Abstract
Alcohol abuse increases the incidence of cerebral accidents, which correlates with cerebrovascular structural changes. The present study was designed to observe the cerebrovascular remodeling of drinking rats with light microscopy and transmission electron microscopy (TEM). Short-term alcohol administration induced apparent amplification of perivascular spaces around small vessels in brain tissue, while long-term administration caused pathological changes of basilar arteries (BAs), including endothelial exfoliation, inner elastic lamina (IEL) fragmentation and thickening of tunica media and adventitia. In addition, the relationship between cerebrovascular remodeling and MMP-2 and MMP-9 synthesized by endothelial cells and vascular smooth muscle cells was explored by immunohistochemistry. The two protein expression in cerebral vessels changed dynamically, peaking at 1-2 weeks after treatment, and decreasing as treatment continued. These results suggest that MMP-2 and MMP-9 may play a significant role in blood-brain barrier disruption after alcohol abuse. But the chronic changes of cerebral arteries resulted from drinking are not coincident with time course of MMP-2 and MMP-9 expression in situ.
Keywords: Alcohol abuse, basilar artery, remodeling, MMP-2, MMP-9
Introduction
Alcohol abuse has been proved increase risk of cerebrovascular events [1-4] by changing cerebral blood flow [5,6], increasing blood pressure (BP) [7,8] and disrupting the blood-brain barrier (BBB) [9,10]. The mechanisms, such as oxidative stress [9], ion channel [11] and apoptosis [12] caught the attention of scientists. But the morphological changes of cerebral vessels have not been systematically described. In the present study, we compared the morphological features of cerebral vessels between control and alcoholic rats, with specific staining, transmission electron microscope (TEM) and immunohistochemistry (IHC). We found alcohol abuse induced vascular remodeling, involved both cells and the extracellular matrix (ECM).
MMPs are a family of zinc-binding proteolytic enzymes that can degrade structural proteins of ECM and have an important role on tissue remodeling. MMPs are synthesized by diverse cell types including mesenchymal cells, macrophages, monocytes, and fibroblasts. But MMPs expressed in vascular wall cells are always ignored. We focused on MMP-2 and MMP-9 synthesized by endothelial cells (ECs) and vascular smooth muscle cells (VSMCs) after drinking and their correlation with pathological changes of cerebral vessels.
Materials and methods
Experimental animals
This study was approved by the Institutional Review Board for Animal Experiments at Hebei Medical University. In alcoholic group, alcoholic beverage (56% v/v, Hongxing, Beijing) was injected into the stomach of Sprague-Dawley (SD) male rats (220 g ± 10 g) using a blunt tipped needle. The injection was processed two times each day at interval of over 10 hours, and the rats were starved 4 hours before each injection. Increasing dose of alcoholic beverage was administered until the end of the 3rd week, and then the full dose (1.2 ml/100 g, 56% v/v) was supplied. The control animals were given water instead. Systolic blood pressure (BP) was measured weekly using the tail-off method with noninvasive BP monitor (LE5001 Pressure Meter).
Blood alcohol concentration (BAC)
Six rats were treated with 1.2 ml/100 g of 56% v/v alcoholic beverage, and their blood samples were collected at the baseline and 0.5 h, 1 h, 2 h, 3 h, 6 h, 12 h after treated. BCA was estimated by headspace gas chromatography (Agilent Technologies).
Tissue preparation
The rats were anesthetized with 10% chloraldurat (0.4 ml/100 g, i.p) and then decapitated. The whole brain with basilar artery (BA) was removed and preserved in the fixation solution for 48 h. Serial coronal paraffin sections were used for Weigert elastic fiber staining, Masson’s trichrome staining and IHC.
The whole brain for TEM with basilar artery was removed and immersed immediately into 4°C 4% glutaric dialdehyde. With the aid of a stereomicroscope, the BA and brain tissue were carefully dissected free from each brain and preserved in 4°C 4% glutaric dialdehyde. Ultrathin sections were observed with TEM (H-7500, Hitachi, Japan).
Specific staining
Weigert elastic fiber staining and Masson’s trichrome staining were performed using standard protocol. The average areas of BA were surveyed by a pathologic image analysis system (Cellsen Standard). The area of the lumina (A1) was measured from the inner boundary of tunica intima, and the gross area of the crosssection (A2) was from the external boundary of tunica adventitia. The area of the wall (A3) was calculated with A1 and A2 (A3 = A2 - A1). The ratio of wall and lumina was achieved with A1 and A3 (the ratio = A3/A1).
Immunohistochemistry
These tissues were incubated respectively with antibodies against rat MMP-2 (Eptomics) and MMP-9 (Eptomics) overnight at 4°C. After rinsed with PBS, the sections were incubated for 1 h with the secondary antibody (Zhonghan Goldenbridge Biotech), and were subsequently incubated with avidin and horseradish peroxidase (HRP)-conjugated biotin (Zhonghan Goldenbridge Biotech) for 30 min. Finally, 0.1 mg/ml 3,3’-diaminobenzidine (DAB) reagent (Tiangen Biotech) was applied to sections for 5 min. In order to identify immunostaining, the sections were counterstained with haematoxylin. The number of MMP-2 and MMP-9-stained small vessels in each section was counted (per 400 × view field) in five view fields. Two independent observers who were blinded to the experimental conditions performed counts and calculated the average number of positive vessels.
Cell culture
Human brain microvascular endothelial cells (HBMECs) (Sciencell) and human brain vascular smooth muscle cells (HBVSMC) (Sciencell) were grown in 25 cm2 flasks until 80% confluent. Cells were treated with 200 mM alcohol (Sigma) for different lengths of time.
Western blotting
Cell extracts were loaded, separated by SDS-PAGE and transferred to NT membranes. The membranes were incubated overnight at 4°C with anti-MMP-2 (Epitomics), MMP-9 (Epitomics) and GAPDH (Genetex.) antibodies. Then, incubation with the secondary antibodies was performed. The secondary antibodies were bound to the fluorophore which underwent excitation by light. The emitted light was then detected by an imager (Odyssey).
Statistical analysis
The data were expressed as mean ± SEM. The biochemical data were analyzed statistically by one-way ANOVA with SPSS statistical software. Statistical significance was set at P < 0.05.
Results
BCA
After alcoholic beverage treatment, BCA increased rapidly, and peaked to (225.06 ± 43.10) mg/dL at 1 h. And then BCA decreased gradually to (25.37 ± 18.21) mg/dL at 12 h (Figure 1).
Figure 1.
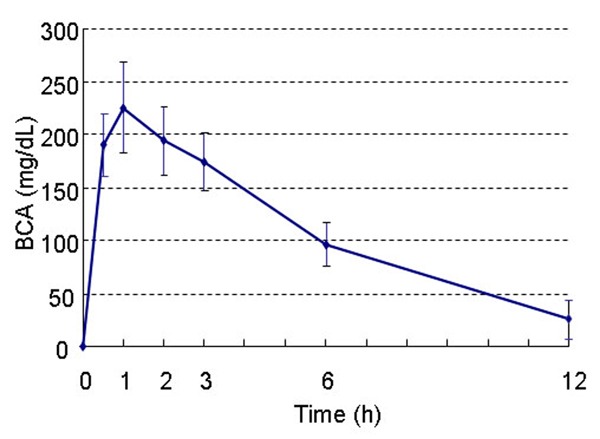
BCA versus time profiles of alcoholic beverage (1.2 ml/100 g of 56% v/v). BCA got the peak after 1 h, and then decreased gradually.
Systolic BP changes
Systolic BP fluctuated smoothly in the control group, while it was profoundly increased in alcoholic rats. A significantly increase in systolic BP was observed in the rats with alcoholic beverage treatment for 10-12 weeks compared to the control rats (P < 0.05). The Systolic BP at the end of 12 week treatment was (135.6 ± 6.9) mmHg (P < 0.01) (Figure 2).
Figure 2.

Systolic BP changes in control and alcoholic groups. Systolic BP increased gradually in alcoholic group, and was significantly different from that in control group since the 10th week. *P < 0.05 vs. control.
Morphological changes of BAs
With Weigert staining (Figure 3A, 3B), elastic fibers were stained in purple. With Masson’s trichrome staining (Figure 3C, 3D), SMCs were stained in red, collagen was blue, and red cells were orange. Specific staining showed the BAs had a typical character of resistance arteries. There was a sharp, solid and undulate IEL in the internal layer. There were a few elastic fibers, not elastic lamina, in tunica media and adventitia. The tunica media had 3-5 layers of VSMCs. In control rats, IEL appeared as a thick continuous and compact layer (Figure 3A). But in alcoholic rats, local IEL appeared as separate thinner elastic fibers (Figure 3B). The SMCs in tunica media and collagen in adventitia were increased in alcoholic rats compared with control (Figure 3C, 3D). The ratio between vascular wall and lumina area was increased after chronic drinking (Table 1).
Figure 3.

Morphological images of BAs of control (A, C) and alcoholic (B, D) rats. Weigert staining (A, B), illustrating elastin (purple), showed the IEL fragmentation (arrow) after chronic alcohol abuse. Masson’s trichrome staining (C, D), illustrating collagen (blue), SMCs (red) and red cells (orange), showed SMCs hyperplasia and collagen increase in tunica adventitia. Scale bar: 50 μm.
Table 1.
The Area of BAs Cross-section (n = 6)
| A1 (μm2) | A2 (μm2) | A3 = A2-A1 (μm2) | A3/A1 | |
|---|---|---|---|---|
| Con | 6516.488 ± 845.12 | 17579.210 ± 1289.87 | 11062.722 ± 1039.91 | 1.695 ± 0.061 |
| Alcoholic | 5871.814 ± 793.33 | 18317.725 ± 1197.68 | 12445.911 ± 938.17 | 2.119 ± 0.046* |
A1: the area of vascular lumina. A2: the area of vascular cross-section. A3: the area of vascular wall.
p < 0.05 vs con.
Ultrastructural changes of cerebral vessels
The ECs of control rats, in both capillaries and basilar arteries, had affluent organelle and microvillus (Figure 4A, 4C). The perivascular space around capillaries was enlarged after 2 week alcohol treatment (Figure 4B). Normal IEL appeared a thick lamina (Figure 4C). After alcoholic beverage-treatment, the vessels were structurally different from control group. The cristae of mitochondria in ECs disrupted and dissolved (Figure 4B), and both microvillus and pinocytosis vesicles were decreased (Figure 4B, 4D). At the end of 12 week treatment, vacuoles were observed between ECs and IEL. IEL appeared as separate thinner layers, and SMCs protruded into these layers (Figure 4D).
Figure 4.

TEM images of capillaries (A, B) and BAs (C, D) of control (A, C) and alcoholic (B, D) rats. Amplification of perivascular spaces (asterisk) were occurred at the 2nd week after treatment (B). Subendothelial vacuoles (black arrow) and separated IEL were shown at the end of 12 week alcohol treatment (D). And SMCs broke through into elastic fibers (white arrow). Cap: capillaries (A, B) × 20 k, (C, D) × 5 k.
Protein expression of MMP-2 and MMP-9 in cerebral vessels
MMP-2 and MMP-9 were rarely found in capillaries, small veins and basilar arteries without alcoholic beverage-treatment (Figures 5A, 5E, and 7A). After treatment, MMP-2 and MMP-9 were observed in capillaries and small veins (Figure 5B, 5C, 5F, 5G). MMP-2 expression peaked at the 1st week (Figures 5B and 6), and MMP-9 at the 2nd week (Figures 5G and 6) paralleling apparent amplification of perivascular spaces. As treatment progressed, the expression decreased. MMP-2 or MMP-9-positive vessels could be hardly observed at the end of the 4th week (Figure 5D, 5H). MMP-2, not MMP-9, was expressed in the ECs of subarachnoid arteries after 1-2 week treatment (Figure 7B). MMP-2 protein level was also transiently increased in cultured HBMECs with alcohol treatment (Figure 8). Neither MMP-2 nor MMP-9 was detected in HBVSMCs (not shown).
Figure 5.

Expression of MMP-2 (A-D), MMP-9 (E-H) in small vessels of rat cerebral cortex. Rarely MMP-2 (A) and MMP-9 (E) expressed in control rats. As alcoholic beverage treated, both proteins expressed at 1st and 2nd week, accompanied with amplification of perivascular spaces (B, C, F, G). There were few positive vessels at 4th week (D, H). The images at the corner were magnifications from the area in the frames. Scale bar: 50 μm.
Figure 7.

Expression of MMP-2 in subarachnoid arteries. There were no expression in control group (A), and endothelial expression in alcoholic group after 2 week-treatment (B). Scale bar: 50 μm.
Figure 6.
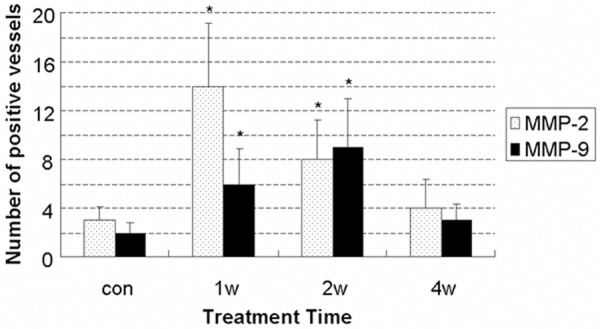
Quantitation of MMP-2, MMP-9 expression in small vessels of rat cerebral cortex after alcoholic beverage treatment (per × 400). MMP-2 expression peaked at the 1st week, and MMP-9 at the 2nd week. *P < 0.05 vs. con.
Figure 8.

Expression of MMP-2 and MMP-9 in HBMECs. A. Western blot analysis of MMP-2 and MMP-9 expression. B. Quantitation of expression. *P < 0.05 vs. con.
Discussion
Alcohol is the most commonly used and abused drug in the world. Deleterious alcohol-related health effects attributed to the internal organ toxicity include vascular injury. We established the model of alcoholic rats with alcoholic beverages purchased from market, which was close to the development of alcoholic intoxication in vessels. The peak of BCA in our model was 225.06 ± 43.1 mg/dL. After chronic alcoholic consumption, both vascular wall cells and ECM were involved in the histological changes of BAs.
The IEL has been suggested a key component of the arterial wall to modulate vascular tone and diameter. It also represents a flexible barrier between the endothelium and inner SMCs. EC proliferation [13] and SMC migration [14-16] are dependent on IEL. Elastic laminate fragmentation and elastin degradation are observed in the progress of aging [17], aneurysm [18] and hypertension [19-22]. Partridge [23] found disruption of the elastic fibers in tunica media of aorta after 72 week alcohol consumption. This present study suggested chronic alcohol abuse could also lead to IEL injury of BA.
Besides of IEL fragmentation, the wall: lumen ratio increasing was another feature of vascular remodeling caused by alcohol intoxication, which might be resulted from SMC hyperplasia in tunica media and collagen deposition in tunica adventitia. Other studies had similar results [24]. These events induced losing of elasticity and stiffening of arteries [25].
MMPs, a family of zinc-dependent extracellular proteinase, are believed to cause disorganization of the vascular wall. MMPs were consistently associated with atherosclerosis and lesion vulnerability [26-29]. Arteries with temporal arteritis had clearly enhanced immunostaining for MMP-9 compared with normal arteries, and MMP-9 was specifically localized to the regions of IEL disruption [30]. Up-regulation of MMP-2 activity was coincident with the degradation of vascular elastin components and proliferation of SMCs in vascular injury [16,23].
The protein level and activity of MMP-2 and MMP-9 could be affected by alcohol. Intraperitoneal alcohol treatment increased MMP-9 activity in the hippocampus and prefrontal cortex [31]. Lieber-DeCarli liquid diet for 72 weeks increased MMP-2 activity in aorta [23]. Both protein level and activity of MMP-9 was elevated in serum of alcohol abusers [32]. In this study, however, only MMP-2 expressed in ECs of intracranial arteries after 1-2 week treatment, which was not in concordance with the time course of vascular remodeling. We supposed that MMP-2 and MMP-9 expression in vascular wall were not the capital factor to vascular remodeling induced by alcohol. The remodeling might related with other factors, such as proteinases in blood circulation, toxicity of alcohol and acetaldehyde, and hypertension. The systolic BP was confirmed to significantly increase after chronic drinking in both our and others’ studies [8]. Hypertension and remodeling of resistance arteries might be each other’s essential prerequisites.
Interestingly, the relationship between BBB disruption and expression of MMP-2 and MMP-9 was more definite. Drinking for 1-2 weeks, the rats appeared obvious vasogenic edema, paralleling with transient up-regulation of MMP-2 and MMP-9. MMPs-mediated disruption of BBB has proved in diverse diseases, such as subarachnoid hemorrhage, cerebral ischemia and diabetes [28-30]. Alcohol could elevate MMPs expression and activity in a PTK-dependent manner [10], followed by tight junction proteins disruption [31,32]. The tight junction proteins, such as ZO, occludin and claudin, were considered as the key components of BBB. We also studied the two protein expression in the isolated cells. Neither MMP-2 nor MMP-9 was detected in HBVSMCs. In HBMECs, MMP-2 expression, not MMP-9, increased transiently after alcohol treatment. Shapira [33] found that in rat head trauma model, the brain edema after chronic alcohol pretreatment was not more serious than that after acute alcohol pretreatment. This result is possible to be associated with the reduction of the proteinase after long-term alcohol treatment.
According to expression patterns of MMP-2 and MMP-9 in vascular wall cells, ECs were the primary origin of vascular MMP-2 and MMP-9 after alcohol treatment, and MMP-2 might play a more important role on vascular pathological change.
In summary, short-term alcohol abuse results in BBB injury, which is closely related with MMP-2 and MMP-9 synthesized by ECs. Long-term alcohol abuse leads to endothelial exfoliation, IEL fragmentation, and inward remodeling, which is a complicated process, not mainly dependent on MMP-2 and MMP-9 in vascular wall.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 81172899) and the Key Researh Items of the Ministry of Public Security (Grant NO. 2011ZDYJGAHBST008).
Disclosure of conflict of interest
None.
References
- 1.Jimenez M, Chiuve SE, Glynn RJ, Stampfer MJ, Camargo CA Jr, Willett WC, Manson JE, Rexrode KM. Alcohol consumption and risk of stroke in women. Stroke. 2012;43:939–945. doi: 10.1161/STROKEAHA.111.639435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ikehara S, Iso H, Yamagishi K, Kokubo Y, Saito I, Yatsuya H, Inoue M, Tsugane S. Alcohol consumption and risk of stroke and coronary heart disease among Japanese women: the Japan Public Health Center-based prospective study. Prev Med. 2013;57:505–510. doi: 10.1016/j.ypmed.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Rantakomi SH, Laukkanen JA, Sivenius J, Kauhanen J, Kurl S. Alcohol consumption and the risk of stroke among hypertensive and overweight men. J Neurol. 2013;260:534–539. doi: 10.1007/s00415-012-6672-6. [DOI] [PubMed] [Google Scholar]
- 4.Anderson C, Ni Mhurchu C, Scott D, Bennett D, Jamrozik K, Hankey G. Triggers of subarachnoid hemorrhage: role of physical exertion, smoking, and alcohol in the Australasian Cooperative Research on Subarachnoid Hemorrhage Study (ACROSS) Stroke. 2003;34:1771–1776. doi: 10.1161/01.STR.0000077015.90334.A7. [DOI] [PubMed] [Google Scholar]
- 5.Gundersen H, van Wageningen H, Gruner R. Alcohol-induced changes in cerebral blood flow and cerebral blood volume in social drinkers. Alcohol Alcohol. 2013;48:160–165. doi: 10.1093/alcalc/ags121. [DOI] [PubMed] [Google Scholar]
- 6.Trim RS, Simmons AN, Tolentino NJ, Hall SA, Matthews SC, Robinson SK, Smith TL, Padula CB, Paulus MP, Tapert SF, Schuckit MA. Acute ethanol effects on brain activation in low- and high-level responders to alcohol. Alcohol Clin Exp Res. 2010;34:1162–1170. doi: 10.1111/j.1530-0277.2010.01193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Husain K, Vazquez-Ortiz M, Lalla J. Down regulation of aortic nitric oxide and antioxidant systems in chronic alcohol-induced hypertension in rats. Hum Exp Toxicol. 2007;26:427–434. doi: 10.1177/0960327106072993. [DOI] [PubMed] [Google Scholar]
- 8.Husain K, Mejia J, Lalla J, Kazim S. Dose response of alcohol-induced changes in BP, nitric oxide and antioxidants in rat plasma. Pharmacol Res. 2005;51:337–343. doi: 10.1016/j.phrs.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Haorah J, Knipe B, Leibhart J, Ghorpade A, Persidsky Y. Alcohol-induced oxidative stress in brain endothelial cells causes blood-brain barrier dysfunction. J Leukoc Biol. 2005;78:1223–1232. doi: 10.1189/jlb.0605340. [DOI] [PubMed] [Google Scholar]
- 10.Haorah J, Schall K, Ramirez SH, Persidsky Y. Activation of protein tyrosine kinases and matrix metalloproteinases causes blood-brain barrier injury: Novel mechanism for neurodegeneration associated with alcohol abuse. Glia. 2008;56:78–88. doi: 10.1002/glia.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhlmann CR, Li F, Ludders DW, Schaefer CA, Most AK, Backenkohler U, Neumann T, Tillmanns H, Waldecker B, Erdogan A, Wiecha J. Dose-dependent activation of Ca2+-activated K+ channels by ethanol contributes to improved endothelial cell functions. Alcohol Clin Exp Res. 2004;28:1005–1011. doi: 10.1097/01.alc.0000130811.92457.0d. [DOI] [PubMed] [Google Scholar]
- 12.Li W, Li J, Liu W, Altura BT, Altura BM. Alcohol-induced apoptosis of canine cerebral vascular smooth muscle cells: role of extracellular and intracellular calcium ions. Neurosci Lett. 2004;354:221–224. doi: 10.1016/j.neulet.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 13.Masuda H, Zhuang YJ, Singh TM, Kawamura K, Murakami M, Zarins CK, Glagov S. Adaptive remodeling of internal elastic lamina and endothelial lining during flow-induced arterial enlargement. Arterioscler Thromb Vasc Biol. 1999;19:2298–2307. doi: 10.1161/01.atv.19.10.2298. [DOI] [PubMed] [Google Scholar]
- 14.Spencer JA, Hacker SL, Davis EC, Mecham RP, Knutsen RH, Li DY, Gerard RD, Richardson JA, Olson EN, Yanagisawa H. Altered vascular remodeling in fibulin-5-deficient mice reveals a role of fibulin-5 in smooth muscle cell proliferation and migration. Proc Natl Acad Sci U S A. 2005;102:2946–2951. doi: 10.1073/pnas.0500058102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karnik SK, Brooke BS, Bayes-Genis A, Sorensen L, Wythe JD, Schwartz RS, Keating MT, Li DY. A critical role for elastin signaling in vascular morphogenesis and disease. Development. 2003;130:411–423. doi: 10.1242/dev.00223. [DOI] [PubMed] [Google Scholar]
- 16.Zahradka P, Harding G, Litchie B, Thomas S, Werner JP, Wilson DP, Yurkova N. Activation of MMP-2 in response to vascular injury is mediated by phosphatidylinositol 3-kinase-dependent expression of MT1-MMP. Am J Physiol Heart Circ Physiol. 2004;287:H2861–2870. doi: 10.1152/ajpheart.00230.2004. [DOI] [PubMed] [Google Scholar]
- 17.Fonck E, Feigl GG, Fasel J, Sage D, Unser M, Rufenacht DA, Stergiopulos N. Effect of aging on elastin functionality in human cerebral arteries. Stroke. 2009;40:2552–2556. doi: 10.1161/STROKEAHA.108.528091. [DOI] [PubMed] [Google Scholar]
- 18.Goto Y, Hojo M, Yamagata S, Kikuta K. Fatal bleeding from arterial dissection after clipping of a ruptured aneurysm--case report. Neurol Med Chir (Tokyo) 2003;43:608–611. doi: 10.2176/nmc.43.608. [DOI] [PubMed] [Google Scholar]
- 19.Briones AM, Gonzalez JM, Somoza B, Giraldo J, Daly CJ, Vila E, Gonzalez MC, McGrath JC, Arribas SM. Role of elastin in spontaneously hypertensive rat small mesenteric artery remodelling. J Physiol. 2003;552:185–195. doi: 10.1113/jphysiol.2003.046904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arribas SM, Hinek A, Gonzalez MC. Elastic fibres and vascular structure in hypertension. Pharmacol Ther. 2006;111:771–791. doi: 10.1016/j.pharmthera.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Aiello VD, Gutierrez PS, Chaves MJ, Lopes AA, Higuchi ML, Ramires JA. Morphology of the internal elastic lamina in arteries from pulmonary hypertensive patients: a confocal laser microscopy study. Mod Pathol. 2003;16:411–416. doi: 10.1097/01.MP.0000067685.57858.D7. [DOI] [PubMed] [Google Scholar]
- 22.Liu SQ. Alterations in structure of elastic laminae of rat pulmonary arteries in hypoxic hypertension. J Appl Physiol (1985) 1996;81:2147–2155. doi: 10.1152/jappl.1996.81.5.2147. [DOI] [PubMed] [Google Scholar]
- 23.Partridge CR, Sampson HW, Forough R. Long-term alcohol consumption increases matrix metalloproteinase-2 activity in rat aorta. Life Sci. 1999;65:1395–1402. doi: 10.1016/s0024-3205(99)00381-1. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Yu X, Xu G, Gao G, Xu X. Alcoholism and traumatic subarachnoid hemorrhage: an experimental study on vascular morphology and biomechanics. J Trauma. 2011;70:E6–12. doi: 10.1097/TA.0b013e3181cda3b9. [DOI] [PubMed] [Google Scholar]
- 25.Sell DR, Monnier VM. Molecular basis of arterial stiffening: role of glycation - a mini-review. Gerontology. 2012;58:227–237. doi: 10.1159/000334668. [DOI] [PubMed] [Google Scholar]
- 26.Silvello D, Narvaes LB, Albuquerque LC, Forgiarini LF, Meurer L, Martinelli NC, Andrades ME, Clausell N, dos Santos KG, Rohde LE. Serum levels and polymorphisms of matrix metalloproteinases (MMPs) in carotid artery atherosclerosis: higher MMP-9 levels are associated with plaque vulnerability. Biomarkers. 2014;19:49–55. doi: 10.3109/1354750X.2013.866165. [DOI] [PubMed] [Google Scholar]
- 27.Napoli C. MMP inhibition and the development of cerebrovascular atherosclerosis: The road ahead. Stroke. 2002;33:2864–2865. [PubMed] [Google Scholar]
- 28.Pradhan-Palikhe P, Vikatmaa P, Lajunen T, Palikhe A, Lepantalo M, Tervahartiala T, Salo T, Saikku P, Leinonen M, Pussinen PJ, Sorsa T. Elevated MMP-8 and decreased myeloperoxidase concentrations associate significantly with the risk for peripheral atherosclerosis disease and abdominal aortic aneurysm. Scand J Immunol. 2010;72:150–157. doi: 10.1111/j.1365-3083.2010.02418.x. [DOI] [PubMed] [Google Scholar]
- 29.Tarin C, Gomez M, Calvo E, Lopez JA, Zaragoza C. Endothelial nitric oxide deficiency reduces MMP-13-mediated cleavage of ICAM-1 in vascular endothelium: a role in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:27–32. doi: 10.1161/ATVBAHA.108.169623. [DOI] [PubMed] [Google Scholar]
- 30.Nikkari ST, Hoyhtya M, Isola J, Nikkari T. Macrophages contain 92-kd gelatinase (MMP-9) at the site of degenerated internal elastic lamina in temporal arteritis. Am J Pathol. 1996;149:1427–1433. [PMC free article] [PubMed] [Google Scholar]
- 31.Wright JW, Masino AJ, Reichert JR, Turner GD, Meighan SE, Meighan PC, Harding JW. Ethanol-induced impairment of spatial memory and brain matrix metalloproteinases. Brain Res. 2003;963:252–261. doi: 10.1016/s0006-8993(02)04036-2. [DOI] [PubMed] [Google Scholar]
- 32.Sillanaukee P, Kalela A, Seppa K, Hoyhtya M, Nikkari ST. Matrix metalloproteinase-9 is elevated in serum of alcohol abusers. Eur J Clin Invest. 2002;32:225–229. doi: 10.1046/j.1365-2362.2002.00975.x. [DOI] [PubMed] [Google Scholar]
- 33.Shapira Y, Lam AM, Paez A, Artru AA, Laohaprasit V, Donato T. The influence of acute and chronic alcohol treatment on brain edema, cerebral infarct volume and neurological outcome following experimental head trauma in rats. J Neurosurg Anesthesiol. 1997;9:118–127. doi: 10.1097/00008506-199704000-00003. [DOI] [PubMed] [Google Scholar]


