Abstract
Fecal incontinence and constipation after procedures for anorectal malformations (ARMs) are closely related to the maldevelopment of the striated muscle complex (SMC). Previous studies have demonstrated that myogenic regulatory factors (MRFs) play a significant role in muscle development. Wnt signal pathway is extremely important for MRFs regulation. This study was designed to investigate the spatiotemporal expression pattern of Wnt5a in SMC in ARMs rat embryos. Materials and Methods: Anorectal malformation embryos were induced by ethylene thiourea on embryonic day 10 (E10). Expression levels of protein and mRNA of Wnt5a were confirmed by immunohistochemistry staining, western blot and quantitative real-time PCR (qRT-PCR) between normal rat embryos and embryos with ARMs. Results: Immunostaining revealed that, on embryonic day 17 (E17), the Wnt5a protein was initially expressed in the SMC in normal embryos. With the growth of pregnancy, the positive staining cells gradually increased. The same time-dependent changes of Wnt5a protein were detected in ARMs embryos. Besides, immunostaining showed that Wnt5a had a significant increase in normal embryos compared with ARMs embryos. Similarly, in Western blot and qRT-PCR, the higher expression of Wnt5a protein and mRNA were remarkable in normal embryos during the SMC development, relatively. Conclusion: Our study demonstrated that the downregulation of Wnt5a at the time of SMC development might partly be related to the dysplasia of SMC in ARMs.
Keywords: Anorectal malformations, Wnt5a, striated muscle complex, expression
Introduction
Anorectal malformations comprise a variety of congenital disorders in which the anus fails to open onto the perineum, occurring in approximately 1 per 5000 live births [1]. In spite of numerous technical advances in the surgical treatment of ARMs, some patients with intermediate-type and high-type ARMs continue to have postoperative anal dysfunctions [2-6]. Poor postoperative anorectal function is subject to many factors, such as the abnormality of innervation of pelvic floor musculature (PMF), maldeveloped PMF, enteric nervous system developmental disorders and spinal cord anomalies [7-12].
Previous studies have indicated that various changes in striated muscle complex (SMC) which also influences defecation function are observed in intermediate-type and high-type ARMs [13-15]. And the morphological changes of SMC take place after the occurrence of abnormal anorectum in rats with ARMs [16]. Developmental studies have given insight into the origins of skeletal muscle, however, the molecular characterization of muscle formation remains poorly determined. Previous studies have demonstrated that the muscle regulatory factors (MRFs) including MyoD, Myf5, Mrf4, and myogenin play a significant role in muscle regulation [17]. Wnt signal pathway is extremely important for MRFs regulation. And the regulation function has developed from initial single linear regulation to the current network-like modulation [18-20].
Wnt5a, a significant member of Wnt family, has been implicated in the regulation of development, proliferation, and cell differentiation [21-26]. Besides, it was revealed that Wnt5a played an important role in human hindgut and the down regulation of Wnt5a might partly be related to the maldevelopment of terminal hindgut in ARMs [27,28]. However, the pattern of expression of Wnt5a has not been described in SMC development in ARMs ever before. To determine the pattern of expression of Wnt5a and the possible role of Wnt5a in SMC development, in the current study, we analyzed the distribution of Wnt5a protein and mRNA in the rat SMC at different developmental stages.
Materials and methods
Animal model and tissue collection
Ethical approval was obtained from the China Medical University Animal Ethics Committee prior to the start of the study. Eighty time-mated pregnant Wistar rats were gavage fed a single dose of either 125 mg/kg of 1% ethylene thiourea (ETU; 2-imidazolidinethione, Aldrich Chemical Co., Germany) or an equal dose of saline on E10 (E0-sperm in vaginal smear after overnight mating). Then embryos can be divided into normal and ARMs group. The embryos were harvested via cesarean delivery on E16, E17, E19 and E21, because the SMC on E16 is invisible under the dissection microscope, embryos only after E17 were used in Western blot and qRT-PCR analysis. For hematoxylin and eosin staining and immunohistochemical studies, the embryos were fixed overnight in 4% paraformaldehyde/0.1 mol/L phosphate buffered saline at 4°C, then embedded in paraffin in a routine manner. Embryos were sectioned sagittally at 4 μm thickness. For Western blot and qRT-PCR analysis, the SMC were dissected under magnification and immediately frozen and stored at -80°C until use. The SMC are thinner in female fetal rats; therefore, only male fetuses were selected in this study. We determined the sex of rats by observing the gonad under the light microscope. In detail, the testis that has a characteristic “striped” appearance is different from the ovary that has a characteristic “spotty” appearance under the light microscope.
Immunohistochemical staining
Immunohistochemical stainings were performed as described previously [29]. For antigen retrieval, slides were incubated in boiling 0.01 mol/L citrate buffer (pH 6.0) for 10 minutes, cooled at room temperature, blocking endogenous peroxidase activity with 3% H2O2, and then 10% normal goat serum was applied to prevent nonspecific binding sites. The sections were incubated overnight at 4°C with the primary antibody at dilutions of 1:100 for Wnt5a (Rabbit polyclonal, Abcam Co. code ab72583). After the primary antibody was washed off, the sections were incubated with biotinylated goat antirabbit IgG (1:200 dilution; Santa Cruz Biotechnology, Santa Cruz, Calif) for 15 minutes at room temperature. Immunoreactivity was visualized by 3’,3-diaminobezidine (Sigma Chemical Co., St. Louis, MO, USA) reaction and then the sections were counterstained with hematoxylin. The specimens were mounted and photographed using a digitized microscope camera (Nikon E800, Japan). Negative controls were performed by either omitting the primary or secondary antibodies or incubating with equivalent concentrations of nonimmune goat antiserum.
Protein preparation and western blot
SMC samples collected from normal and ARMs rat embryos were sonicated in ddH2O containing protease inhibitors. Protein extracts (50 μg) were heated at 90°C for 10 min and size fractionated on Bis-Tris sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) gels (Invitrogen, Carlsbad, CA, USA). Protein samples were denatured, separated by SDS/PAGE, and transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA), blocked with 5% fat-free milk in Tris-buffered saline (1 h, RT) and incubated overnight at 4°C in primary antibody against Wnt5a (diluted 1:500). The membrane was incubated with secondary antibody (diluted 1:2,000), and immunostained bands were detected with a Proto Blot II AP System with a stabilized substrate (Promega). Protein levels in each lane were normalized to those of β-actin as an internal standard.
RNA extraction, reverse transcription and quantitative real-time PCR (qRT-PCR)
Approximate 100 mg tissues from normal and ARMs specimens were used for total RNA extraction using RNA extraction reagent TRIZOL (Invitrogen Life Technologies), according to manufacturer’s instructions. The harvested RNA was diluted to a concentration of 1 μg/μl, aliquoted and stored at -80 temperature. Single strand cDNA was prepared with SYBR Prime Script RT-PCR Kit (Takara, Dalian, China) per manufacturers’ instructions. The housekeeping gene β-actin (Takara, code D3783) was used as an endogenous control. The primers of Wnt5a used for qRT-PCR were as follows: sense 5’AGT TTC ACT GGT GCT GCT A-3’, and antisense 5’-ATA TGT GGG TCC TGG GAG-3’. The qRT-PCR was performed with a 12.5 μl reaction system in triplicate for each specimen in the presence of SYBR green PCR Master mix (Takara Biotechnology Co.) in a Lightcycler (Roche Molecular Biochemicals, Co.). The reaction program was: 5 min pre-denaturation at 95°C and 45 cycles of 5 s of denaturation at 95°C, 30 s of annealing at 55°C. After the termination of qRT-PCR, the production was analyzed by the Lightcyclersystem automatically. The amplification process was followed by a melting curve analysis and CT value was recorded. The average CT value was the extreme CT value of the sample. The expression difference of the gene was calculated by the 2-ΔΔct method [30].
Statistical analysis
The Statistical Program for Social Sciences, version 13.0 (SPSS, Chicago, IL), was used for statistical analysis. A t test was used to compare the Wnt5a levels between the normal and ARM group. All results were expressed as means ± standard deviation (S.D.), where P values less than 0.05 were considered statistically significant.
Results
General observation
In this study, malformations were not observed in the 168 male normal embryos. A total of 212 ARMs embryos were obtained from 416 ETU-treated male rat embryos. Among the ETU-treated embryos, none of the embryos died in utero. In all ETU-treated embryos, the tail was short or absent, and externally visible spinal bifida and/or meningocele could be observed in 14.6% (31/212) embryos. In this study, all specimens with anorectal malformations were determined by means of observing the fistula between the rectum and the urethra in sagittal planes on different embryonic days under the light microscope, respectively (Figure 1). The incidence of ARMs in embryos of the ETU-treated group on E16 to E21 was 62.8%. Both ARMs and neurologic defect could be detected in 4.2% (9/212) embryos. Because denervation might affect the development of SMC, specimens with neurologic defects were excluded [31].
Figure 1.
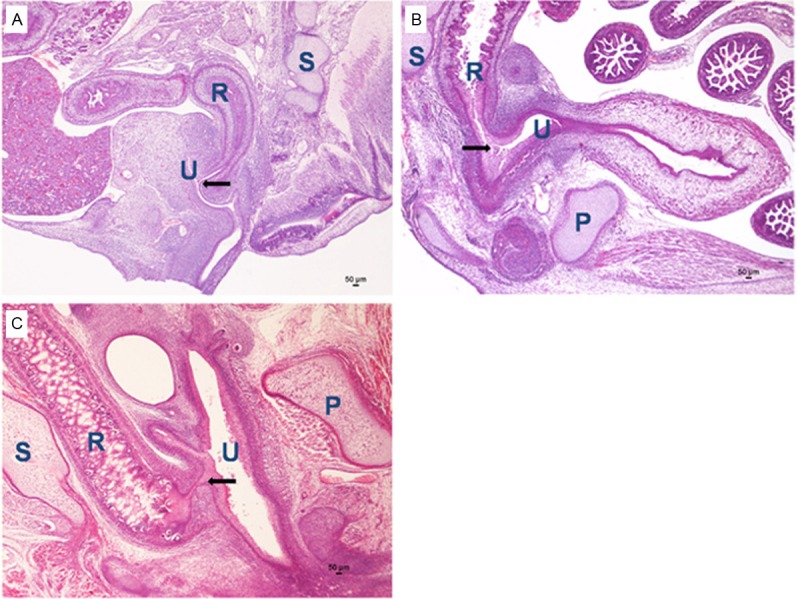
The rectourethral fistula on E17 (A), E19 (B), E21 (C) in ARMs rats (H&E staining, original magnification×40). R indicates rectum; U, urethra; P, pubis; S, sacrum. The black arrow indicated fistula.
Immunohistochemical results
Before immunohistochemical to Wnt5a, we selected slides with SMC using H&E (Figure 2). In normal embryos, on E16, no evidence of Wnt5a-positive staining was detected in SMC. The Wnt5a protein was initially expressed in the SMC on E17 (Figure 3A(a)). The number of positive staining cells increased on E19 and E21. Sporadic positive staining cells were mainly localized in SMC on E19 (Figure 4A(a)). More and more immunoreactivity specific to Wnt5a was detected in SMC and bulbocavernosus muscle on E21 (Figure 5A(a)). Nevertheless, in ARMs embryos, no positive cells could be detected in SMC on E17 (Figure 3B(b)). Little Wnt5a-labeled cells were observed from E19 to E21 (Figures 4B(b) and 5B(b)). However, Wnt5a had an obvious decrease in ARMs embryos compared with normal ones.
Figure 2.
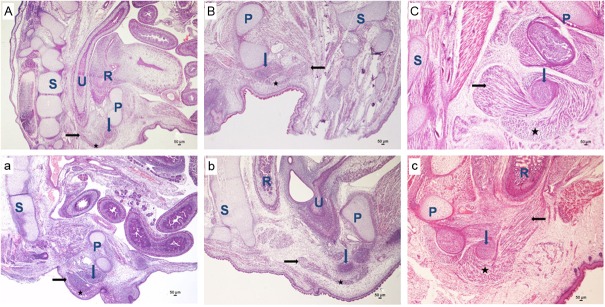
Embryogenesis of SMC in sagittal sections. (A-C) Indicate the normal group; (a-c) Indicate the ARMs group. On E17 (A and a), H&E staining, original magnification×40. On E19 (B and b), H&E staining, original magnification×40.On E21 (C and c), H&E staining, original magnification×40. The black arrows indicate SMC, the blue arrows indicate the bulb of penis, and black five-pointed star indicates the bulbocavernosus muscle. R indicates rectum; U, urethra; P, pubis; S, sacrum.
Figure 3.
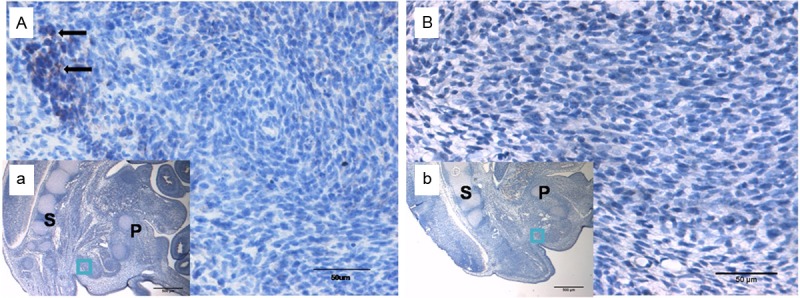
Immunohistochemical staining of Wnt5a on E17. (B and b) Indicate ARMs group; no positive cells could be detected in SMC on E17. (A and a) Indicate the normal group; the Wnt5a protein was initially expressed in the SMC on E17. (A and B) Original magnification×400; (a and b) Original magnification×40. The black arrows indicate positive cells. P, pubis; S, sacrum. The region marked with a square in (a and b) is magnified in (A and B).
Figure 4.
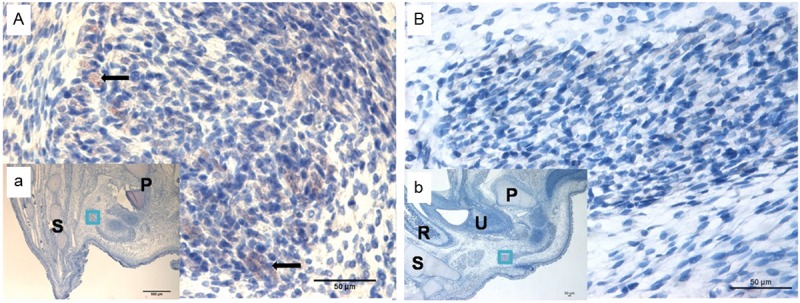
Immunohistochemical staining of Wnt5a on E19. (B and b) Indicate ARMs group; little Wnt5a-labeled cells were observed. (A and a) Indicate the normal group; sporadic positive staining cells were mainly localized in SMC. (A and B) Original magnification×400; (a and b) Original magnification×40. The black arrows indicate positive cells. R, rectum; U, urethra; P, pubis; S, sacrum. The region marked with a square in (a and b) is magnified in (A and B).
Figure 5.
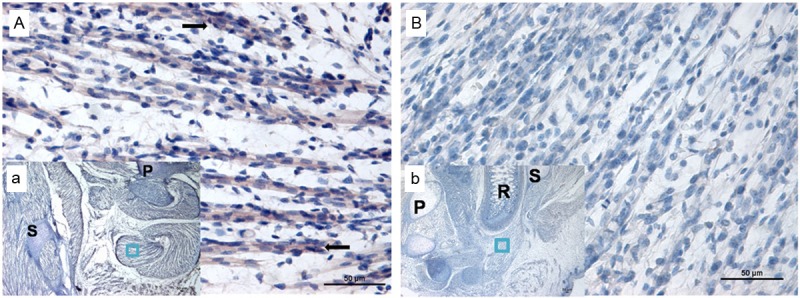
Immunohistochemical staining of Wnt5a on E21. (B and b) Indicate ARMs group; little Wnt5a-labeled cells were observed. (A and a) Indicate the normal group; more and more immunoreactivity specific to Wnt5a was detected in SMC and bulbocavernosus muscle. (A and B) Original magnification×400; (a and b) Original magnification×40. The black arrows indicate positive cells. R, rectum; P, pubis; S, sacrum. The region marked with a square in (a and b) is magnified in (A and B).
Western blot analysis
The expressions of Wnt5a protein were evaluated by western blotting with specific antibodies in normal and ARMs SMC. Wnt5a was detected as an approximately 40 kDa band on Western blots of protein extracted from both the normal and ARMs specimen analyzed. Each protein band was normalized by a corresponding β-actin band. In the normal group, the expression of Wnt5a gradually increased on E17, 19 and 21. while in ARMs group, Wnt5a protein expression was faint. Significant decrease expression of Wnt5a protein was detected in ARMs SMC compared with the normal SMC in each time point (0.24 ± 0.01 versus 0.56 ± 0.03; 0.38 ± 0.04 versus 0.82 ± 0.02; 0.51 ± 0.03 versus 1.16 ± 0.05; respectively; P<0.05; Figure 6).
Figure 6.
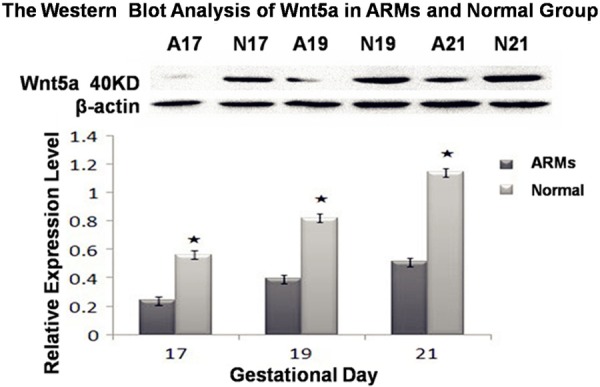
The expressions of Wnt5a protein were evaluated by western blotting in normal and ARMs SMC. Each protein band was normalized by a corresponding β-actin band. Significant decrease expression of Wnt5a protein was detected in ARMs SMC compared with the normal SMC in each age group. N, normal group, A, ARMs group. Results are presented as mean ± SD, significant differences are marked with asterisks (P<0.05).
qRT-PCR analysis
The OD value of total RNA calculated by A260/A280 was from 1.8 to 2.0. The expression level of Wnt5a was normalized to the mRNA level of β-actin from the same specimen. Consistent with the results of western blot analysis, significant increase expression pattern of Wnt5a was detected in normal group compared to ARMs group in each time point. It was showed that the mRNA levels of Wnt5a were 1.97 fold, 2.41 fold and 3.14 fold higher in normal group than those in ARMs group on E17, E19 and E21 (P<0.05), respectively (Tables 1, 2 and 3).
Table 1.
The relative quantity of Wnt5a mRNA on E17
| Group | Wnt5a average Ct value | β-actin average Ct value | ΔCt | ΔΔCt | Times of gene (compared to ARMs group) |
|---|---|---|---|---|---|
| A17 | 24.89 ± 1.43 | 23.95 ± 1.68 | 0.94 | 0 | 1 |
| N17 | 24.05 ± 1.65 | 24.01 ± 1.21 | -0.04 | -0.98 | 1.97 |
Table 2.
The relative quantity of Wnt5a mRNA on E19
| Group | Wnt5a average Ct value | β-actin average Ct value | ΔCt | ΔΔCt | Times of gene (compared to ARMs group) |
|---|---|---|---|---|---|
| A19 | 24.35 ± 1.56 | 23.72 ± 1.25 | 0.63 | 0 | 1 |
| N19 | 23.21 ± 1.71 | 23.85 ± 1.52 | -0.64 | -1.27 | 2.41 |
Table 3.
The relative quantity of Wnt5a mRNA on E21
| Group | Wnt5a average Ct value | β-actin average Ct value | ΔCt | ΔΔCt | Times of gene (compared to ARMs group) |
|---|---|---|---|---|---|
| A21 | 23.87 ± 1.57 | 23.49 ± 1.68 | 0.38 | 0 | 1 |
| N21 | 22.41 ± 1.63 | 23.68 ± 1.34 | -1.27 | -1.65 | 3.14 |
Discussion
SMC is one of the most important factors that influence postoperative defecation. Our previous study documented that in ARMs rat embryos, SMC shifted obviously cephalad, ventrally, and medianward from E18, and considerable connective tissue was observed among intermuscular bundles under high-power view [16]. Chen QJ et al found that dysregulation of apoptosis was implicated as one of the fundamental factors in the pathogenesis of SMC maldevelopment in ARMs rats [32]. However, the mechanism of SMC development in ARMs still remains poorly understood. Previous results provided evidence that Wnt5a was related to development of anorectal malformation [27,28,33]. However, the functions of Wnt5a during SMC development when the ARM presented or not have not yet been elucidated. In this study, to determine the possible role of Wnt5a in SMC development, we explored the expression pattern of Wnt5a in the rat SMC at different developmental stages.
In the current study, we investigated the spatiotemporal expression pattern of Wnt5a during SMC development by immunohistochemistry staining, Western blot and qRT-PCR analysis. Time and space dependent changes could be shown from the results of immunohistochemistry. In normal group, from E17 to E19, sporadic positive staining cells were only localized in SMC. More and more positive cells were detected in SMC and bulbocavernosus muscle on E21. Nevertheless, in ARMs embryos, only little Wnt5a staining was noted in these areas from E19 to E21, and the intensity of the immunohistochemistry of Wnt5a expression in the SMC is lower than in normal embryos. Therefore, there was relative spatiotemporal imbalance between the normal and ARMs embryos during the embryogenesis of the SMC. Our previous studies have demonstrated that the critical period of SMC morphogenesis was from E17 to E19, and original skeletal muscle fibers gradually fused into mature skeletal muscle fibers after E19. In normal embryos, from E17 to E21, the expression of Wnt5a increased, indicating that Wnt5a was extremely important for the development of SMC. In contrast, the down expression of Wnt5a in ARMs embryos may affect the conformation of original skeletal muscle fibers, resulting in the maldevelopment of SMC.
Furthermore, based on the results of Western blot analysis and qRT-PCR analysis, in normal embryos, Wnt5a expression increased greatly at the determinant time of SMC development (E17-E21), further suggesting that Wnt5a may plays a significant role in the development of the SMC. However, at the same stage, the expression level of Wnt5a increased slowly from E17 to E21 and was reduced in ARMs embryos compared with the normal embryos of the same gestational age. The results implied Figure 6. The expressions of Wnt5a protein were evaluated by western blotting in normal and ARMs SMC. Each protein band was normalized by a corresponding β-actin band. Significant decrease expression of Wnt5a protein was detected in ARMs SMC compared with the normal SMC in each age group. N, normal group, A, ARMs group. Results are presented as mean ± SD, significant differences are marked with asterisks (P<0.05). However, the functions of Wnt5a during SMC development when the ARM presented or not have not yet been elucidated. In this study, to determine the possible role of Wnt5a in SMC development, we explored the expression pattern of Wnt5a in the rat SMC at different developmental stages. In the current study, we investigated the spatiotemporal expression pattern of Wnt5a during SMC development by immunohistochemistry staining, Western blot and qRT-PCR analysis. Time and space dependent changes could be shown from the results of immunohistochemistry. In normal group, from E17 to E19, sporadic positive staining cells were only localized in SMC. More and more positive cells were detected in SMC and bulbo that this special down regulation of Wnt5a expression may affect SMC development during the essential stage of SMC development.
The genetics of ARMs is an extremely complex event. Many genes may be involved in this process including Shh, Hox and BMP4 [34-38]. Up to now, there are no reports concerning the signal pathways that mediate the development of SMC. Important signal pathways that initiate the expression of MRFs in regulating the development of skeletal muscle such as Wnt, Shh and BMPs [39-41] may be involved in the mediation of SMC development. However, the relationship between these signals and formation of SMC still remains to be elucidated.
The current study demonstrated that spatiotemporal expression of Wnt5a was imbalanced during the development of SMC in ARMs embryos, suggesting that this imbalanced expression may contribute to the poor development of SMC. Combined with previous studies, we conclude that Wnt5a is extremely important for the development of terminal hindgut and SMC in ARMs embryos. However, this study was unable to substantiate whether Wnt5a was the initial event that lead to SMC malformation, and numerous signaling molecules have recently been shown to be involved in the different phases of the development of SMC. Further studies are required to confirm the signal pathways regulating SMC formation during embryonic development and to clarify the underlying molecular mechanisms mediating the maldevelopment of SMC. Understanding these mechanisms may help us to establish potential therapeutic interventions that could reduce skeletal muscle wasting and preserve physiologic function.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (Grant Nos. 81270436 and 81170334).
Disclosure of conflict of interest
There are no interests of conflicts about this paper.
References
- 1.van der Putte SC. Normal and abnormal development of the anorectum. J Pediatr Surg. 1986;21:434–440. doi: 10.1016/s0022-3468(86)80515-2. [DOI] [PubMed] [Google Scholar]
- 2.Levitt MA, Peña A. Outcomes from the correction of anorectal malformations. Curr Opin Pediatr. 2005;17:394–401. doi: 10.1097/01.mop.0000163665.36798.ac. [DOI] [PubMed] [Google Scholar]
- 3.Peña A, Guardino K, Tovilla JM, Levitt MA, Rodriguez G, Torres R. Bowel management for fecal incontinence in patients with anorectal malformations. J Pediatr Surg. 1998;33:133–137. doi: 10.1016/s0022-3468(98)90380-3. [DOI] [PubMed] [Google Scholar]
- 4.Sonnino RE, Reinberg O, Bensoussan AL, Laberge JM, Blanchard H. Gracilis muscle transposition for anal incontinence in children: long-term follow-up. J Pediatr Surg. 1991;26:1219–1223. doi: 10.1016/0022-3468(91)90338-t. [DOI] [PubMed] [Google Scholar]
- 5.Bai YZ, Yuan Z, Wang WL, Zhao YR, Wang HZ, Wang W. Quality of life for children with fecal incontinence after surgically corrected anorectal malformation. J Pediatr Surg. 2000;35:462–464. doi: 10.1016/s0022-3468(00)90215-x. [DOI] [PubMed] [Google Scholar]
- 6.Rintala R, Lindahl H. Is normal bowel function possible after repair of intermediate and high anorectal malformations. J Pediatr Surg. 1995;30:491–494. doi: 10.1016/0022-3468(95)90064-0. [DOI] [PubMed] [Google Scholar]
- 7.Rintala R. Postoperative internal sphincter function in anorectal malformation: a manometric study. Pediatr Surg Int. 1990;5:127–130. [Google Scholar]
- 8.Li L, Li Z, Wang LY, Xiao FD. Anorectal anomaly: neuropathological changes in the sacral spinal cord. J Pediatr Surg. 1993;28:880–885. doi: 10.1016/0022-3468(93)90687-g. [DOI] [PubMed] [Google Scholar]
- 9.Yuan ZW, Bai YZ, Zhang ZB, Ji SJ, Li Z, Wang WL. Neural electrophysiological studies on the external anal sphincter in children with anorectal malformation. J Pediatr Surg. 2000;35:1052–1057. doi: 10.1053/jpsu.2000.7770. [DOI] [PubMed] [Google Scholar]
- 10.Fernández-Fraga X, Azpiroz F, Malagelada JR. Significance of pelvic floor muscles in anal incontinence. Gastroenterology. 2002;123:1441–1450. doi: 10.1053/gast.2002.36586. [DOI] [PubMed] [Google Scholar]
- 11.Yuan ZW, Lui VC, Tam PK. Deficient motor innervation of the sphincter mechanism in fetal rats with anorectal malformation: a quantitative study by fluorogold retrograde tracing. J Pediatr Surg. 2003;38:1383–1388. doi: 10.1016/s0022-3468(03)00401-9. [DOI] [PubMed] [Google Scholar]
- 12.Jia HM, Zhang KR, Zhang SC, Yuan ZW, Bai YZ, Wang WL. Quantitative analysis of sacral parasympathetic nucleus innervating the rectum in rats with anorectal malformation. J Pediatr Surg. 2007;42:1544–1548. doi: 10.1016/j.jpedsurg.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 13.Kablar B, Krastel K, Ying C, Asakura A, Tapscott SJ, Rudnicki MA. MyoD and Myf-5 differnatially regulate the development of limb versus trunk skeletal muscle. Development. 1997;124:4729–4738. doi: 10.1242/dev.124.23.4729. [DOI] [PubMed] [Google Scholar]
- 14.Sassoon D, Lyons G, Wright WE, Lin V, Lassar A, Weintraub H, Buckingham M. Expression of two myogenic regulatory factors myogenin and MyoD1 during mouse embryogenesis. Nature. 1989;341:303–307. doi: 10.1038/341303a0. [DOI] [PubMed] [Google Scholar]
- 15.McHugh KM. Molecular analysis of smooth muscle development in the mouse. Dev Dyn. 1995;204:278–290. doi: 10.1002/aja.1002040306. [DOI] [PubMed] [Google Scholar]
- 16.Zhang SW, Bai YZ, Zhang SC, Wang DJ, Zhang T, Zhang D, Wang WL. Embryonic development of the striated muscle complex in rats with anorectal malformations. J Pediatr Surg. 2008;43:1452–1458. doi: 10.1016/j.jpedsurg.2008.02.059. [DOI] [PubMed] [Google Scholar]
- 17.Tapscott SJ. The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Development. 2005;132:2685–2695. doi: 10.1242/dev.01874. [DOI] [PubMed] [Google Scholar]
- 18.Ridgeway AG, Petropoulos H, Wilton S, Skerjanc IS. Wnt signaling regulates the function of MyoD and myogenin. J Biol Chem. 2000;275:32398–32405. doi: 10.1074/jbc.M004349200. [DOI] [PubMed] [Google Scholar]
- 19.Ouko L, Ziegler TR, Gu LH, Eisenberg LM, Yang VW. Wnt11 signaling promotes proliferation, transformation, and migration of IEC6 intestinal epithelial cells. J Biol Chem. 2004;279:26707–26715. doi: 10.1074/jbc.M402877200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Topoi L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang YZ. Wnt5a inhibits the canonical Wnt pathway by promoting GSK-3-independent β-catenin degradation. J Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 22.Kurayoshi M, Oue N, Yamamoto H, Kishida K, Inoue A, Asahara T, Yasui W, Kikuchi A. Expression of Wnt5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res. 2006;66:10439–10448. doi: 10.1158/0008-5472.CAN-06-2359. [DOI] [PubMed] [Google Scholar]
- 23.Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through β-catenin. Science. 2002;296:1644–1646. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- 24.Nusse R. Wnts and Hedgehogs: lipid-modified proteins and similarities in signaling mechanisms at the cell surface. Development. 2003;130:5297–5305. doi: 10.1242/dev.00821. [DOI] [PubMed] [Google Scholar]
- 25.Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y, Topol L, Lee H, Wu JL. Wnt5a and Wnt5b exhibit distinct activities in coordinating chondrocyte proliferation and differentiation. Development. 2003;130:1003–1015. doi: 10.1242/dev.00324. [DOI] [PubMed] [Google Scholar]
- 27.Li FF, Zhang T, Bai YZ, Yuan ZW, Wang WL. Spatiotemporal expression of Wnt5a during the development of the hindgut and anorectum in human embryos. Int J Colorectal Dis. 2011;26:983–988. doi: 10.1007/s00384-011-1191-y. [DOI] [PubMed] [Google Scholar]
- 28.Jia HM, Chen QJ, Zhang T, Bai YZ, Yuan ZW, Wang WL. Wnt5a expression in the hindgut of fetal rats with chemically induced anorectal malformations-studies in the ETU rat model. Int J Colorectal Dis. 2011;26:493–499. doi: 10.1007/s00384-010-1125-0. [DOI] [PubMed] [Google Scholar]
- 29.Pluznick JL, Wei P, Grimm PR, Sansom SC. BK-b1 subunit: immunolocalization in the mammalian connecting tubule and its role in the kaliuretic response to volume expansion. Am J Physiol Renal Physiol. 2005;288:F846–54. doi: 10.1152/ajprenal.00340.2004. [DOI] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔct method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Adhihetty PJ, O’Leary MF, Chabi B, Wick KL, Hood DA. Effect of denervation on mitochondrially mediated apoptosis in skeletal muscle. J Appl Physiol. 2007;102:1143–1151. doi: 10.1152/japplphysiol.00768.2006. [DOI] [PubMed] [Google Scholar]
- 32.Chen QJ, Jia HM, Zhang SW, Zhang SC, Bai YZ, Yuan ZW, Wang WL. Apoptosis during the development of pelvic floor muscle in anorectal malformation rats. J Pediatr Surg. 2009;44:1884–1891. doi: 10.1016/j.jpedsurg.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Tai CC, Sala FG, Ford HR, Wang KS, Li CG, Minoo P, Grikscheit TC, Bellusci S. Wnt5a knock-out mouse as a new model of anorectal malformation. J Surg Res. 2009;156:278–282. doi: 10.1016/j.jss.2009.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mo R, Kim JH, Zhang J, Chiang C, Hui CC, Kim PC. Anorectal malformations caused by defects in sonic hedgehog signaling. Am J Pathol. 2001;159:765–774. doi: 10.1016/S0002-9440(10)61747-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127:2763–2772. doi: 10.1242/dev.127.12.2763. [DOI] [PubMed] [Google Scholar]
- 36.Kimmel SG, Mo R, Kim PC. New mouse models of congenital anorectal malformations. J Pediatr Surg. 2000;35:227–230. doi: 10.1016/s0022-3468(00)90014-9. [DOI] [PubMed] [Google Scholar]
- 37.Warot X, Fromental-Ramain C, Fraulob V, Chambon P, Dollé P. Gene dosage-dependent effects of the Hoxa-13 and Hoxd-13 mutations on morphogenesis of the terminal parts of the digestive and urogenital tracts. Development. 1997;124:4781–4791. doi: 10.1242/dev.124.23.4781. [DOI] [PubMed] [Google Scholar]
- 38.Sasaki Y, Iwai N, Tsdua T, Kimura O. Sonic hedgehog and bone morphogenetic protein 4 expressions in the hindgut region of murine embryos with anorectal malformations. J Pediatr Surg. 2004;39:170–173. doi: 10.1016/j.jpedsurg.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Tajbakhsh S, Borelle U, Vivarelli E, Kelly R, Papkoff J, Duprez D, Buckingham M, Cossu G. Differential activation of Myf5 and MyoD by different Wnts in explants of mouse paraxial mesoderm and the later activation of myogenesis in the absence of Myf5. Development. 1998;125:4155–4162. doi: 10.1242/dev.125.21.4155. [DOI] [PubMed] [Google Scholar]
- 40.Gustaffsson MK, Pan H, Pinney DF, Liu YL, Lewandowski A, Epstein DJ, Emerson CP. Myf5 is a direct target of long-range Shh signaling and Gli regulation for muscle specification. Genes Dev. 2002;16:114–126. doi: 10.1101/gad.940702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reshef R, Maroto M, Lassar AB. Regulation of dorsal somitic cell fates: BMPs and Noggin control the timing and pattern of myogenic regulator expression. Genes Dev. 1998;12:290–303. doi: 10.1101/gad.12.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]


