Abstract
Objective: To investigate the migratory path of stem cells in pancreatic tissues damaged by pancreatitis and to preliminarily identify stem cells that efficiently contribute to the repair of damaged pancreatic tissues. Methods: An animal model of acute pancreatitis was established, in which rats in the experimental group were given intraperitoneal (IP) injections of caerulein. Before the rats were sacrificed, 5-bromo-2’-deoxyuridine (BrdU) was administered by IP injection to label proliferating pancreatic cells. The localization and distribution of the stem cell-specific marker proteins nestin and c-kit in pancreatic tissues were examined using an immunohistochemical approach, and proliferation-specific BrdU incorporation was also analyzed. Results: (1) The nestin-positive cells first appeared in the pancreatic interlobar vessels, and then, were observed in the pancreatic acinar and islet tissues. (2) C-kit-positive cells were located only in the pancreatic islets. (3) BrdU-positive cells first appeared in the area surrounding the interlobular region, and then were diffusely distributed and filled the pancreatic lobules. Conclusions: (1) The stem cells, participated in the repair of damaged pancreatic tissue, appear firstly in the pancreatic interlobar vessels, then migrate toward the pancreatic lobules by using the interlobar vessels as channels and penetrate through the vascular endothelium into the pancreatic acinar tissues. A portion of the stem cells eventually penetrate into the islet tissue. (2) Exogenous stem cells, rather than the tissue-resident stem cells, efficiently contribute to the repair of damaged pancreatic tissues.
Keywords: Acute pancreatitis, stem cells, migratory path
Introduction
Numerous studies have demonstrated that adult pancreatic ductal epithelium [1], exocrine gland tissue [1-3] and islet tissue [4,5] contain multiple populations of tissue-resident stem cells. However, the precise population of tissue-resident stem cells that contributes to the efficient repair of pancreatic damage has not been described in the literature. The ultimate goal of our research on stem cells is to identify multipotent stem cells that efficiently participate in this type of damage repair. Investigation of the migratory path of stem cells in damaged pancreatic tissues may provide important clues for the discovery of these multipotent stem cells. However, few studies in the literature have focused on the migratory path of pancreatic stem cells. Nestin and c-kit are 2 major marker proteins for pancreatic stem cells [3,6,7]. In the present study, we continuously monitored the localization of stem cells at different time points during pancreatic damage repair following experimentally induced pancreatitis using both of these stem cell markers. In addition, we examined the incorporation of the proliferation and differentiation-specific marker 5-bromo-2’-deoxyuridine (BrdU) [8] and investigated the migratory path of stem cells in damaged pancreatitis tissues. Our findings may provide important clues for the discovery of stem cells capable of contributing to the efficient repair of pancreatic damage.
Materials and methods
Materials
Anti-caerulein, anti-nestin and anti-c-kit polyclonal antibodies, BrdU and an anti-BrdU monoclonal antibody were purchased from Sigma-Aldrich Co. LLC. The ready-to-use immunohistochemistry kit (Strepavidin-Biotin Complex (SABC) method) was purchased from Wuhan Boster Biological Technology, Ltd., and the 3,3’-diaminobenzidine (DAB) chromogenic kit was purchased from Beijing Zhongshan Biotechnology Co., Ltd. The high-powered microscope was manufactured by the Olympus Corporation.
Grouping of experimental animals and establishment of the animal model
A total of 42 clean-grade Sprague-Dawley (SD) rats, randomly selected from males and females weighing 120 ± 20 g, were provided by the Experimental Animal Center of Huaxi Medical University. The rats were randomized into an experimental group (30 rats) and a control group (12 rats). The rats were fasted from 12 h prior to the establishment of the pancreatitis model until 24 h after the model was established, but drinking water was made continuously available. Subsequently, the rats resumed their regular intake of food and water. The experimental group was given 4 successive intraperitoneal (IP) injections of caerulein at 50 µg/kg body weight/h, while the control group was given 0.5 mL of saline by IP injection at the same time. At 6 h, 1 d, 2 d, 3 d, 5 d and 7 d after pancreatitis was induced, 5 rats in the experimental group and 2 rats in the control group were sacrificed by cervical dislocation. At 6 h and 3 h before the rats were sacrificed and tissue samples were collected, BrdU was administered via IP injection at 100 mg/kg body weight.
Target detection
Immunohistochemical staining for nestin, c-Kit and BrdU was performed using the SABC method. Paraffin-embedded tissue sections (5 μm) were dewaxed, rehydrated, washed 3 times with 0.01 mol/L PBS for 2 min and incubated with 3 mol/L H2O2 for 10 min. Antigen retrieval was achieved by immersing the sections in 0.01 mol/L citrate buffer and then boiling the sections in a microwave for 10 min at 100°C. The sections were then blocked with normal goat serum for 10 min at room temperature. Upon completion of the protein-blocking step, excess liquid was shaken off, and the sections were incubated with rabbit anti-rat IgG primary antibodies at 4°C overnight. After washing 3 times with 0.01 mol/L PBS for 2 min, the sections were incubated with biotinylated goat anti-rabbit IgG secondary antibody at 37°C for 20 min and then washed 3 times with 0.01 mol/L PBS for 2 min. The SABC reagent was added drop-wise to the tissue sections and incubated at 37°C for 20 min. The sections were washed 3 times with 0.01 mol/L PBS for 2 min, stained with the DAB chromogenic reagent and then double-stained briefly with hematoxylin for 50 s. After staining, the sections were dehydrated, cleared and mounted. PBS buffer was used in place of the primary antibodies as a negative control. The standard positive reaction consisted of yellow-brown granules distributed in the cytoplasm and nucleus.
Results
Distribution characteristics of nestin-positive cells
Nestin protein was expressed at low levels in normal pancreatic tissues, mainly scattered across the pancreatic acinar and islet tissues. At 6 h after establishment of the acute pancreatitis model, nestin-positive cells became concentrated mainly in the walls of pancreatic interlobar vessels. Tissue cross-sections showed that nestin-positive cells were arranged in a circular manner along the interlobar vessel walls. In addition, nestin-positive cells were observed in the lumen of certain interlobar vessels. However, few nestin-positive cells were present in the pancreatic acinar and islet tissues. At 1 d after the establishment of pancreatitis, nestin-positive cells had migrated along the interlobar vessels towards the pancreatic lobules, and some nestin-positive cells were arrayed in 2 parallel lines along the interlobar vessel walls. In addition, a few scattered nestin-positive cells were detected in the pancreatic acinar and islet tissues. At 2 d after the establishment of pancreatitis, a significant increase in scattered nestin-positive cells was observed in the pancreatic lobules. In addition, increased numbers of nestin-positive cells were distributed to the pancreatic islets, although some nestin-positive cells were still distributed along the interlobar vessels toward the pancreatic lobules. At 3 d, the nestin-positive cells were diffusely distributed and filled the pancreatic lobules, and the number of nestin-positive cells that were distributed along the pancreatic interlobar vessels was decreased significantly. At 5-7 d after the establishment of pancreatitis, the number of nestin-positive cells in the pancreatic tissues was significantly reduced, and very few nestin-positive cells were distributed along the interlobar vessels (Figure 1).
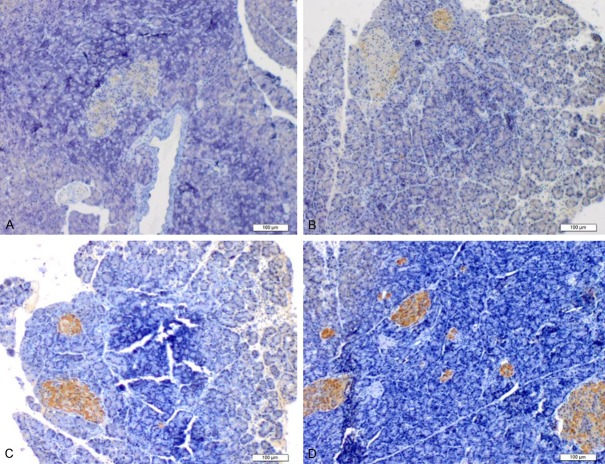
Figure 1.
The distribution of nestin-positive cells. The distribution of nestin-positive cells. At 6 h after establishment of the acute pancreatitis model, nestin-positive cells were concentrated in the walls of the interlobar vessels. Tissue cross-sections showed that nestin-positive cells were arranged in a circular manner along the vessel walls. Nestin-positive cells (Arrow) were also observed in the lumen of certain interlobar vessels (A). At 1 d after the establishment of pancreatitis, nestin-positive cells had migrated along the interlobar vessels toward the pancreatic lobules. A portion of the nestin-positive cells was also distributed along the walls of interlobar vessels in 2 parallel lines (Arrow), and a few nestin-positive cells were scattered in the pancreatic acinar and islet tissues (B, C). At 2 d, the number of scattered nestin-positive cells in the pancreatic lobules had increased significantly. In addition, nestin-positive cells gathered in the pancreatic islets (Arrow). A portion of the nestin-positive cells remained distributed along the interlobar vessels (D).
Distribution characteristics of c-kit-positive cells
Very few c-kit-positive cells were detected in normal pancreatic tissues. C-kit-positive cells first appeared in the pancreatic islet tissue at 6 h after establishment of the acute pancreatitis model. However, the c-kit staining in these positive cells was extremely week and patchy, and no c-kit-positive cells were detected outside of the pancreatic islets. At 1 d after the establishment of pancreatitis, the c-kit staining in the pancreatic islets was slightly enhanced, and the c-kit-positive areas were more widespread and connected. However, no c-kit-positive cells were detected in the pancreatic tissues, with the exception of the pancreatic islets. At 2-3 d after the establishment of pancreatitis, increased numbers of c-kit-positive cells covered large areas of the pancreatic islets, and the c-kit staining became very strong. However, no c-kit-positive cells were found outside of the pancreatic islets or in the areas of inflammatory cell infiltration. At 5-7 d, the c-kit staining in the pancreatic islet tissue had diminished gradually (Figure 2).
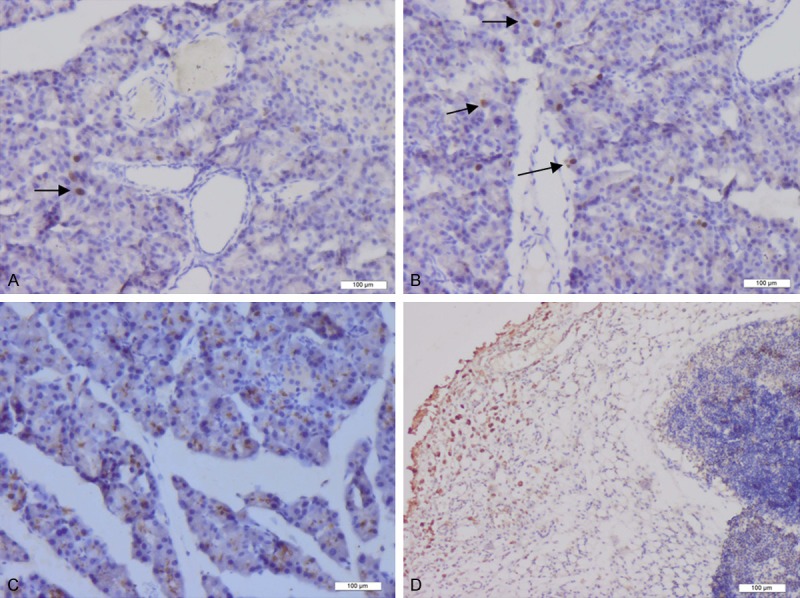
Figure 2.
The distribution of c-kit-positive cells. The distribution of c-kit-positive cells. During the process of establishing the animal model of acute pancreatitis, no c-kit-positive cells were detected outside of the pancreatic islets. At 6 h after the establishment of pancreatitis, c-kit-positive cells first appeared in the pancreatic islet tissue, although this c-kit staining was extremely weak (A). At 1 d, the c-kit staining in the pancreatic islets was slightly enhanced, and c-kit-positive areas became more widespread and connected (B). At 2-3 d, c-kit-positive cells were detected in larger areas of the pancreatic islets, and this c-kit staining was very strong (C, D).
Distribution characteristics of BrdU-positive cells
BrdU-positive cells were very rare in normal pancreatic tissues. At 6 h after the establishment of acute pancreatitis, BrdU-positive cells remained sparse. At 1 d, few BrdU-positive cells were scattered in the periphery of the pancreatic interlobar region. At 2 d, the numbers of BrdU-positive cells located at the periphery of the pancreatic interlobar region were increased slightly, and additional BrdU-positive cells were observed scattered in the pancreatic acinar tissue. At 3-5 d, the number of BrdU-positive cells located in the pancreatic acinar and islet tissues had increased dramatically, and greater numbers of BrdU-positive cells were distributed throughout the “dissipation zone” of inflammatory cells. At 7 d, very few BrdU-positive cells were present in pancreatic tissues, although BrdU-positive cells were observed to form cell sheets in the area of inflammatory cell dissipation (Figure 3).
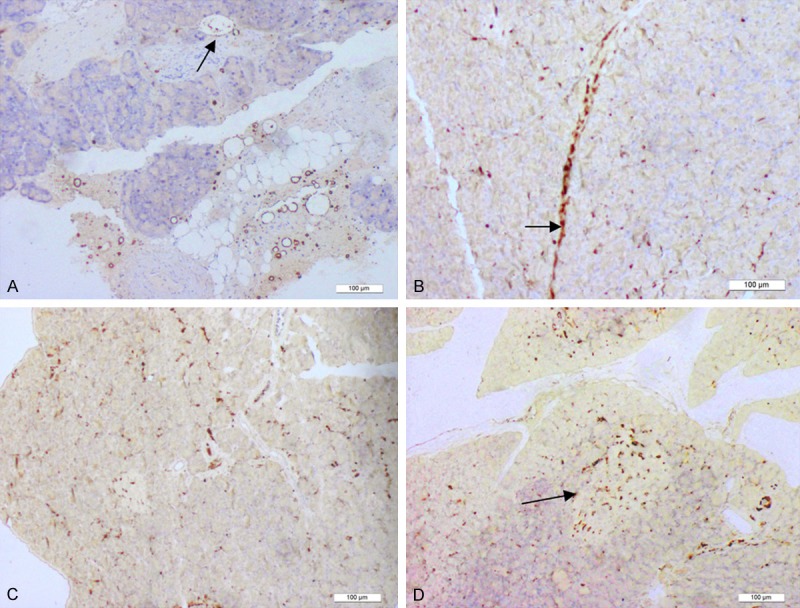
Figure 3.
The distribution of BrdU-positive cells. The distribution of BrdU-positive cells. At 1 d after establishment of the animal model of acute pancreatitis, a small number of BrdU-positive cells (Arrow) was scattered in the periphery of the pancreatic interlobar region (A). At 2 d after the establishment of pancreatitis, the number of BrdU-positive cells located in the periphery of the interlobar region had increased slightly (Arrow). In addition, scattered BrdU-positive cells were also visible in the pancreatic acinar tissue (B). At 5 d, the numbers of BrdU-positive cells located in the pancreatic acinar and islet tissues were increased significantly (C). At 7 d, BrdU-positive cells formed cell sheets in the area of inflammatory cell dissipation (D).
Discussion
Migratory path of stem cells during the repair of pancreatitis-induced damage
High expression of the nestin protein was first observed in neuroepithelial cells during embryonic development [9]. Subsequently, nestin expression was detected in bone marrow stem cells [10] and embryonic stem cells [11]. Recently, the expression of nestin protein was also observed in embryonic pancreatic tissues [12], and nestin has been proposed to serve as a specific marker of pancreatic stem cells by many scholars. C-kit, also known as CD117, is a type of receptor and surface differentiation antigen expressed on stem cells. C-kit interacts with specific antigens to form dimeric complexes, which activate corresponding signal transduction pathways and regulate the biological behaviors of cells [13]. In recent years, c-kit expression has been investigated in pancreatic stem cell research [5,14]. BrdU is well recognized as a specific marker of cell proliferation and differentiation and has been widely used in the study of cell proliferation and differentiation in multiple organs [15,16].
In the present study, we monitored the localization of the above stem cell makers in damaged pancreatic tissues at different time points after the induction of acute pancreatitis. The results showed that stem cells involved in pancreatic tissue repair first appeared in the pancreatic interlobar vessels, then migrated toward the pancreatic lobules using the interlobar vessels as channels and eventually penetrated through the vascular endothelium to reach the pancreatic acinar tissue. Some stem cells also penetrated through the blood-pancreatic barrier and entered the pancreatic islet tissue. The specific findings were as follows. (1) At 6 h after establishment of the acute pancreatitis model, nestin-positive cells first appeared in the interlobar vascular endothelium. Tissue cross-sections showed that nestin-positive cells were also present in the lumen of the interlobar vessels. At 1 d after the establishment of pancreatitis, large numbers of nestin-positive cells had migrated toward the pancreatic lobules, using the interlobar vessels as channels. In addition, a portion of the nestin-positive cells had penetrated the vascular endothelium and entered the pancreatic acinar tissue at this time point. A few nestin-positive cells also penetrated the blood-pancreatic barrier and reached the pancreatic islet tissue. At 2 d after the establishment of pancreatitis, large numbers of neatly arranged nestin-positive cells were observed in the interlobar vascular endothelium, whereas scattered nestin-positive cells were detected in the pancreatic acinar tissue. In addition, numerous nestin-positive cells were found in the pancreatic islet tissue. Ishiwata et al. [17] also utilized nestin as a stem cell-specific marker protein in pancreatic stem cell research and found that nestin-positive cells first appeared in the capillary endothelium, which is consistent with our experimental results. (2) C-kit-positive cells were localized exclusively within the pancreatic islet tissue, and no c-kit-positive cells were detected outside of the pancreatic islets. Over time, c-kit expression gradually became stronger in the pancreatic islets. These results suggested that c-kit-positive cells were pancreatic islet stem cells, which were virtually devoid of any migration capacity or multi-directional differentiation potential. At 1 d after the establishment of acute pancreatitis, c-kit-positive cells showed weak expression, whereas this expression was significantly increased at 2 d. In addition, the expression pattern of c-kit was consistent with that of nestin in the pancreatic islets. Therefore, we hypothesized that, in a strict sense, these c-kit-positive cells were not stem cells but merely progenitor cells within the pancreatic islets, which were likely subordinated to the nestin-positive cells. (3) At 1 d after the establishment of acute pancreatitis, BrdU-positive cells were scattered around the pancreatic interlobar region. At 2 d, the number of BrdU-positive cells had increased slightly, and scattered BrdU-positive cells were found in the pancreatic acinar tissue. At 5-7 d, diffuse BrdU staining was observed in the pancreatic tissue and necrotic areas. Moreover, the distribution of BrdU-positive cells was consistent with the migratory path of nestin-positive cells and was related to the repair of pancreatic damage mediated by stem cells.
Exogenous stem cells, rather than tissue-resident stem cells, were involved in the repair of pancreatitis-induced damage
A large number of studies have shown that stem cells are involved in the repair of damaged organs and tissues [18]. Thus, many researchers have investigated whether resident stem cells in adult pancreatic tissues are involved in damage repair. For example, Lardon et al. [1] reported that human pancreatic duct epithelial cells and acinar cells in damaged pancreatic tissues could be transformed into embryonic cells expressing the stem cell-specific marker pancreatic and duodenal homeobox 1 (PDX-1). In vitro cell culture experiments have also shown that pancreatic acinar cells can be transformed into islet-like cells that display endocrine functions. In another study, Baertschiger et al. [3] isolated and cultured human pancreatic exocrine tissues and found that exocrine cells exhibited typical stem cell characteristics, with a portion of these cells secreting insulin and glucagon. In a similar report, after performing animal experiments, Pinho et al. reached the same conclusion [2]. A large number of studies have also shown that resident stem cells in adult pancreatic tissues have the potential for multi-directional differentiation. However, whether these tissue-resident stem cells are involved in damage repair remains to be elucidated. In the present study, we examined the migratory path of stem cells and found that exogenous stem cells, rather than resident stem cells in pancreatic tissues, were involved in the repair of pancreatitis-induced tissue damage. The specific findings were as follows. (1) At 6 h after the establishment of acute pancreatitis, nestin-positive cells first appeared in interlobar vessels. In addition, nestin-positive cells were found in the lumen of certain interlobar vessels. Furthermore, nestin-positive cells migrated toward the pancreatic lobules, using the interlobar vessels as channels. These results indicated that nestin-positive cells originated from cells in the peripheral blood circulation, likely from bone marrow mesenchymal stem cells, as previous studies have shown that bone marrow stem cells also express nestin [10]. (2) c-kit-positive cells were only found in the pancreatic islet tissue and did not migrate to acinar tissue. Based on the time course of c-kit expression, c-kit positive cells appeared to be subordinated to nestin-positive cells. (3) Cellular proliferative responses were examined, and the results showed that BrdU-positive cells first appeared in the periphery of the pancreatic interlobar region. With time, however, larger numbers of BrdU-positive cells were found in the pancreatic acinar tissue as well as necrotic areas. The distribution of BrdU-positive cells was consistent with the location of the first appearance and migratory path of nestin-positive cells. (4) If tissue-resident stem cells efficiently participated in the repair of pancreatic damage, stem cell-specific marker proteins would be first expressed in the pancreatic acinar tissue, pancreatic duct epithelial tissue and pancreatic islet tissue. In addition, cells expressing these stem cells markers would display migratory behaviors. In the present study, the expression of the pancreatic stem cell-specific marker proteins was not detected in the above tissues at the first time point following the successful establishment of pancreatitis. Therefore, the present study does not support the hypothesis that tissue-resident stem cells effectively participate in the repair of pancreatitis-induced damage.
Disclosure of conflict of interest
None.
References
- 1.Lardon J, Corbeil D, Huttner WB, Ling ZD, Bouwens L. Stem Cell Marker Prominin-1/AC133 Is Expressed in Duct Cells of the Adult Human Pancreas. Pancreas. 2008;36:e1–6. doi: 10.1097/mpa.0b013e318149f2dc. [DOI] [PubMed] [Google Scholar]
- 2.Pinho AV, Rooman I, Reichert M, De Medts N, Bouwens L, Rustgi AK, Real FX. Adult pancreatic acinar cells dedifferentiate to an embryonic progenitor phenotype with concomitant activation of a senescence programme that is present in chronic pancreatitis. Gut. 2011;60:958–966. doi: 10.1136/gut.2010.225920. [DOI] [PubMed] [Google Scholar]
- 3.Baertschiger RM, Bosco D, Morel P, Serre-Beinier V, Berney T, Buhler LH, Gonelle-Gispert C. Mesenchymal stem cells derived from human exocrine pancreas express transcription factors implicated in beta-cell development. Pancreas. 2008;37:75–84. doi: 10.1097/MPA.0b013e31815fcb1e. [DOI] [PubMed] [Google Scholar]
- 4.Wilson LM, Wong SH, Yu N, Geras-Raaka E, Raaka BM, Gershengorn MC. Insulin but not glucagon gene is silenced in human pancreas-derived mesenchymal stem cells. Stem Cells. 2009;27:2703–2711. doi: 10.1002/stem.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montanucci P, Pennoni I, Pescara T, Blasi P, Bistoni G, Basta G, Calafiore R. The functional performance of microencapsulated human pancreatic islet-derived precursor cells. Biomaterials. 2011;32:9254–9262. doi: 10.1016/j.biomaterials.2011.08.052. [DOI] [PubMed] [Google Scholar]
- 6.Kim HS, Yoo SY, Kim KT, Park JT, Kim HJ, Kim JC. Expression of the stem cell markers CD133 and nestin in pancreatic ductal adenocarcinoma and clinical relevance. Int J Clin Exp Pathol. 2012;5:754–761. [PMC free article] [PubMed] [Google Scholar]
- 7.Koblas T, Zacharovová K, Berková Z, Mindlová M, Girman P, Dovolilová E, Karasová L, Saudek F. Isolation and characterization of human CXCR4-positive pancreatic cells. Folia Biol (Praha) 2007;53:13–22. [PubMed] [Google Scholar]
- 8.Lee SH, Hao E, Levine F, Itkin-Ansari P. Id3 upregulates BrdU incorporation associated with a DNA damage response, not replication, in human pancreatic β-cells. Islets. 2011;3:358–366. doi: 10.4161/isl.3.6.17923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Messam CA, Hou J, Berman JW, Major EO. Analysis of the temporal expression of nestin in human fetal brain derived neuronal and glial progenitor cells. Brain Res Dev Brain Res. 2002;134:87–92. doi: 10.1016/s0165-3806(01)00325-x. [DOI] [PubMed] [Google Scholar]
- 10.Sauerzweig S, Munsch T, Lessmann V, Reymann KG, Braun H. A population of serum deprivation-induced bone marrow stem cells (SD-BMSC) expresses marker typical for embryonic and neural stem cells. Exp Cell Res. 2009;315:50–66. doi: 10.1016/j.yexcr.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Singh MD, Kreiner M, McKimmie CS, Holt S, van der Walle CF, Graham GJ. Dimeric integrin alpha5beta1 ligands confer morphological and differentiation responses to murine embryonic stem cells. Biochem Biophys Res Commun. 2009;390:716–721. doi: 10.1016/j.bbrc.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 12.Yue F, Cui L, Johkura K, Ogiwara N, Sasaki K. Glucagon-like peptide-1 differentiation of primate embryonic stem cells into insulin-producing cells. Tissue Eng. 2006;12:2105–2116. doi: 10.1089/ten.2006.12.2105. [DOI] [PubMed] [Google Scholar]
- 13.Miettinen M, Lasota J. KIT (CD117): a review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol. 2005;13:205–220. doi: 10.1097/01.pai.0000173054.83414.22. [DOI] [PubMed] [Google Scholar]
- 14.Zhang M, Ma Q, Hu H, Zhang D, Li J, Ma G, Bhat K, Wu E. Stem cell factor/c-kit signaling enhances invasion of pancreatic cancer cells via HIF-1α under normoxic condition. Cancer Lett. 2011;303:108–117. doi: 10.1016/j.canlet.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Ji YQ, Zhang YQ, Li MQ, Du MR, Wei WW, Li da J. EPO improves the proliferation and inhibits apoptosis of trophoblast and decidual stromal cells through activating STAT-5 and inactivating p38 signal in human early pregnancy. Int J Clin Exp Pathol. 2011;4:765–774. [PMC free article] [PubMed] [Google Scholar]
- 16.Liboska R, Ligasová A, Strunin D, Rosenberg I, Koberna K. Most anti-BrdU antibodies react with 2’-deoxy-5-ethynyluridine -- the method for the effective suppression of this cross-reactivity. PLoS One. 2012;7:e51679. doi: 10.1371/journal.pone.0051679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishiwata T, Kudo M, Onda M, Fujii T, Teduka K, Suzuki T, Korc M, Naito Z. Defined localization of nestin-expressing cells in L-arginine-induced acute pancreatitis. Pancreas. 2006;32:360–368. doi: 10.1097/01.mpa.0000220860.01120.21. [DOI] [PubMed] [Google Scholar]
- 18.Burdon TJ, Paul A, Noiseux N, Prakash S, Shum-Tim D. Bone marrow stem cell derived paracrine factors for regenerative medicine: current perspectives and therapeutic potential. Bone Marrow Res. 2011;2011:207326. doi: 10.1155/2011/207326. [DOI] [PMC free article] [PubMed] [Google Scholar]