Abstract
Objective: To investigate the inducible effect of hemin on exogenous neuroglobin (Ngb) in focal cerebral hypoxic-ischemia in rats. Methods: 125 healthy SD rats were randomly divided into five groups: sham-operation control group, operation group, hemin treatment group, exogenous Ngb treatment group, and hemin and exogenous Ngb joint treatment group. Twenty-four hours after focal cerebral hypoxic-ischemia, Ngb expression was evaluated by immunocytochemistry, RT-PCR, and western blot analyses, while the brain water content and infarct volume were examined. Results: Immunocytochemistry, RT-PCR, and western blot analyses showed more pronounced Ngb expression in the hemin and exogenous Ngb joint operation group than in the hemin or exogenous Ngb individual treatment groups, thus producing significant differences in brain water content and infarct volume (p < 0.05). Conclusions: Hemin may be beneficial in protecting against focal cerebral hypoxic-ischemia through inducing the expression of exogenous Ngb.
Keywords: Hemin, neuroglobin, focal cerebral hypoxic-ischemia, neuroprotection
Introduction
Hemin, also known as hematin chloride, porphyrin iron chloride, and ferriheme, is a product of the oxidation of heme and has a wide range of biological functions. Hemin is a substrate and an inducing agent for heme oxygenase (HO) [1,2]. By inducing the expression of HO-1, it promotes the production of endogenous CO, which prevents damage caused by radicals, reduces excitatory amino acid toxicity and has important protective functions against hypoxic-ischemia (H-I) damage in organs such as brain, heart, lung and intestine [1,2].
Neuroglobin (Ngb) [5] was the third type of globin discovered after myoglobin and hemoglobin, first reported by German scientists Burmester et al. [6] in 2000. Its name reflects its main expression in the nervous system. Research showed that Ngb mRNA-positive neurons are distributed across the cerebral cortex, all subdivisions of the hippocampus (CA1-4), forebrain nuclei, thalamus, and hypothalamus. Mitral cells in the olfactory bulb stain positive, with large cell bodies and relatively weak staining intensity. The Ngb mRNA-positive cells in the cerebellum stain strongly and are largely Purkinje cells. Ngb mRNA-positive neurons were also found in the brainstem, the reticular formation of the pons, and the pontine nuclei, albeit in small amounts. Current research indicates that Ngb plays an important role in the pathological and biological mechanisms of cerebral ischemic disease.
Stroke is a common cause of death and a leading cause of adult disability worldwide. Ischemic stroke constitutes 85% of all stroke cases. No effective treatment has been found to prevent damage to the brain except tissue plasminogen activator, which has a narrow therapeutic window [7]. The mechanisms of ischemic stroke have been reported to involve apoptosis, necrosis, inflammation, oxidative stress, excitotoxicity, and withdrawal of trophic factors [7]. Such complex pathogenesis means that no drug yet exists that both prevent onset and reverses all damage from ischemic stroke. Therefore, it is extremely important to expand research on the prevention and control of ischemic cerebrovascular disease.
In the present study, we aim to investigate the protective functions of Ngb in cerebral ischemia by examining the increase in exogenous Ngb expression after cerebral ischemia in rats. In addition, we provide new information pertinent to the prevention and control of ischemic disease in the future.
Material and methods
Drugs, reagents and antibodies
Hemin and triphenyltetrazolium chloride (TTC) were purchased from Sigma-Aldrich Co., USA (hemin was dissolved in NaOH and its pH adjusted to 7.4 using a phosphate buffer solution; the concentration was 12.5 g/L). The recombinant plasmid pCDNA3.1 (+)/Ngb was purchased from Genechem, Shanghai. Immunohistochemistry kits were purchased from Bioss biological reagents Biotechnology Co., Ltd., Beijing. Goat-anti-Ngb polyclonal antiserum was purchased from Zhongshan Biological Reagent Co., Beijing.
Animals and drug treatments
Healthy adult Sprague Dawley (SD) male rats (n = 125) with body weights 200~250 g were purchased from the Experimental Animal Center of Chongqing Medical University. They were divided into five groups of 25 each: the sham-operation control group, the operation group, the hemin treatment group, the exogenous Ngb treatment group, and the hemin and exogenous Ngb joint treatment group. For the sham-operation control group, the skin around the neck was cut open, the carotid artery and its branches were separated, but no artery ligation or occlusion was performed. For the operation group, the permanent focal right cerebral middle artery occlusion (MCAO) procedure was carried out. For the hemin treatment group, hemin (50 mg/kg) was injected intraperitoneally 12 h before the right cerebral MCAO. For the exogenous Ngb treatment group, exogenous Ngb was stereotactically positioned in the cerebral cortex 24 h before the right cerebral MCAO. Similarly, for the hemin and exogenous Ngb joint treatment group, exogenous Ngb was stereotactically positioned in the cerebrum 24 h before the operation and hemin was injected intraperitoneally 12 h before the operation. The rats in each group were divided into five sub-groups (five in each sub-group), used for immunocytochemistry, RT-PCR, western blot analysis, TTC staining and brain water content measurement 24 h after the operations.
MCAO model
A permanent focal right cerebral middle artery cerebral ischemia model was reproduced according to the Longa method [5]. The occlusion was inserted from the right common carotid artery bifurcation to stop the blood flow to the right cerebrum. Pre-intraperitoneal injections of 10% chloral hydrate (0.3 mL/100 g) were administered to the rats for anesthesia. Their breathing and heart rate were monitored during the operations. Rats in all groups were sacrificed by decapitation 24 h after the operation.
Brain infarct volume measurement
Five rats from each group were selected and sacrificed by decapitation 24 h after the operations. After removing the olfactory bulb, the cerebellum and the lower brain stem, the rest of the brain was frozen at -20°C before six consecutive coronal sections were taken at 2-mm intervals. They were put into 2% TTC phosphate buffer solution (pH = 7.4) and incubated in 37°C incubators in the dark for 20 min. Normal brain tissues were stained deep red, and infarcted brain tissues appeared white. Photos of the sections were taken, and Photoshop 7.0 image-processing software was used to calculate the volume of the brain infarct.
Neurobehavioral scores
Rats were scored according to the Longa 5-mark scoring [5] standard when they regained consciousness after the operation. The following criteria were used to score the behavior of the rats: 0 marks: no neurological impairment; 1 mark: not being able to fully stretch the left front paw; 2 marks: turning to the left in a circle; 3 marks: toppling to the left while walking; 4 marks: not being able to walk spontaneously, 5 marks: showing disturbances in consciousness.
Brain water content measurement
Five rats from each group were selected and sacrificed by decapitation 24 h after the operation. The brain tissue was taken out and weighed immediately (using the dry-wet method) before being dried in an oven until the weight became constant. The brain water content was calculated using the formula: Brain tissue water content (%) = (wet weight-dry weight)/wet weight × 100%.
Immunohistochemistry
The ABC method was used for immunohistochemical reactions. The sections were dewaxed using xlyene and an alcohol gradient placed in 3% peroxide and incubated for 30 min to stop the activity of the endogenous catalase. Sections were then washed three times with 0.01 mol/L PBS for 5 min each time. The antigens were heat-revived, and the sections were incubated in normal goat serum working solution at 37°C for 30 min. The normal goat serum was discarded, and the goat-anti-Ngb polyclonal antiserum (working concentration 1:500) was added to the sections and incubated overnight at 4°C. The sections were washed three times with 0.01 mol/L PBS for 5 min each time and incubated with horseradish peroxide (HRP)-labeled active rabbit-anti-goat IgG working solution at 37°C for 30 min and washed three times with 0.01 mol/L PBS for 5 min each time. The sections were then stained for 3-5 min in DAB (3, 3’-diaminobenzidine) staining solution, re-stained in hematoxylin, differentiated using hydrochloric alcohol, and gradient dehydrated. After the sections were sealed with neutral balsam, photos were taken and analyzed.
RT-PCR
The total RNA in each sample mentioned was extracted using the TRlzol reagent kit. The A260/A280 values were measured using a UV spectrophotometer. The primers were designed according to rat Ngb cDNA sequence with upstream: 5-CGTTGACATCCGTAAAGACCTC-3, downstream: 5-TAGGAGCCAGGGCAGTAATCT-3. The amplification product was 110 bp long. GAPDH was used as an internal control with its primers as: upstream: 5-GTGAGCCGCAGCCCTCTGGAACAT-3 and downstream: 5-AGCAGGGACTCACCTACTGTCG-3. The amplification product was 296 bp long. Equal amounts (1 μg) of total RNA were taken from each of the samples mentioned above and cNDA was produced using the AMV reverse-transcription reagent kit. 1 μL of each of the resultant cDNA was used as a template in PCR reactions together with Ngb and GAPDH primers (the volume of the reaction was 25 L). PCR amplification conditions were 95°C pre-denaturation for 1 min, 95°C for 15 s, 58°C for 15 s, and 72°C for 45 s, repeated for 40 cycles. To analyze the result, 5 μL of the PCR product was subjected to agarose gel electrophoresis. The result was recorded using a UV gel imaging analysis system. ImageJ software was used to measure the average grayscale and area of each electrophoresis band. The integrated absorbance (IA) of each band was calculated using the equation (IA) = -log10 [255/(255-gray)] × area. This value represented the relative concentration of the substances within the measured regions. Thus, the calculated IA values (cIA) of Ngb enabled us to semi-quantitatively analyze Ngb expression.
Western blot
Five rats were randomly selected from each group. Their brains were extracted, placed in cell lysis buffer, and homogenized at low temperature. They were then left standing for 10 min and 90 μL of 100 g/L NP-40 was added. They were shaken vigorously for 30 seconds and centrifuged at 4°C, 13,000 rpm for 15 min. The supernatant was removed and stored separately at -80°C. After the volume of protein was determined, four times its volume of sample buffer was added, and the samples were denatured at 95°C for 5 min. Approximately 30 μg samples were electrophoresed on 10% SDS-polyacrylamide gel and transferred to nitrocellulose membranes. Band strips were cut according to molecular weights. Rabbit-anti-Ngb polyclonal antibody (1:200) and β-actin monoclonal antibody (1:400) were added to the corresponding strips and incubated overnight at 4°C. After washing with TBST, the strips were incubated separately with biotin-labeled goat-anti-rabbit IgG anti serum (1:100) at room temperature with vibration for 1.5 h. They were then washed with TBST, incubated with avidin-HRP complex at room temperature for 2 h and stained with DAB. The specific protein bands were scanned using β-actin as an internal control. The grayscale of the bands was determined using the Quantity-One image analysis system.
Statistical analysis
Data were analyzed with SPSS13.0, and the significance level was set at p < 0.05. The results of semi-quantitative pathological evaluation were compared among hemin and exogenous Ngb joint treatment group and hemin treatment group and the exogenous Ngb treatment group with Student-Newman-Keuls Test.
Results
Hemin offers neuroprotection in a rat model of cerebral ischemia
Experiments were conducted to measure brain infarct volume. After TTC staining, all the rats from the operation group, the hemin treatment group, the exogenous Ngb treatment group, and the hemin and exogenous Ngb joint treatment group showed white regions in the right cerebral hemisphere cortex and subcortex, while no white regions were seen in the sham-operation group. Brain infarct volume was calculated using Image Pro Plus 4.5 (IPP) image processing software. The vehicle-only group showed a remarkable high infarct volume (0.426 ± 0.021), while the hemin and exogenous Ngb joint treatment group (0.248 ± 0.029) showed significantly different results compared with the hemin single treatment (0.301 ± 0.019) or Ngb treatment (0.299 ± 0.032) groups (*p < 0.05; Figure 1, Table 1). Our results demonstrate that hemin decreases infarct volume produced by cerebral ischemia-induced injury. After the MCAO operations, all rats achieved different neurobehavioral scores (*p < 0.05; Table 2). Our results demonstrate that hemin decreases neurobehavioral scores between different groups.
Figure 1.
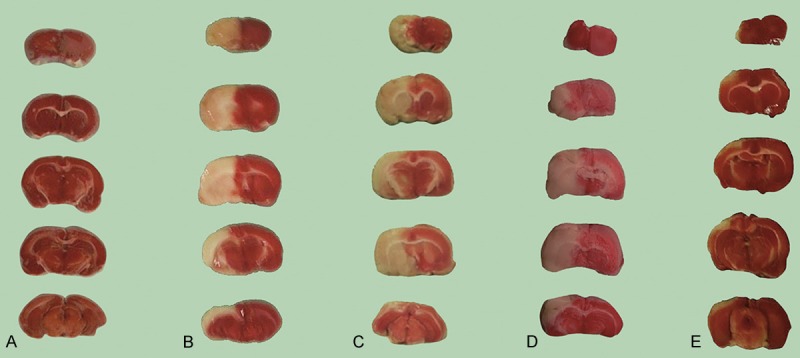
Hemin, exogenous Ngb, and Hemin plus exogenous Ngb decrease infract volume by cerebral ischemia-induced injury. Five rats of each group were treated as indicated and sacrificed 24 h after a permanent focal right cerebral middle artery cerebral ischemia operation. The brains were frozen at -20°C and a total of six consecutive coronal sections were taken at 2-mm intervals and submitted for TTC stain. Photos of the brain sections were taken and the volume of the brain infarct were calculated. A: Sham-operation group; B: Operation group; C: Hemin treatment group; D: Exogenous Ngb treatment group; E: Hemin and exogenous Ngb joint treatment group.
Table 1.
Comparison between the rat brain infarct volumes among groups
| Group | Brain infarct volume (cm3) |
|---|---|
| Sham-operation control group | 0 |
| Operation group | 0.426 ± 0.021 |
| Hemin treatment group | 0.301 ± 0.019 |
| Ngb treatment group | 0.299 ± 0.032 |
| Hemin and Ngb treatment group | 0.248 ± 0.029 |
Rats were treated as in Figure 1. The infract volume (average ± standard deviation) were shown. The operation group, the hemin treatment group, the exogenous Ngb treatment group and the hemin and exogenous Ngb joint treatment group (n = 5/per group) all had right-side homers symptoms after the operations, that is, they were unable to stretch the front left paw forward.
Table 2.
Comparison of neurological scores among groups
| Group | Neurological scores |
|---|---|
| Sham-operation control group | 0 |
| Operation group | 3.53 ± 0.027 |
| Hemin treatment group | 2.96 ± 0.019 |
| Ngb treatment group | 3.02 ± 0.032 |
| Hemin and Ngb treatment group | 2.48 ± 0.029 |
Rats were treated as in Figure 1 (n = 5). Neurological scores were recorded (average ± standard deviation).
Hemin decreases brain water content in a rat model of cerebral ischemia
All the other groups showed significant differences in brain water content compared to the control group (**p < 0.01); the hemin and exogenous Ngb joint treatment group showed less water content compared with the hemin treatment group and the exogenous Ngb treatment group (*p < 0.05); however no significant difference was seen between the hemin treatment group and the exogenous Ngb joint treatment group (Table 3).
Table 3.
Comparison of rat brain water content among indicated groups
| Group | Brain water content (%) |
|---|---|
| Sham-operation control group | 76.65 ± 0.06 |
| Operation group | 79.17 ± 0.73 |
| Hemin treatment group | 78.31 ± 0.21 |
| Ngb treatment group | 78.23 ± 0.37 |
| Hemin and Ngb treatment group | 77.82 ± 0.19 |
Rats were treated as in Figure 1 (n = 5). Brain water content were measured (average ± standard deviation).
Hemin increases expression of exogenous Ngb in rats under focal cerebral H-I conditions
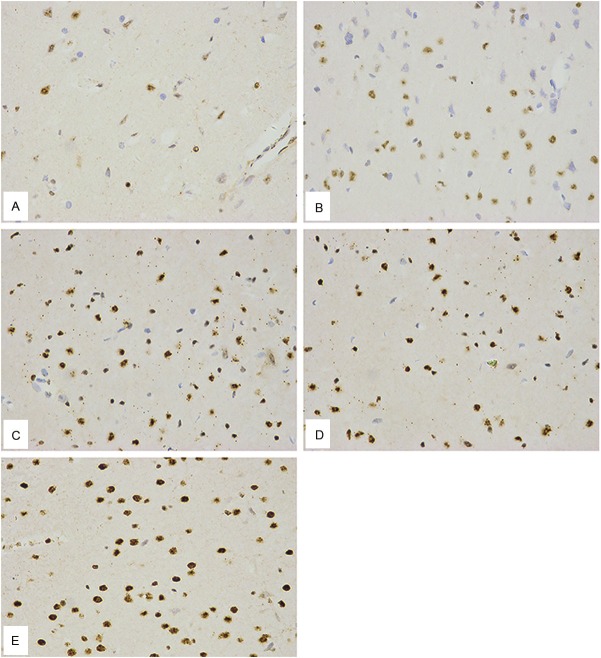
The immunohistochemical staining of brain sections showed Ngb immune-positive cells as brown, with positive areas located in the neuron cytoplasm. The nuclei of the negative controls were stained blue, whereas the cytoplasm and whole pieces of tissue appeared either colorless or light blue, like the background. The hemin and exogenous Ngb-joint treatment group showed many more positive neurons in ischemic regions compared to the two single-treatment groups. The hemin treatment group and the exogenous Ngb treatment group did not significantly differ in the number of positive neurons, which in both cases was higher than in the operation group (Figure 2, Table 4).
Figure 2.
Hemin increases expression of exogenous Ngb in rats under focal cerebral Hypoxic-Ischemic conditions. Cerebral cortex Ngb immunohistochemical examinations of indicated group were shown. A: Sham-operation group; B: Operation group; C: Hemin treatment group; D: Exogenous Ngb treatment group; E: Hemin and exogenous Ngb joint treatment group (The brown positive substances in the cytoplasm were Ngb immune-positive neuron cells; the neuron nuclei were blue; the light blue or colorless cytoplasm was the negative control).
Table 4.
Number of Ngb positive cells in the cerebral cortex of rats
| Group | Number of Ngb positive cells |
|---|---|
| Sham-operation control group | 11.55 ± 3.90 |
| Operation group | 23.75 ± 5.25 |
| Hemin treatment group | 41.58 ± 2.46 |
| Ngb treatment group | 43.21 ± 4.74 |
| Hemin and Ngb treatment group | 64.92 ± 3.66 |
The numbers of Ngb positive cells in the cerebral cortex of indicated rat group (n = 5) were shown (average ± standard deviation).
After the MCAO operation, cerebral cortex Ngb mRNA expression in the hemin and exogenous Ngb joint treatment group was much more than the two single-treatment groups (versus the hemin group, **p < 0.01; versus the exogenous Ngb treatment group, **p < 0.01). There was no significant difference (p > 0.05) between the hemin treatment group and the exogenous Ngb treatment group, which had significantly higher expression than the operation group (versus the hemin group, **p < 0.01; versus the exogenous Ngb treatment group, **p < 0.01; Figures 3 and 4).
Figure 3.
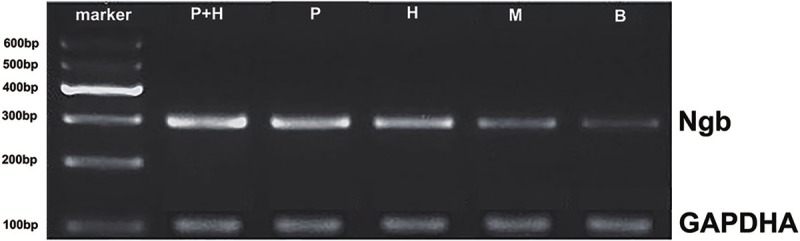
The expression of Ngb mRNA in cerebral cortex of different treatments. Rat cerebral cortex of indicated groups were obtained and total RNA were extracted from tissue homogenate and were analyzed by RT-PCR using primers appropriate for the Ngb transcripts. GAPDH were used as the internal control. P+H: Hemin and exogenous Ngb joint treatment group; P: Exogenous Ngb treatment group; H: Hemin treatment group; M: Operation group; B: Blank; GAPDH: internal control.
Figure 4.
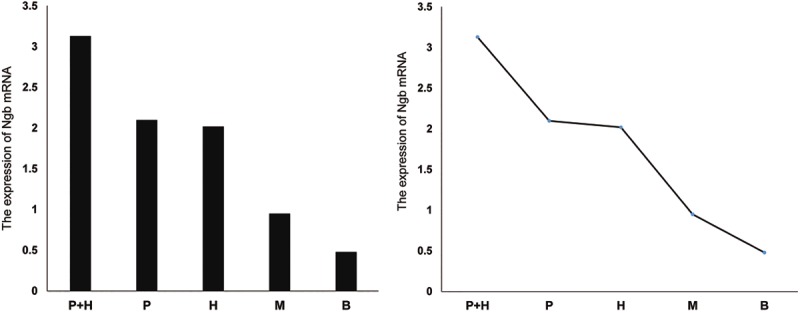
Variation in the expression of Ngb mRNA in the rat cerebral tissues of each group. Rat cerebral cortex of indicated groups were obtained and total RNA were extracted and analyzed by RT-PCR using Ngb primers. P+H: Hemin and exogenous Ngb joint treatment group; P: Exogenous Ngb treatment group; H: Hemin treatment group; M: operation group; B: Blank control.
After the MCAO operations, Ngb protein expression was significantly higher in the hemin and exogenous Ngb joint treatment group than in either of the two single-treatment groups (versus the hemin group, **p < 0.01; versus the exogenous Ngb treatment group, p < 0.01). No statistical significance (p > 0.05) was found between the hemin treatment group and the exogenous Ngb treatment group, both of which had significantly higher expression than the operation group (versus the hemin group, **p < 0.01; versus the exogenous Ngb treatment group, **p < 0.01; Figure 5).
Figure 5.
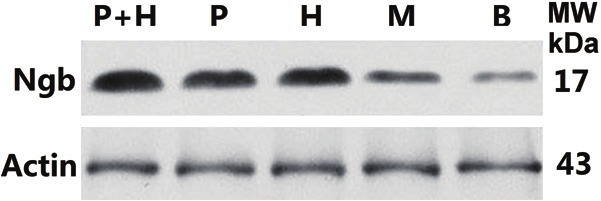
Hemin, exogenous Ngb, and Hemin plus exogenous Ngb increases the expression of Ngb protein in cortex of MCAO rat. Rat cerebral cortex of indicated groups were obtained and proteins were extracted from tissue homogenate and analyzed by Western blots using antibodies against Ngb. β-actin was used as the internal control. P+H: Hemin and exogenous Ngb joint treatment group; P: Exogenous Ngb treatment group; H: Hemin treatment group; M: Operation group; B: Blank control; β-actin: internal control.
Discussion
In situ hybridization has indicated that Ngb mRNA is located inside the neuron cytoplasm [8]. Immunohistochemistry demonstrates that Ngb-positive cells are widely distributed in rat cerebral tissues, with varying densities in different regions and Ngb expression positively correlated with the degree of tolerance to hypoxia [9]. In recent years, murine experiments have proven that in acute cerebral hypoxia-ischemia, the expression of Ngb at the mRNA and protein levels is increased, which indicates that Ngb is very sensitive to H/I and simultaneously produces protective stress responses [7]. We have conducted the first study jointly applying hemin and Ngb recombinant plasmid to cerebral ischemia injury, providing experimental evidence for the capability of exogenous Ngb to prevent H/I nerve damage, and laying the foundation not only for clinical trials, but also for related social and economic benefits [10]. Wang et al. previously showed that Ngb expression increases during the acute phase of cerebral ischemic injury, which similar effects exist in the chronic phase, and that increased in expression can persist for 2 weeks. They also indicated that the induction of Ngb expression had important protective functions on neurons [11].
Experiments showed that hemin had a substantial inducible effect on Ngb expression in vitro [12], dependent on both concentration and time. The induction of Ngb by hypoxia and hemin are controlled by different signaling pathways, indicating that these two factors have different underlying mechanisms [12].
In the present study, we used traditional methods to build an MCAO model, used western blotting and RT-PCR to examine the changes in Ngb protein and mRNA expression in rat cortex, and used TTC staining to measure the area affected by cerebral ischemia. The results showed that when comparing the hemin and exogenous Ngb joint treatment groups with the two single treatments, Ngb expression was significantly higher at both genetic and protein levels, whereas the differences in the Ngb gene and protein expression levels were not profound compared to the hemin treatment group and the exogenous Ngb treatment group, but were significant relative to the operation group. Thus, under focal cerebral H-I conditions in rats, hemin induced the increase of exogenous Ngb expression, yielding neuroprotective effects.
Our data shows that hemin induces the increase of exogenous expression of Ngb, a type of globin present in the nervous system, whose role has been studied extensively. It has been found to provide oxygen to mitochondria to increase the neuron’s tolerance to hypoxemia, to remove active oxygen to protect tissues from oxidizing effects, to detoxify various reactions in the body, to play a role in the signaling pathways, and to participate in redox reactions in the body [13-16]. Our experiment demonstrates that hemin induces increased exogenous Ngb expression in focal cerebral H-I in rats, paving the way for further research concerning the inductive mechanisms.
H-I cerebrovascular disease threatens health and shortens life expectancy, affecting quality of life worldwide. With an increasing aging population, its incidence and mortality rate are steadily increasing. However, due to its complex pathogenesis, a drug to prevent the onset and reverse all of the effects of H-I stroke does not exist. Therefore, the investigation of hemin plays an extremely important part in the prevention and control of H-I cerebrovascular disease. Our present study provides important experimental data on hemin’s neuroprotective functions in the strong potential for future clinical application in supporting patients suffering from acute H-I disease.
Acknowledgements
This work was supported by funds from Chongqing Natural Science Project Fund (2010JJ0254 to J. Z.), National Clinical Key Specialty Construction Project (to J. Z.), the Bill & Melinda Gates Foundation (to X. W.), the ALS Therapy Alliance (to X. W.), and the Muscular Dystrophy Association (to X. W.).
Disclosure of conflict of interest
None.
References
- 1.Zijlstra GS, Brandsma CA, Harpe MF, Van Dam GM, Slebos DJ, Kerstjens HA, De Boer AH, Frijlink HW. Dry powder inhalation of hemin to induce heme oxygenase expression in the lung. Eur J Pharm Biopharm. 2007;67:667–675. doi: 10.1016/j.ejpb.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 2.Hualin C, Wenli X, Dapeng L, Xijing L, Xiuhua P, Qingfeng P. The anti-inflammatory mechanism of heme oxygenase-1 induced by hemin in primary rat alveolar macrophages. Inflammation. 2012;35:1087–1093. doi: 10.1007/s10753-011-9415-4. [DOI] [PubMed] [Google Scholar]
- 3.Ahanger AA, Prawez S, Leo MD, Kathirvel K, Kumar D, Tandan SK, Malik JK. Pro-healing potential of hemin: an inducer of heme oxygenase-1. Eur J Pharmacol. 2010;645:165–170. doi: 10.1016/j.ejphar.2010.06.048. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Ari Z, Issan Y, Katz Y, Sultan M, Safran M, Michal LS, Nader GA, Kornowski R, Grief F, Pappo O, Hochhauser E. Induction of heme oxygenase-1 protects mouse liver from apoptotic ischemia/reperfusion injury. Apoptosis. 2013;18:547–555. doi: 10.1007/s10495-013-0814-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 6.Burmester T, Weich B, Reinhardt S, Hankeln T. A vertebrate globin expressed in the brain. Nature. 2000;407:520–523. doi: 10.1038/35035093. [DOI] [PubMed] [Google Scholar]
- 7.Pandya RS, Mao L, Zhou H, Zhou S, Zeng J, Popp AJ, Wang X. Central nervous system agents for ischemic stroke: neuroprotection mechanisms. Cent Nerv Syst Agents Med Chem. 2011;11:81–97. doi: 10.2174/187152411796011321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang C, Wang C, Deng M, Li L, Wang H, Fan M, Xu W, Meng F, Qian L, He F. Full-length cDNA cloning of human neuroglobin and tissue expression of rat neuroglobin. Biochem Biophys Res Commun. 2002;290:1411–1419. doi: 10.1006/bbrc.2002.6360. [DOI] [PubMed] [Google Scholar]
- 9.Sun YJ, Jin KL, Mao XO, Zhu YH, Greenberg DA. Neuroglobin is up-regulated by and protects neurons from hypoxic-ischemic injury. Proc Natl Acad Sci U S A. 2001;98:15306–15311. doi: 10.1073/pnas.251466698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shang A, Zhou D, Wang L, Gao Y, Fan M, Wang X, Zhou R, Zhang C. Increased neuroglobin levels in the cerebral cortex and serum after ischemia-reperfusion insults. Brain Res. 2006;1078:219–226. doi: 10.1016/j.brainres.2006.01.064. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Liu J, Zhu H, Tejima E, Tsuji K, Murata Y, Atochin DN, Huang PL, Zhang C, Lo EH. Effects of neuroglobin overexpression on acute brain injury and long-term outcomes after focal cerebral ischemia. Stroke. 2008;39:1869–1874. doi: 10.1161/STROKEAHA.107.506022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu Y, Sun Y, Jin K, Greenberg DA. Hemin induces neuroglobin expression in neural cells. Blood. 2002;100:2494–2498. doi: 10.1182/blood-2002-01-0280. [DOI] [PubMed] [Google Scholar]
- 13.Burmester T, Hankeln T. What is the function of neuroglobin? J Exp Biol. 2009;212:1423–1428. doi: 10.1242/jeb.000729. [DOI] [PubMed] [Google Scholar]
- 14.Brittain T, Skommer J, Henty K, Birch N, Raychaudhuri S. A role for human neuroglobin in apoptosis. IUBMB Life. 2010;62:878–885. doi: 10.1002/iub.405. [DOI] [PubMed] [Google Scholar]
- 15.Giuffre A, Moschetti T, Vallone B, Brunori M. Neuroglobin: enzymatic reduction and oxygen affinity. Biochem Biophys Res Commun. 2008;367:893–898. doi: 10.1016/j.bbrc.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Hundahl C, Kelsen J, Kjaer K, Ronn LC, Weber RE, Geuens E, Hay-Schmidt A, Nyengaard JR. Does neuroglobin protect neurons from ischemic insult? A quantitative investigation of neuroglobin expression following transient MCAo in spontaneously hypertensive rats. Brain Res. 2006;1085:19–27. doi: 10.1016/j.brainres.2006.02.040. [DOI] [PubMed] [Google Scholar]