Ju J. Prognostic significance of miR-194 in endometrial cancer. Biomark Res. 2013;1:12. doi: 10.1186/2050-7771-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fearon AE, Gould CR, Grose RP. FGFR signalling in women’s cancers. Int J Biochem Cell Biol. 2013;45:2832–42. doi: 10.1016/j.biocel.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Gadducci A, Sergiampietri C, Guiggi I. Antiangiogenic agents in advanced, persistent or recurrent endometrial cancer: a novel treatment option. Gynecol Endocrinol. 2013;29:811–6. doi: 10.3109/09513590.2013.801446. [DOI] [PubMed] [Google Scholar]
- 21.Santacana M, Yeramian A, Velasco A, Bergada L, Gatius S, García V, Azueta A, Palacios J, Dolcet X, Oliva E, Matias-Guiu X. Immunohistochemical features of post-radiation vaginal recurrences of endometrioid carcinomas of the endometrium: role for proteins involved in resistance to apoptosis and hypoxia. Histopathology. 2012;60:460–71. doi: 10.1111/j.1365-2559.2011.04106.x. [DOI] [PubMed] [Google Scholar]
- 22.SCCA Gynecologic Cancer Trials Clinical. Radiation vs Brachytherapy for Endometrial Carcinoma (GOG 249). A Phase III Trial of Pelvic Radiation Therapy Versus Vaginal Cuff Brachytherapy Followed By Paclitaxel/Carboplatin Chemotherapy in Patients With High Risk, Early Stage Endometrial Carcinoma. Ongoing trial. (available at: http://www.seattlecca.org/clinical-trials/gyncancer- NCT00807768.cfm) [Google Scholar]
- 23.CRUK/08/001: PORTEC-3: Randomised Phase III Trial Comparing Concurrent Chemoradiation and Adjuvant Chemotherapy with Pelvic Radiation Alone in High Risk and Advanced Stage Endometrial Carcinoma. (available at: http://www.cancerresearchuk.org/science/research/who-and-what-we-fund/browse-by-location/london/barts-and-the-london-nhs-trust/grants/melanie-powell-8659-cruk-08-001- portec-3-randomised-phase-iii) [Google Scholar]
- 24.Loizzi V, Cormio G, Lorusso M, Latorre D, Falagario M, Demitri P, Scardigno D, Selvaggi LE. The impact of lymph vascular space invasion on recurrence and survival in patients with early stage endometrial cancer. Eur J Cancer Care (Engl) 2014;23:380–4. doi: 10.1111/ecc.12115. [DOI] [PubMed] [Google Scholar]
- 25.Patrelli TS, Gizzo S, Di Gangi S, Guidi G, Rondinelli M, Nardelli GB. Cervical Mullerian adenosarcoma with heterologous sarcomatous overgrowth: a fourth case and review of literature. BMC Cancer. 2011;11:236. doi: 10.1186/1471-2407-11-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patrelli TS, Silini EM, Gizzo S, Berretta R, Franchi L, Thai E, Lukanovic A, Nardelli GB, Modena AB. Extragenital Müllerian adenosarcoma with pouch of Douglas location. BMC Cancer. 2011;11:171. doi: 10.1186/1471-2407-11-171. [DOI] [PMC free article] [PubMed] [Google Scholar]


