Abstract
Kimura’s disease (KD) is a rare chronic disease with unknown origin. It remains controversial in KD’s diagnosis, treatment, transformation and need further research. The aim of this study is to investigate the clinicopathologic features of KD and the relationship between the expression of Notch-1, Ki-67 receptor and the recurrence of KD. The hematoxylin and eosin sections and clinical data of 40 patients diagnosed with KD were examined retrospectively. Specimens were available in these 40 cases. Notch-1 and Ki-67 expression were examined using IHC (immunohistochemistry staining) analysis. Of 40 cases of KD (average age, 38.4 years; median age, 36.0 years), 34 cases (85.0%) were clinically seen to involve swelling of the head and neck region. Notch-1 and Ki-67 have a high expression in recurrent patients. High expression of Notch-1 receptor and Ki-67 tended to be found in patients who relapsed. This is the first study to discuss the correlation among Notch-1, Ki-67 and recurrent KD. These results suggest both of the markers may act as promising predictors for the recurrence and prognosis of KD. However, Notch-1 immunoexpression had no statistically significant association with the Ki-67 proliferation index.
Keywords: Notch-1 receptor, Ki-67 proliferation index, recurrence, Kimura’s disease (KD)
Introduction
Kimura’s disease (KD), a chronic inflammatory disorder of unknown cause, is found mainly in East Asian men aged in their twenties and thirties [1]. It usually presents in the head and neck regions with painless, slow-growing subcutaneous masses [2]. KD can coexist with nephritic syndrome and bronchial asthma [3,4].
Using ultrasound and CT alone, it is possible for experienced radiologists to misdiagnose KD as other kinds of malignant disease [5]. Through microscopic findings, KD manifests marked eosinophilic counts, and surrounding fibrosis may extend into the lesions. As for the approaches illustrated above, pathology is the most sensitive way to confirm this disease.
Nowadays routine treatment for KD includes surgical excision, steroid medication, and radiation therapy. Despite its benign characteristics, KD has a high recurrence rate, 14-44%, and there is no optimal treatment to date [6]. Moreover, some published literature asserts the coexistence of cancer with KD [7]. It is unknown whether KD is directly associated with carcinoma or instead is subject to a malignant transformation. Further research will be needed to verify it. According to KD, the presence of peripheral eosinophils, increased mast cells, and increased levels of IL5 and immunoglobulin E (IgE) suggests an abnormal T cell stimulation similar to a hypersensitivity-type reaction [1]. We may hope to find markers of KD would predict its recurrence, thereby helping the prognosis of patients.
Notch-1 is a multifunctional transmembrane receptor and has a crucial double role in human cancer cells by regulating cell-fate decision, proliferation, differentiation, and apoptosis [8]. It was first found in human T-cell neoplasia for its oncogenic role [9]. It has since been found expressed in many kinds of cancer, including colon cancer, renal cell carcinoma, pancreatic cancer, prostate carcinoma, and lymphomas [10]. It is highly expressed in tumor cells of Hodgkin and anaplastic large cell lymphoma [11]. Because KD shares clinical and pathological characteristics with lymphoma, the two conditions are easily misdiagnosed.
Ki-67 is an immunoenzyme-labeled tumor-proliferating antigen and cell-cycle-regulating protein. The Ki-67 index (fraction of Ki-67-positive nuclei in IHC) is higher in metastatic disease than disease without metastasis. It is suggested that a high Ki-67 index could define a group of patients with poor prognosis [12]. Ki-67 has diverse positive expressions in different kinds of lymphoma and acts as a predictor of the prognosis of lymphoma [13].
The aim of this present study was to evaluate the factors that affect the recurrence of KD. IHC staining was used to investigate the expression of Notch-1 and Ki-67 to find out whether these can serve as predictors of the treatment of recurrence of KD.
Materials and methods
Patient selection and available specimens
This study includes 40 patients diagnosed with KD at the Sun Yat-Sen University Cancer Center from January 2000 to July 2013. The cases selected were based on the following criteria: histologically proven KD with available biopsy specimens, no previous treatment, and regular follow-up. The mass biopsy specimens were retrieved from paraffin blocks of the 40 KD patients from the Department of Pathology of our cancer center, and 20 samples of chronic lymphnoditis were used for controls. The study was approved by the medical ethics committee of our cancer center. We retrospectively examined patients’ clinicopathological characteristics including gender, mass location, size, status of recurrence, disease-free survival (DFS), and the expression of Notch-1 receptor and Ki-67 proliferation index.
Evaluation and follow-up
Information was collected on 40 cases of KD treated in Sun Yat-Sen University Cancer Center via telephone conversations and outpatient follow-up visits up to December 2013. The follow-up period was from the date of the definitive diagnosis to the final visit. The diagnostic examinations consisted of ultrasonography and blood examination, X-ray, and computed tomography (CT) to diagnose the recurrence.
Immunohistochemistry procedure
Immunohistochemistry (IHC) staining was performed on sections (5 μm thick) and deparaffinized through graded alcohols. Endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide for 15 min. For antigen retrieval, tissue slides were heated in Tris (hydroxymethyl) aminomethane-EDTA buffer (pH 8.0) in a microwave and then either treated for 10 min (Ki-67) and a pressure cooker for 12 min (Notch-1). Nonspecific binding was blocked with 10% normal rabbit serum for 20 min. The tissue slides were incubated with anti-Notch-1 (Cell Signaling Technology, Inc., US, 1:100 dilution) or anti-Ki-67 (Dako, Glostrup, Denmark, 1:50 dilution) in a moist chamber for 60 min at 37°C. The slides were sequentially incubated with biotinylated rabbit anti-mouse immunoglobulin with a concentration of 1:100 at 37°C for 30 min, then reacted with a streptavidin-peroxidase conjugate at 37°C for 30 min. For a negative control, the primary antibody was omitted in the staining procedure. Human chronic lymphnoditis samples were used as positive controls.
The sections were analyzed using an Olympus BX43 microscope. The stained slices were evaluated at 200X magnification. Two experienced pathologists analyzed these results independently. The intensity of the immunoreaction for Notch-1 was measured using a descriptive scale as follows: negative staining (“−”, score = 0), weak staining (“+”, score = 1), moderate and strong staining (“++”, score = 2) [14] (Figure 1). Ki-67 was denoted by the proliferation index, which is the ratio of positive nuclei expression and the total number of neoplastic cells. Inside the KD’s germinal centers, Ki-67 was highly expressed with a range of 70% to 90% (Figure 2). Outside the germinal centers, low proliferation (≤ 3%) and high proliferation (> 3%) were measured [15,16].
Figure 1.
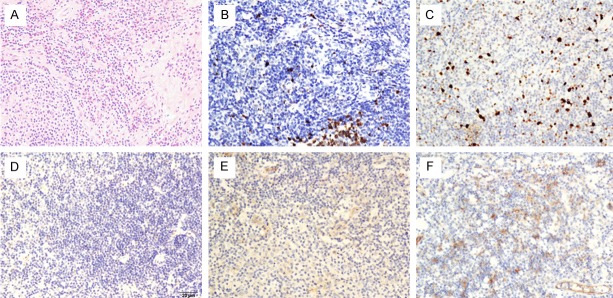
Immunohistochemical staining of Notch-1 and Ki-67 expression in Kimura’s disease. A. Hematoxylin and eosin staining in KD (X200); B. Low expression of Ki-67 in KD (X200); C. High expression of Ki-67 in KD (X200); D. Negative expression of Notch-1 in KD (X200); E. Low expression of Notch-1 in KD (X200); F. High expression of Notch-1 in KD (X200).
Figure 2.
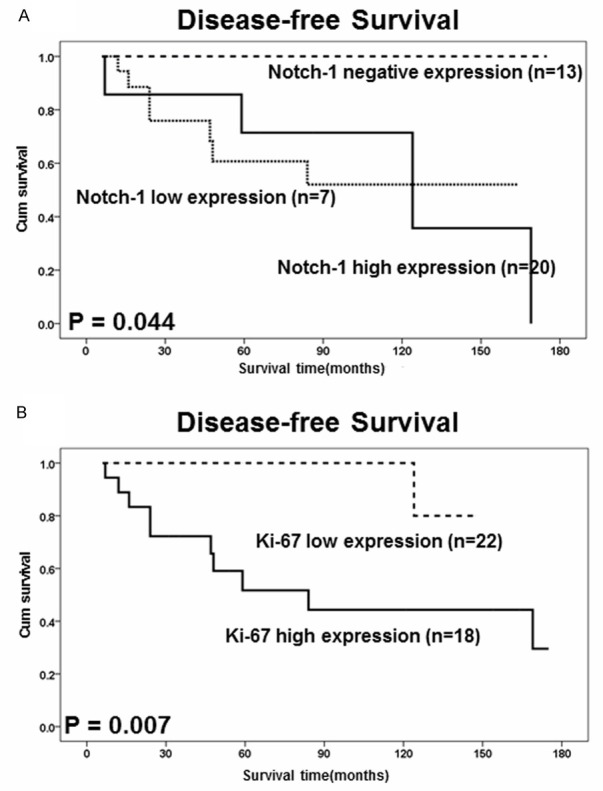
Kaplan-Meier estimates of disease-free survival (DFS) with respect to Notch-1 and Ki-67 expression in 40 KD patients. A. DFS curves stratified by Notch-1 expression in KD tissues. B. DFS curves stratified by Ki-67 expression in KD tissues.
Statistical analysis
Statistical analysis was performed using SPSS software (SPSS version 16.0, Chicago, IL, USA). A binary logistic regression model was used to analyze the correlation of variables with Notch-1 expression. The association of Notch-1 expression and the Ki-67 proliferation index (the index from outside the germinal centers) with KD patients’ clinicopathological features was assessed by the χ2 test. The disease-free survival (DFS) rates were assessed with the Kaplan-Meier method and compared by the log-rank test. Multivariate survival analysis was performed on all the factors that were found to be significant different in univariate analysis through the Cox regression model. A P-value of less than 0.05 was considered statistically significant.
Results
Clinical findings
The patient characteristics for both groups are summarized in Table 1. Of the 40 patients pathologically diagnosed with KD, 3 were male and 37 were male, with a female-to-male ratio of 1:12.3. The average age of KD patients was 38.4 years old, with a range from 10 to 75 years old (median: 36.0 years). Follow-up time ranged from 6 months to 175 months (mean: 69.8 months). For the relapse patients, the mean of follow-up time is 55.8 months with a range from 7 to 169 months (median: 47.0 months).
Table 1.
Clinical characteristics of 40 patients with Kimura’s disease
| Clinical characteristics | No. (%) |
|---|---|
| Total cases | 40 |
| Age, years | |
| Median | 36 |
| Range | 10-75 |
| Gender | |
| Male | 37 (92.5%) |
| Female | 3 (7.5%) |
| Peripheral eosinophilia (%) | |
| < 20% | 18 (45%) |
| 20-35% | 17 (42.5%) |
| > 35% | 5 (12.5%) |
| Initial location | |
| Head and neck regions | 34 (85.0%) |
| Other regions | 6 (15.0%) |
| Largest sizes | |
| < 3.5 cm | 25 (62.5%) |
| ≥ 3.5 cm | 15 (37.5%) |
| Multiplicity | |
| Single | 11 (27.5%) |
| Multiple | 29 (72.5%) |
| Laterality | |
| Unilateral | 36 (90.0%) |
| Bilateral | 4 (10.0%) |
The most common lesion locations at initial presentation were the head and neck regions (85%), while other regions made up 15%. All the patients had peripheral eosinophilia (mean: 21.0%, range: 5%-46%) (normal reference: 0.5%-5%) on initial admission. The average largest size in initial mass was 3.5 cm with a range from 1.0 to 6.5 cm (median: 3.0 cm). 11 patients have single mass (27.5%) and other 29 patients with multiple masses (72.5%). As for mass laterality, 90.0% of the KD patients are unilateral and 10.0% are bilateral.
A statistically significant correlation was found between Notch-1 receptor expression and DFS of KD (P = 0.044) in Kaplan-Meier and log-rank survival tests. Statistical significance was also found for peripheral eosinophilia (%) (p = 0.001), Ki-67 proliferation index (p = 0.007), and largest mass sizes (p = 0.004) (Figures 2, 3). Quantitative analysis of IHC showed Notch-1 and Ki-67 expression in KD was statistical higher than that in chronic lymphnoditis samples (p < 0.05). χ2 test suggested correlations between Notch-1 and peripheral eosinophilia (%) and recurrence (Table 2). No significant association between expression of Notch-1 and other clinicopathological parameters such as gender, age, multiplicity, largest mass sizes. Statistical significant revealed between Ki-67 and recurrence with no other parameters associate with Ki-67 (Table 2).
Figure 3.
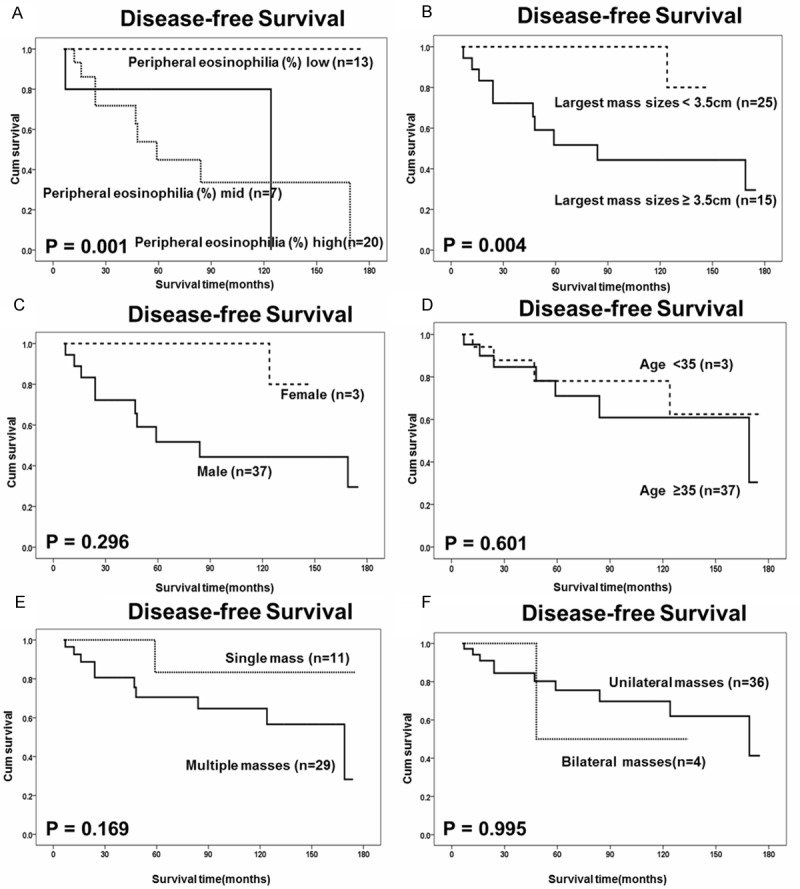
Kaplan-Meier estimates of disease-free survival (DFS) with respect to other parameters. A. DFS curves stratified by peripheral eosinophilia (%) in KD tissues. B. DFS curves stratified by largest mass sizes in KD tissues. C. DFS curves stratified by gender in KD tissues. D. DFS curves stratified by age in KD tissues. E. DFS curves stratified by multiplicity in KD tissues. F. DFS curves stratified by laterality in KD tissues.
Table 2.
Association of the expression of Notch-1 receptor, Ki-67 proliferation index and clinicopathological characters in 40 KD patients
| Variables | N | Notch-1 receptor | P-values | Ki-67 proliferation index | P-values | |||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| - | + | ++ | < 3% | ≥ 3% | ||||
| Gender | ||||||||
| Male | 37 | 12 | 7 | 18 | 0.688 | 19 | 18 | 0.103 |
| Female | 3 | 1 | 0 | 2 | 3 | 0 | ||
| Age, years | ||||||||
| < 35 | 19 | 6 | 2 | 11 | 0.480 | 10 | 9 | 0.775 |
| ≥ 35 | 21 | 7 | 5 | 9 | 12 | 9 | ||
| Multiplicity | ||||||||
| Single | 11 | 5 | 3 | 3 | 0.204 | 8 | 3 | 0.165 |
| Multiple | 29 | 8 | 4 | 17 | 14 | 15 | ||
| Largest size (cm) | ||||||||
| < 3.5 | 25 | 9 | 3 | 13 | 0.483 | 15 | 10 | 0.412 |
| ≥ 3.5 | 15 | 4 | 4 | 7 | 7 | 8 | ||
| Peripheral eosinophilia (%) | ||||||||
| < 20% | 17 | 8 | 2 | 7 | 0.025* | 10 | 7 | 0.847 |
| 20-35% | 18 | 2 | 3 | 13 | 9 | 9 | ||
| 35-50% | 5 | 3 | 2 | 0 | 3 | 2 | ||
| Recurrence status | ||||||||
| Recurrence | 11 | 0 | 4 | 7 | 0.014* | 1 | 10 | 0.001* |
| No recurrence | 29 | 13 | 3 | 13 | 21 | 8 | ||
In our univariate analysis, variables showed that significant prognosis factors included Notch-1 expression, Ki-67 proliferation index, largest mass sizes and peripheral eosinophilia (%). Moreover, multivariate survival analysis showed indeed predictive of the DFS of KD patients were found for the following items: Notch-1 receptor expression (95% CI: 2.258-449.207, p = 0.010), Ki-67 proliferation index (95% CI: 3.129-6.433 × 104, p = 0.016), peripheral eosinophilia (%) (95% CI: 4.282-1.207 × 105, p = 0.012) (Table 3).
Table 3.
Univariate and multivariate analyses of prognostic parameters in 40 patients with Kimura’s disease by Cox-regression analysis
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Hazard ratios | 95% CI | P-values | Hazard ratios | 95% CI | P-values | |
| Age (< 35/≥ 35) | 1.387 | 0.404-4.767 | 0.604 | - | - | - |
| Gender (male/female) | 0.042 | 0.000-412.779 | 0.500 | - | - | - |
| Initial location (head and neck/others) | 0.424 | 0.054-3.346 | 0.416 | - | - | - |
| Multiplicity (single/multiple) | 3.932 | 0.501-30.888 | 0.193 | - | - | - |
| Laterality (unilateral/bilateral) | 1.006 | 0.127-7.985 | 0.995 | - | - | - |
| Peripheral eosinophils (< 20%/20%-35%/≥ 35%) | 3.595 | 1.567-8.248 | 0.003* | 718.810 | 4.282-1.207 × 105 | 0.012* |
| Largest mass sizes (< 3.5/≥ 3.5) | 5.883 | 1.534-22.565 | 0.010* | 3.519 | 0.639-19.381 | 0.148 |
| Notch-1 expression (-/+/++) | 2.939 | 1.020-8.467 | 0.046* | 31.848 | 2.258-449.207 | 0.010* |
| Ki-67 proliferation index (< 3%/≥ 3%) | 9.918 | 1.256-78.323 | 0.030* | 448.684 | 3.129-6.433 × 104 | 0.016* |
One patient was reported positive for urine protein. One patient was diagnosed with KD with a mass located in cervical level II after 3 months when he underwent thyroidectomy and modified lymph nodes dissection for thyroid papillary carcinoma. Another patient was once diagnosed with the Steven Johnson syndrome before KD. For other patients, no complications were reported.
Immunohistochemical features and statistic analysis
Relationship between expression of Notch-1 and clinicopathological factors
IHC analyses were performed in 40 KD cases, of which 20 were strongly or moderately positive, 7 weakly positive, and 13 negative for the expression of Notch-1 receptor. All the recurrent patients (N = 11) had positive Notch-1 receptor expression (Table 2, Figure 1); among them, 4 were weakly positive, while the other 7 were strongly or moderately positive. Of the 27 patients with positive Notch-1 expression, 16 (59.3%) were nonrecurrent (Table 2).
Relationship between expression of Ki-67 and clinicopathological factors
All cases showed positive Ki-67 expression in germinal centers with a range of 70-90%. Outside the germinal centers, 22 patients were in the range of 0-3% (recurrent patients vs. nonrecurrent patients: 1:21), and 10 relapsed patients were included in the group (18 patients) that had a Ki-67 proliferation index > 3% (Table 2, Figure 1).
Association of KD recurrence and expression of Notch-1 and Ki-67
Notch-1 receptor (χ2 = 8.580, p = 0.0014) and Ki-67 proliferation index (χ2 = 10.489, p = 0.001) are statistically significant for recurrence of KD. However, there is no statistically significant correlation between these two indicators (χ2 = 0.440, p = 0.803) (Table 2).
Distribution of KD in China
Among the 40 cases of KD, they all came from the eastern region of China: 37 patients came from Guangdong Province, and 1 each from Guangxi, Sichuan, and Anhui Provinces. Thirty-one (77.5%) of the patients lived in rural areas where the living conditions were relatively poor. Among the 11 relapsed patients, 8 (73%) patients lived in rural areas.
Discussion
KD is a rare chronic inflammatory disorder with unknown cause and pathogenesis [17]. It was first described in Chinese in 1937 by Kim H, Szeto C. Kimura et al. [18] described it as “an unusual granulation combined with hyperplastic changes of lymphatic tissue”. They firstly published literature in English in 1948 and the disease is known as Kimura’s disease. To date, it is about 400 cases of KD reported in the world, mainly are case reports [23]. Previous literature attributes it to autoimmune or allergic reactions. Typically, KD occurs in the head and neck regions with slowly growing masses and more easily happens in men than in women. However, it is still unclear in the correlation between clinicopathological characters and prognosis of KD.
The patients who are diagnosed as KD have different prognosis. Some don’t experience reoperation or other therapies after initial operations, others suffer several times of recurrence. Effective biomarkers are necessary to evaluate the potential patients with recurrent risk of KD.
Notch-1 was first found in human T-cell neoplasia as an oncogene. It express upregulated in some lymphomas and as a potential role in cellular differentiation, proliferation, etc [9]. Ki-67 is a common pathological detection marker and related to the prognosis of disease [12]. As KD’s pathological character showed the T cells are increase in the lesions and resemble lymphoma, we may infer whether these markers altogether can predict the prognosis of KD and act as a guidance for better treatment.
The standard therapies for KD include surgery, radiation and steroids. Moreover, some studies document attempted treatments by cryotherapy, electrodesiccation, and chemotherapy [17], the effects of which still need much more data to verify. Some research reported that the most effective approach is by surgery, but it suffers a problem of high recurrence rates [19]. The main treatment for recurrent KD is reoperation or systematic steroid medication. It is recommended to undergo radiotherapy of moderate radiation doses (20-45 Gy within 3-5 weeks) to reach the goal of local control [17]. But the optimal treatment for KD is still controversial.
In our present study, the mean age of KD patients was 38.4 years old, which is consistent with the literature. The female-to-male ratio is 1:12.3, whereas the published literature has 60 cases in a ratio of 1:5.7 [20]; the discrepancy is probably attributable to our relatively small sample size. Peripheral eosinophilia (%), Ki-67 proliferation index, largest mass sizes and Notch-1 receptor are related to the recurrence of KD (all p < 0.05). It is generally recognized that tumors with high Ki-67 expression tend to respond less to treatment and have a poorer prognosis [17,20,21]. Interestingly, though it is known that KD is not malignant disease, the 40 cases all have high Ki-67 expression in germinal centers. Outside the germinal centers, Ki-67 proliferation index tended to achieve a higher expression in the relapsed patients. It suggested that Ki-67 can be a biomarker for the recurrence of KD. As for Notch-1, as a paradoxical role, it is mainly reported its different expression levels in various types of carcinoma. Up-regulated expressions of Notch-1 are reported in colon cancer, renal cell carcinoma, human melanoma, cervical cancer [9]. However, medullary thyroid carcinoma, hematologic malignancies, skin cancer have low expression of Notch-1 [9,23,24]. In our present research, high expressions of Notch-1 were found in the recurrent KD patients. It indicated that activation of Notch-1 signaling may provide a therapeutic role in KD. Since it has been some reports about the simultaneity of KD and carcinoma and our study showed a case with papillary thyroid carcinoma and KD, we may further wonder KD’s possibility of carcinoma transformation [7]. With no published studies about the correlation among Ki-67, Notch-1 and prognosis of KD. More data will be needed to analysis. Initial location, gender, age, laterality and multiplicity appear to be unrelated to the recurrence of KD. Our study showed that high expression of Ki-67 proliferation index and Notch-1 receptor are associated to the recurrence and undesirable prognosis of KD. We suggest to provide a more intense follow-up and radiation therapy after the initial operation when immunohistochemical staining showed the high expression of these markers and indicated the potentially possibility of recurrence. However, this hypothesis need more research.
The Ki-67 proliferation index, Notch-1 receptor and peripheral eosinophilia (%), largest mass sizes are independent factors for recurrence of KD. Multivariate survival analysis showed that the 95% CI for the Ki-67 proliferation index and peripheral eosinophilia (%) have a large range, which may be attributable to our small sample size. Besides, serum IgE may act as an independent factor in relapsed KD. Since serum IgE examination was not available in our 40 cases, further study incorporating these factors will be needed to verify this analysis. Besides the obviously increased eosinophil counts and serum IgE that have published as characters in KD, a male who has multiple unilateral masses in head and neck with nonspecific ultrasound imaging description should not rule out the suspect of KD. Performing ultrasound-guided biopsy is recommended in our hospital’s experience. For our present study, Ki-67 and Notch-1 could work as promising markers for the recurrence of KD. We propose patients who finish the initial operations to have excised sections’ immunohistochemical analysis for Notch-1 and Ki-67. High expression of both markers indicate the possible vulnerable relapsed of KD in future. In that case, we suggest these patients can have radiation therapy after operation and have intense follow-up about once 1-3 months. In our study, the median follow-up time of recurrent patients is 47.0 months. After 47.0 months without recurrence, the time could appropriately extend to once 3-6 months.
According to the published literature, common factors contributing to KD include infection, immune system, toxicity, allergic reaction, etc [1,25]. Wang et al [26] show a distribution of 20 cases of KD in eastern China. Our present cases here also come mainly from eastern China, partly because of our hospital’s location in coastal eastern China, and possibly because the disease occurs mainly in coastal areas. Moreover, the patients reported as having KD live in rural areas. Combined with the distribution described above, we may wonder whether the cause of KD has some relationship with a factor from the surrounding environment.
Patients who had the largest size of mass ≥ 3.5 cm, peripheral eosinophilia ≥ 35%, positive expression of Notch-1, or Ki-67 proliferation index ≥ 3% tended to be vulnerable to recurrence. Our present analysis indicates that Notch-1 and Ki-67 show promise as novel predictors for recurrence, thereby enabling patients to choose optimal treatment. Further studies that include IgE data and more patients are needed.
Acknowledgements
The study was supported by the Natural Science Foundation of Guangdong Province (No. 7301082).
References
- 1.Chen H, Thompson LD, Aguilera NS, Abbondanzo SL. Kimura disease: a clinicopathologic study of 21 cases. Am J Surg Pathol. 2004;28:505–513. doi: 10.1097/00000478-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Abuel-Haija M, Hurford MT. Kimura disease. Arch Pathol Lab Med. 2007;131:650–651. doi: 10.5858/2007-131-650-KD. [DOI] [PubMed] [Google Scholar]
- 3.Yamada A, Mitsuhashi K, Miyakawa Y, Kosaka K, Takehara K, Iijima M, Tanaka K, Shibata S. Membranous glomerulonephritis associated with eosinophilic lymphfolliculosis of the skin (Kimura’s disease): report of a case and review of the literature. Clin Nephrol. 1982;18:211–215. [PubMed] [Google Scholar]
- 4.Saxe N, Kahn LB. Angiolymphoid hyperplasia with eosinophilia: report of 3 cases. S Afr Med J. 1977;52:454–7. [PubMed] [Google Scholar]
- 5.Zhang R, Ban XH, Mo YX, Lv MM, Duan XH, Shen J, Li JP, Liu XW, Xie CM. Kimura’s disease: the CT and MRI characteristics in fifteen cases. Eur J Radiol. 2011;80:489–97. doi: 10.1016/j.ejrad.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Deshpande AH, Nayak S, Munshi MM, Bobhate SK. Kimura’s disease. Diagnosis by aspiration cytology. Acta Cytol. 2002;46:357–63. doi: 10.1159/000326734. [DOI] [PubMed] [Google Scholar]
- 7.Akhavan A, Cannon GJ, Sasatomi E, Franks ME. Synchronous unilateral renal cell carcinoma and Kimura disease of the kidney. Urology. 2006;68:673, e21–2. doi: 10.1016/j.urology.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 8.Zhu H, Zhou X, Redfield S, Lewin J, Miele L. Elevated Jagged-1 and Notch-1 expression in high grade and metastatic prostate cancers. Am J Transl Res. 2013;5:368–78. [PMC free article] [PubMed] [Google Scholar]
- 9.Kunnimalaiyaan M, Chen H. Tumor suppressor role of Notch-1 signaling in neuroendocrine tumors. Oncologist. 2007;12:535–42. doi: 10.1634/theoncologist.12-5-535. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita AS, Geraldo MV, Fuziwara CS, Kulcsar MA, Friguglietti CU, da Costa RB, Baia GS, Kimura ET. Notch pathway is activated by MAPK signaling and influences papillary thyroid cancer proliferation. Transl Oncol. 2013;6:197–205. doi: 10.1593/tlo.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jundt F, Anagnostopoulos I, Forster R, Mathas S, Stein H, Dorken B. Activated Notch1 signaling promotes tumor cell proliferation and survival in Hodgkin and anaplastic large cell lymphoma. Blood. 2002;99:3398–403. doi: 10.1182/blood.v99.9.3398. [DOI] [PubMed] [Google Scholar]
- 12.McLoughlin J, Foster CS, Price P, Williams G, Abel PD. Evaluation of Ki-67 monoclonal antibody as prognostic indicator for prostatic carcinoma. Br J Urol. 1993;72:92–7. doi: 10.1111/j.1464-410x.1993.tb06466.x. [DOI] [PubMed] [Google Scholar]
- 13.Shou Y, Lu J, Chen T, Ma D, Tong L. Correlation of fluorodeoxyglucose uptake and tumor-proliferating antigen Ki-67 in lymphomas. J Cancer Res Ther. 2012;8:96–102. doi: 10.4103/0973-1482.95182. [DOI] [PubMed] [Google Scholar]
- 14.Gao J, Liu J, Fan D, Xu H, Xiong Y, Wang Y, Xu W, Wang Y, Cheng Y, Zheng G. Up-regulated expression of Notch1 and Jagged1 in human colon adenocarcinoma. Pathol Biol (Paris) 2011;59:298–302. doi: 10.1016/j.patbio.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Wang YF, Yang YL, Gao ZF, Zhou CJ, Gregg X, Shi YF, Wang J, Yang XF, Ke XY. Clinical and laboratory characteristics of systemic anaplastic large cell lymphoma in Chinese patients. J Hematol Oncol. 2012;5:38. doi: 10.1186/1756-8722-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Determann O, Hoster E, Ott G, Wolfram Bernd H, Loddenkemper C, Leo Hansmann M, Barth TE, Unterhalt M, Hiddemann W, Dreyling M, Klapper W European Mantle Cell Lymphoma Network and the German Low Grade Lymphoma Study Group. Ki-67 predicts outcome in advanced-stage mantle cell lymphoma patients treated with anti-CD20 immunochemotherapy: results from randomized trials of the European MCL Network and the German Low Grade Lymphoma Study Group. Blood. 2008;111:2385–7. doi: 10.1182/blood-2007-10-117010. [DOI] [PubMed] [Google Scholar]
- 17.Chang AR, Kim K, Kim HJ, Kim IH, Park CI, Jun YK. Outcomes of Kimura’s disease after radiotherapy or nonradiotherapeutic treatment modalities. Int J Radiat Oncol Biol Phys. 2006;65:1233–9. doi: 10.1016/j.ijrobp.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 18.Kimura T, Yoshimura S, Ishikwa E. On the unusual granulation combined with hyperplastic changes of lymphatic tissues. Trans Soc Pathol Jpn. 1948;37:179–180. [Google Scholar]
- 19.Olsen TG, Helwig EB. Angiolymphoid hyperplasia with eosinophilia. A clinicopathologic study of 116 patients. J Am Acad Dermatol. 1985;12:781–96. doi: 10.1016/s0190-9622(85)70098-9. [DOI] [PubMed] [Google Scholar]
- 20.Gao Y, Chen Y, Yu GY. Clinicopathologic study of parotid involvement in 21 cases of eosinophilic hyperplastic lymphogranuloma (Kimura’s disease) Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:651–8. doi: 10.1016/j.tripleo.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 21.Buffa FM, Bentzen SM, Daley FM, Dische S, Saunders MI, Richman PI, Wilson GD. Molecular marker profiles predict locoregional control of head and neck squamous cell carcinoma in a randomized trial of continuous hyperfractionated accelerated radiotherapy. Clin Cancer Res. 2004;10:3745–54. doi: 10.1158/1078-0432.CCR-03-0248. [DOI] [PubMed] [Google Scholar]
- 22.Saito B, Shiozawa E, Yamochi-Onizuka T, Adachi D, Nakamaki T, Tomoyasu S, Omine M, Mitsuya T, Takimoto M, Ota H. Efficacy of rituximab plus chemotherapy in follicular lymphoma depends on Ki-67 expression. Pathol Int. 2004;54:667–74. doi: 10.1111/j.1440-1827.2004.01678.x. [DOI] [PubMed] [Google Scholar]
- 23.Kunnimalaiyaan M, Yan S, Wong F, Zhang YW, Chen H. Hairy Enhancer of Split-1 (HES-1), a Notch1 effector, inhibits the growth of carcinoid tumor cells. Surgery. 2005;138:1137–42. doi: 10.1016/j.surg.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 24.Radtke F, Raj K. The role of Notch in tumorigenesis: oncogene or tumour suppressor? Nat Rev Cancer. 2003;3:756–67. doi: 10.1038/nrc1186. [DOI] [PubMed] [Google Scholar]
- 25.Mitsui M, Ogino S, Ochi K, Ohashi T. Three cases of eosinophilic lymphfolliculoid granuloma of the soft tissue originating from the parotid gland. Acta Otolaryngol Suppl. 1996;522:130–2. [PubMed] [Google Scholar]
- 26.Wang DY, Mao JH, Zhang Y, Gu WZ, Zhao SA, Chen YF, Liu AM. Kimura disease: a case report and review of the Chinese literature. Nephron Clin Pract. 2009;111:c55–61. doi: 10.1159/000178980. [DOI] [PubMed] [Google Scholar]


