Abstract
The patient was a 47-year-old man diagnosed as having autoimmune hemolytic anemia (AIHA) in April 2011. He also had a congenital chromosomal abnormality, a balanced translocation. Treatment with prednisolone (PSL) 60 mg/day resulted in resolution of the AIHA, and the treatment was completed in November 2011. While the patient no longer had anemia, the direct and indirect Coombs tests remained positive. In May 2013, he developed recurrent AIHA associated with acute pure red cell aplasia (PRCA) and hemophagocytic syndrome (HPS) caused by human parvovirus B19 (HPV B19) infection. Tests for anti-erythropoietin and anti-erythropoietin receptor antibodies were positive. Steroid pulse therapy resulted in resolution of the AIHA, PRCA, as well as HPS. The serum test for anti-erythropoietin antibodies also became negative after the treatment. However, although the serum was positive for anti-HPV B19 IgG antibodies, the patient continued to have a low CD4 lymphocyte count (CD4, <300/μL) and persistent HPV B19 infection (HPV B19 DNA remained positive), suggesting the risk of recurrence and bone marrow failure.
Keywords: Autoimmune hemolytic anemia, human parvovirus B19, pure red cell aplasia, hemophagocytic syndrome, CD4 lymphocyte count
Introduction
Association of autoimmune hemolytic anemia (AIHA) and pure red cell aplasia (PRCA) is rare, with only single-case reports in the literature [1-34]. Therefore, the underlying diseases associated with their occurrence, and the causes, mechanisms, clinical features and prognosis have not yet been clarified in detail. In addition, there is no established treatment, and at present, treatment is administered taking into consideration the underlying diseases, complications, and clinical features. We encountered a patient who developed AIHA associated with PRCA, immediately complicated by hemophagocytic syndrome (HPS), who was successfully treated by steroid pulse therapy. This is the only case reported so far, in which both direct and indirect Coombs tests and anti-erythropoietin and anti-erythropoietin receptor antibodies were positive in the same patient, suggesting its importance in considering the etiology, mechanisms and treatment. The patient has a low CD4 lymphocyte count and persistent human parvovirus B19 infection, and careful follow-up is considered necessary.
Case report
A 47-year-old man presented to us with the chief complaints of fever, anasarca, generalized malaise and polyarthralgia. He had a history of pollinosis and bronchial asthma. His family history included development in his elementary school-going daughter of erythema infectiosum a few weeks earlier. In April 2011, the patient was found to have anemia, an elevated reticulocyte count, indirect bilirubin-dominant hyperbilirubinemia, elevated serum LDH, decreased serum haptoglobin, and positive direct and indirect Coombs tests (Figure 1), based on which he was diagnosed as having AIHA. Treatment was started with prednisolone (PSL) 60 mg/day, which resulted in resolution of the AIHA. The dose of PSL was gradually reduced and the PSL treatment was completed in November 2011. Thereafter, the clinical course was uneventful, with the patient no longer having anemia, however, the direct and indirect Coombs tests remained positive. In early May 2013, the patient again developed fever, anasarca, generalized malaise and polyarthralgia. He had marked anemia, with a hemoglobin (Hb) level of 4.4 g/dL, and was admitted to our department in mid-May.
Figure 1.
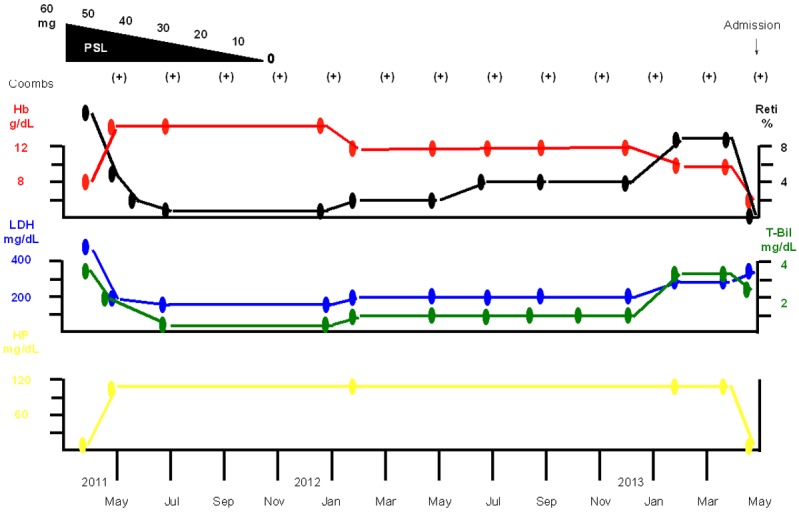
Clinical course from the first examination to admission. The patient was diagnosed as having AIHA in April 2011. Treatment with PSL 60 mg/day was started and the AIHA improved. The dose was gradually tapered and the PSL treatment was completed in November 2011. He no longer had anemia, although the direct and indirect Coombs tests remained positive. He developed marked anemia, with a Hb level of 4.4 g/dL, in early May 2013, and was admitted to our department in mid-May.
The findings at admission were as follows: height 177 cm, weight 67.9 kg, temperature 38.8 degrees Celsius, blood pressure 114/62 mmHg, pulse 80/minute, regular, arterial oxygen saturation under room air 95%, clear consciousness, palpebral conjunctival pallor, mild scleral icterus, no intraoral abnormalities, diminished breath sounds in the left chest regions, no breath sound abnormalities in the right chest regions, normal heart sounds, flat and soft abdomen, no palpable liver or spleen, no abnormal neurological findings, and no palpable superficial lymph nodes. Laboratory findings at the time of admission are shown in Table 1.
Table 1.
Laboratory findings
| CBC | WBC | 2200/µL ↓ |
| Band | 5.0% | |
| Seg | 46.5% | |
| Ly | 26.5% | |
| Mono | 22.0% ↑ | |
| RBC | 111隆脕104/µL ↓ | |
| Hb | 4.4 g/dL ↓ | |
| Ht | 12.6% ↓ | |
| MCV | 113.5 fl ↑ | |
| MCH | 40.2 pg ↑ | |
| Plt | 17.8隆脕104/µL ↓ | |
| Reti | 0.1% ↓ | |
| Coagulation profile | PT activity | 84% |
| APTT | 38.0 sec | |
| Fbg | 244 mg/dL | |
| FDP | ≤5.0 µg/mL | |
| Urinalysis | No abnormalities | |
| Biochemistry | TP | 5.1 g/dL ↓ |
| Alb | 3.3 g/dL ↓ | |
| AST | 35 IU/L ↑ | |
| ALT | 17 IU/L | |
| LDH | 341 IU/L ↑ | |
| γ-GTP | 63 IU/L ↑ | |
| T-Bil | 2.2 mg/dL ↑ | |
| D-Bil | 0.8 mg/dL ↑ | |
| BUN | 18 mg/dL | |
| Cr | 0.90 mg/dL | |
| Ferritin | 1698.6 mg/dL ↑ | |
| Immuno-serological findings | IgG | 792 mg/dL ↓ |
| IgA | 124 mg/dL | |
| IgM | 33 mg/dL ↓ | |
| Antinuclear antibodies | <x40 | |
| C3 | 51 mg/dL ↓ | |
| C4 | 8 mg/dL ↓ | |
| Direct Coombs | Positive ↑ | |
| Indirect Coombs | Positive ↑ | |
| Cold agglutinins | x256 | |
| Haptoglobin | ≤10 mg/dL↓ | |
| Human parvovirus B19 IgM antibody index | 2.79 ↑ | |
| Human parvovirus B19 IgG antibody index | 8.11 ↑ | |
| Human parvovirus B19 DNA qualitative analysis (PCR) | Positive | |
| Others | HIV antibodies | Negative |
| Erythropoietin | 2040 mU/m ↑ | |
| Anti-erythropoietin antibodies | 580 ng/mL (cut point, 223) | |
| IL-6 | 2.9 pg/mL | |
| CD4 | 227.6/µL ↓ | |
| Ferritin | 1698.6 ng/mL ↑ | |
“↑ and ↓ indicate values higher and lower than normal ranges, respectively. Normal ranges are shown in parentheses.”
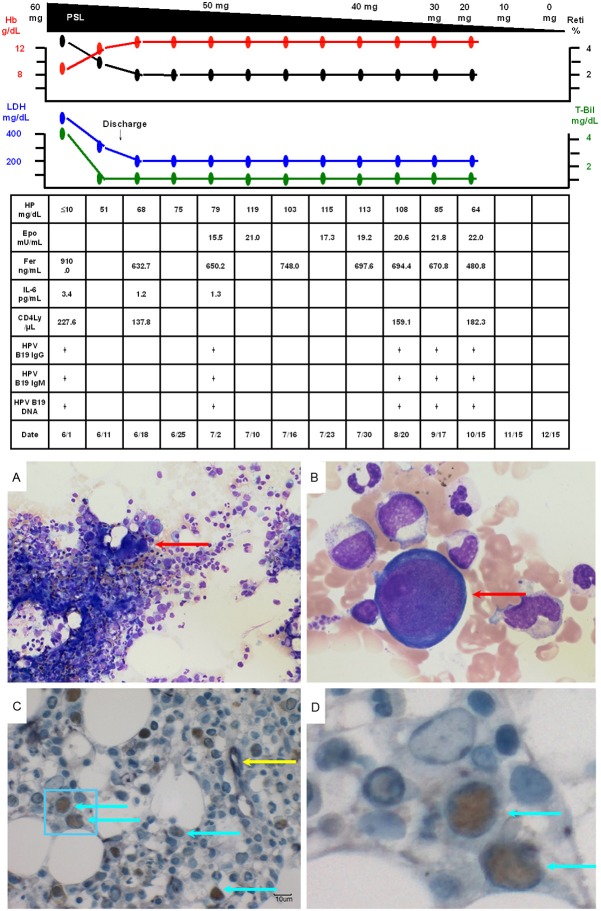
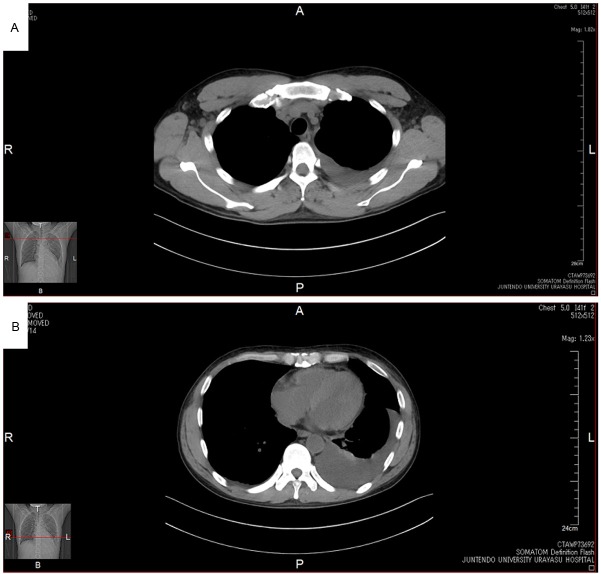
His clinical course after admission is shown in Figure 2A. He had indirect bilirubin-dominant mild hyperbilirubinemia (T-Bil, 2.2 g/dL; D-Bil, 0.8 g/dL), elevated serum LDH (341 mg/dL) and decreased serum haptoglobin (≤10 mg/dL), based on which recurrent AIHA was diagnosed. In addition, the peripheral blood reticulocyte count had decreased to 0.1%, and bone marrow examination revealed normal cellularity, but a marked decrease in erythroblasts, with an M/E ratio of 117.25 (Figure 3A). Giant proerythroblasts were also observed (Figure 3B), and the diagnosis of PRCA was made. Serum was positive for anti-human parvovirus (HPV) B19 IgM and IgG and also for HPV B19 DNA (PCR), which led to the HPV B19 infection being determined as the cause of the PRCA. In addition, double-immunostaining (enzyme-labeled antibody method) of bone marrow biopsy specimens showed positive staining of erythroblasts for anti-HPV B19 and anti-erythropoietin receptor antibodies (Figure 3C, 3D, light blue arrows), while some erythroblasts were positive for only anti-erythropoietin receptor antibodies (Figure 3, yellow arrow). The patient also showed elevated serum levels of erythropoietin (2,040 mU/mL) and anti-erythropoietin antibodies (580 ng/mL) (Table 1). Chest CT revealed left-dominant bilateral pleural effusion, but no thymoma (Figure 4A, 4B). Pleural fluid examination revealed an exudate, although culture and cytology were negative, suggesting exudative reactive pleural effusion due to HPV B19 infection. The recurrent AIHA did not show satisfactory response to treatment with PSL 30 mg/day (no improvement of the serum LDH, T-Bil or HP levels). Blood transfusions were given daily, however, the Hb level did not improve and remained at 4 to 6 g/dL. The reticulocyte count increased to 0.4%, suggesting a tendency towards improvement of the PRCA. The fever subsided immediately after admission, however, one week later, the body temperature rose again to 41 degrees Celsius, with marked increase of the serum LDH (2510 mg/dL) and ferritin (19235.8 ng/mL) levels. Bone marrow examination was performed again, which revealed a hyperplastic bone marrow, recovery of erythroblasts with an M/E ratio of 2.6 (Figure 5A), and hemophagocytosis (Figure 5B). Based on the above, the patient was diagnosed as having HPS. Steroid pulse therapy (1,000 mg/day of methylprednisolone for 3 days) led to resolution of the HPS, as well as of the AIHA and PRCA. The steroid pulse therapy was followed by treatment with PSL at the dose of 60 mg/day; subsequently, the PSL dose was gradually reduced to 55 mg/day. The patient did not show recurrence and was discharged in early June. Thereafter, the dose of PSL was gradually tapered at the outpatient setting and the PSL treatment was completed in January 2014. Since then, the patient has had no recurrence of AIHA, PRCA, or HPS, and his clinical course has been uneventful. Serum became negative for anti-erythropoietin antibodies (59.9 ng/mL).
Figure 2.
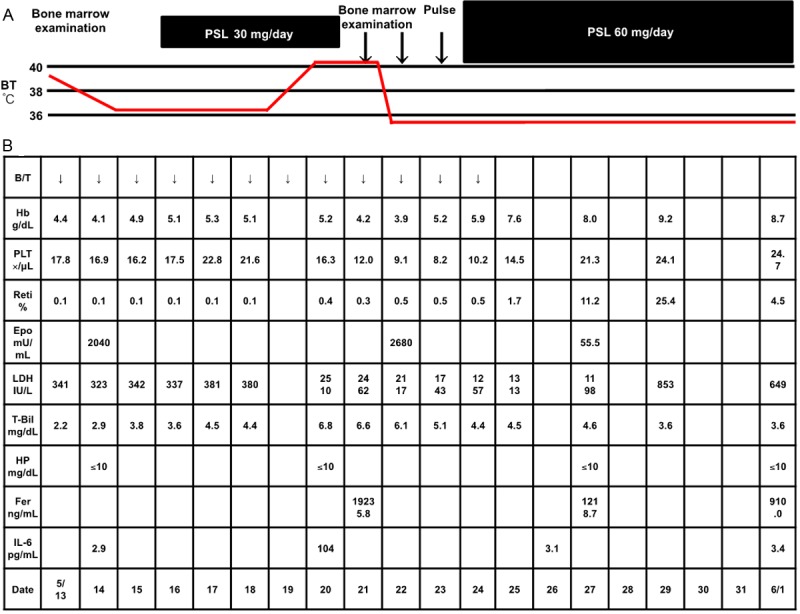
A, B. The patient was diagnosed as having recurrent AIHA associated with PRCA caused by HPV B19 infection. The recurrent AIHA was treated with PSL 30 mg/day, however, the Hb level did not improve, remaining at 4 to 6 g/dL. The reticulocyte count increased to 0.4%, suggesting a tendency towards improvement of the PRCA. One week after admission, the patient again developed fever (body temperature 41 degrees Celsius). Bone marrow examination was performed again, which led to the diagnosis of HPS. Methylprednisolone pulse therapy resulted in resolution of the HPS, as well as of the AIHA and PRCA. Subsequently, the patient was treated with PSL at the dose of 60 mg/day. The PSL treatment was completed in January 2014. The patient has had no recurrence of AIHA, PRCA or HPS, and his clinical course has been uneventful.
Figure 3.
A. Bone marrow examination (smear; ×40) revealed normal cellularity and a marked decrease in the density of erythroblasts, with an M/E ratio of 117.25. B. Bone marrow examination (smear; ×600) also showed giant proerythroblasts (red arrow), suggesting that the patient also had PRCA. C. Double-immunostaining (enzyme-labeled antibody method; ×400) of bone marrow biopsy specimens showed positivity of the erythroblasts for anti-HPV B19 antibodies (brown staining of nuclei) and anti-erythropoietin receptor antibodies (purple staining of the cytoplasm) (light blue arrows). Some erythroblasts showed positivity for only anti-erythropoietin receptor antibodies (yellow arrow). D. An enlarged image of the area in the light blue frame in C.
Figure 4.
A, B. Chest CT revealed left-dominant bilateral pleural effusion, but no thymoma.
Figure 5.
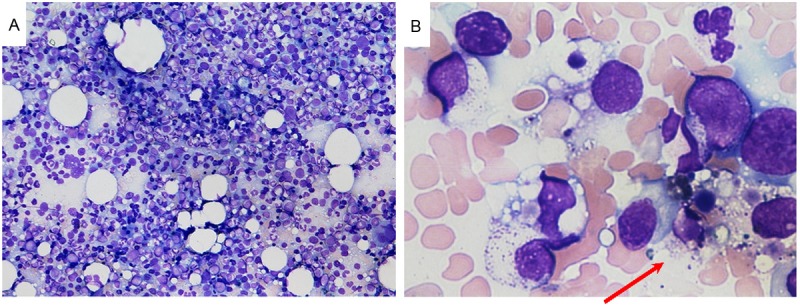
A. Bone marrow examination (smear; ×40) revealed a hyperplastic bone marrow and recovery of erythroblasts with an M/E ratio of 2.6. B. Bone marrow examination (smear; ×600) showed hemophagocytosis (red arrow).
Discussion
To the best of our knowledge, there have been 38 reported cases of AIHA associated with PRCA until now in the literature, including the present one (Table 2). The characteristics of these reported patients are shown in Table 3. The patients were relatively young, with a median age of 51.5 years, and the majority were female, with a male/female ratio of 11:21. Among the complications, lymphoma was the most common (11 cases), followed by Evan’s syndrome (3 cases), systemic lupus erythematosus (SLE, 3 cases), and rheumatoid arthritis (2 cases), in that order. Thus, lymphoma and autoimmune diseases were common complications. In addition, 11 patients had no complications. In regard to the sequence of onset of AIHA and PRCA, the largest number (17) of patients had simultaneous onset of AIHA and PRCA, 8 developed AIHA first, and 5 developed PRCA first. Among the causes of PRCA, HPV B19 was the most common (10 cases), followed by thymoma (2 cases). However, as many as 14 patients had no identifiable cause. The AIHA improved in 29 of the 31 patients with known outcomes. In addition, the PRCA improved in 28 of the 33 patients with known outcomes. A total of 4 patients died, and the causes of death included tuberculosis (Case 7 in Table 2), pneumonia and subacute hepatitis (Case 10 in Table 2), leukemic transformation of lymphoma (Case 22 in Table 2), and sepsis (Case 25 in Table 2). Analysis of the outcomes suggested a relatively good prognosis, which was, however, also dependent on the underlying diseases and complications. Other characteristics included hypergammaglobulinemia in 9 cases, hypogammaglobulinemia in 3 cases, normal serum γ-globulin in 1 case, and cold agglutinins in 1 case. There were no cases of congenital balanced chromosomal abnormalities or HPS, as in the present case.
Table 2.
Reports of AIHA associated with PRCA
| Case | Age/sex | Treatment of AIHA | Outcome of AIHA | Complication | Treatment of complications | Outcome of the complications | Interval between the onset of AIHA and the onset of PRCA | Cause of PRCA | Treatment of PRCA | Outcome of PRCA | Note | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 46/M | ACTH | Improvement | None | None | None | Simultaneous | None | ACTH | Improvement | [1] | |
| Splenectomy | Splenectomy | |||||||||||
| 2 | 58/F | Cortisone | Improvement | None | None | None | -9 M | None | ACTH Cortisone | Improvement | [2] | |
| Splenectomy | Splenectomy | |||||||||||
| 3 | 15/F | Cortisone | Improvement | None | None | None | 8 M | Contrast media? | Cortisone | No improvement | [3] | |
| Splenectomy | ||||||||||||
| 4 | 63/n.a | Cortisone | Improvement | Lymphoma | Cortisone | Improvement | Simultaneous | None | Cortisone | Improvement | Hypergammaglobulinemia | [4] |
| Nitrogen mustard | Nitrogen mustard | Nitrogen mustard | ||||||||||
| 5 | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | [5] | |
| 6 | 76/M | PSL 30 mg | Improvement | None | None | None | -1 M and 3 W | None | PSL 30 mg | Improvement | [6] | |
| 7 | 68/M | PSL 30 mg | Improvement | None | None | None | Simultaneous | None | PSL 30 mg | Recurrence after improvement | [6] | |
| Death from Tb | ||||||||||||
| 8 | 51/M | mPSL 60 mg | Improvement | None | None | None | Simultaneous | None | mPSL 60 mg | Improvement | [7] | |
| ACTH | ACTH | |||||||||||
| 9 | 56/F | PSL 60 mg | Improvement | SLE | PSL 60 mg | n.a | 7 M | SLE | PSL 100 mg | Improvement | [8] | |
| 10 | 71/M | PSL 60 mg | Improvement | Lymphoma | PSL 60 mg | Improvement | Simultaneous | Lymphoma | PSL 60 mg | Improvement | Polyclonal hypergammaglobulinemia | [9] |
| VCR | RA | VCR | Death from pneumonia and subacute hepatitis | Cold agglutinins | ||||||||
| AZP 50 mg | AZP 50 mg | |||||||||||
| Anabolic hormone 200 mg every other day | Anabolic hormone 200 mg every other day | |||||||||||
| 11 | 66/F | COP | Improvement | Lymphoma | COP | Improvement | Simultaneous | Lymphoma | COP | Improvement | [10] | |
| 12 | 67/M | PSL | Improvement | B-CLL | Chlorambucil | Improvement | 32 M | None | PSL | Improvement | [11] | |
| Immunoadsorption | PSL | Immunoadsorption | ||||||||||
| 13 | 12/M | n.a | n.a | n.a | n.a | n.a | Simultaneous | HPV B19 | None | Improvement | [12] | |
| 14 | 1/n.a | n.a | n.a | n.a | n.a | n.a | n.a | None | n.a | n.a | [13] | |
| 15 | 0.5/n.a | n.a | n.a | n.a | n.a | n.a | n.a | None | n.a | n.a | [13] | |
| 16 | 12/n.a | n.a | n.a | n.a | n.a | n.a | n.a | HPV B19 | n.a | n.a | [13] | |
| 17 | 58/M | Cortisone | Improvement | None | None | None | -6 y | Thymoma | Cortisone | Improvement | [14] | |
| Thymectomy | ||||||||||||
| 18 | 33/F | PSL | Improvement | None | None | None | 8 Y | HPV B19 | PSL | Improvement | [15] | |
| CPA | ||||||||||||
| 19 | 41/F | PSL 60 mg | Improvement | HA | n.a | Improvement | Simultaneous | HA | PSL 60 mg | Improvement | [16] | |
| 20 | 61/M | COP | Improvement | Lymphoma | COP | n.a | Simultaneous | Lymphoma | COP | No improvement | Polyclonal hypergammaglobulinemia | [17] |
| CPA 50 mg | CPA 50 mg | CPA 50 mg | ||||||||||
| PSL 80 mg | PSL 80 mg | PSL 80 mg | ||||||||||
| IVIG | IVIG | IVIG | ||||||||||
| MACOPB | MACOPB | MACOPB | ||||||||||
| 21 | 54/F | PSL 60 mg | Improvement | None | None | None | Simultaneous | HPV B19 | PSL 60 mg | Improvement | Normal immunoglobulin | [18] |
| 22 | 52/F | PSL 60 mg | Improvement | Lymphoma | Chemotherapy | Death from leukemic transformation | -6 M | None | PSL 60 mg | Improvement | [19] | |
| 23 | 35/F | PSL 50 mg | Improvement | None | None | None | 4 Y | None | PSL | Improvement | Monoclonal gammopathy | [20] |
| mPSL | ||||||||||||
| AZP 100 mg | ||||||||||||
| 24 | 28/F | PSL 1 mg/kg | n.a | SLE | PSL 1 mg/kg | n.a | Simultaneous | None | PSL 1 mg/kg | Improvement | Polyclonal hypergammaglobulinemia | [21] |
| IVIG | ||||||||||||
| 25 | 62/F | PSL 1 mg/kg | No improvement | Lymphoma | PSL 1 mg/kg | No improvement | Simultaneous | Lymphoma | PSL 1 mg/kg | No improvement | [22] | |
| IVIG | IVIG | IVIG | Death from sepsis | |||||||||
| CsA | CsA | CsA | ||||||||||
| Anti-lymphocyte antibodies | Anti-lymphocyte antibodies | Anti-lymphocyte antibodies | ||||||||||
| Splenectomy | Splenectomy | Splenectomy | ||||||||||
| 26 | 53/M | CHOP | Improvement | Lymphoma | CHOP | Improvement | Simultaneous | Lymphoma | CHOP | Improvement | Hypergammaglobulinemia | [23] |
| Auto | Auto | Auto | ||||||||||
| 27 | 21/F | Cortisone 1.5 mg/kg | Improvement | HA | n.a | Improvement | Simultaneous | HPV B19 | Cortisone 1.5 mg/kg | Improvement | [24] | |
| IVIG | β-thalassemia | IVIG | ||||||||||
| CsA | CsA | |||||||||||
| 28 | 56/F | PSL 50 mg | Improvement | Lymphoma | CHOP | Improvement | Simultaneous | None | PSL 50 mg | Improvement | [25] | |
| 29 | 54/F | CHOP | Improvement | Lymphoma | R-CHOP | Improvement | Simultaneous | Thymoma | CHOP | Improvement | [26] | |
| PSL 1 mg/kg | PSL 1 mg/kg | |||||||||||
| 30 | 40/F | mPSL pulse | Improvement | Evan’s syndrome | mPSL pulse | Improvement | 6 y | HPV B19 | Plasmapheresis | Improvement | Hypogammaglobulinemia | [27] |
| AZP 100 mg/day | AZP 100 mg/day | IVIG | ||||||||||
| CsA 300 mg/day | CsA 300 mg/day | |||||||||||
| Splenectomy | Splenectomy | |||||||||||
| Plasmapheresis | Plasmapheresis | |||||||||||
| 31 | n.a/n.a | PSL 1 mg/kg | Repeated exacerbations and remissions | Evan’s syndrome | Splenectomy | Improvement | n.a | HPV B19 | IVIG | Repeated exacerbations and remissions | Hypogammaglobulinemia | [28] |
| Rituxan | MPGN | |||||||||||
| 32 | 71/F | THP-COP | Improvement | Lymphoma | THP-COP | Improvement | Simultaneous | Lymphoma | THP-COP | Improvement | Polyclonal hypergammaglobulinemia | [29] |
| PSL 40 mg | PSL 40 mg | |||||||||||
| 33 | 54/F | mPSL pulse | Improvement | Lymphoma | R-CHOP | Improvement | Simultaneous | Lymphoma | mPSL pulse | Improvement | Polyclonal hypergammaglobulinemia | [30] |
| Plasmapheresis | Auto | Plasmapheresis | ||||||||||
| R-CHOP | R-CHOP | |||||||||||
| Auto | Auto | |||||||||||
| 34 | 28/F | Cortisone 15-20 mg | n.a | Retinal detachment | Surgery | Improvement | n.a | HPV B19 | Cortisone 15-20 mg | n.a | [31] | |
| IVIG | IVIG | |||||||||||
| 35 | 33/F | mPSL pulse | Improvement | Evan’s syndrome | PSL | Improvement | -22 D | HPV B19 | PSL | Improvement | Polyclonal hypergammaglobulinemia | [32] |
| PSL 60 mg | DM | Insulin | CsA | |||||||||
| Hashimoto’s disease | ||||||||||||
| 36 | 42/F | PSL 40 mg | Improvement | SLE | PSL 40 mg | n.a | Simultaneous | SLE | PSL 40 mg | Improvement | [33] | |
| mPSL pulse | mPSL pulse | mPSL pulse | ||||||||||
| MZB | MZB | MZB | ||||||||||
| 37 | 26/F | PSL 50 mg | Improvement | None | None | None | 3 W | None | PSL 50 mg | Improvement | [34] | |
| AZP | ||||||||||||
| 38 | 47/M | PSL 60 mg | Improvement | Congenital chromosomal abnormality/balanced reciprocal translocation | None | None | 24 M | HPV B19 | PSL 60 mg | Improvement | Hypogammaglobulinemia | The present case |
| Simultaneous with recurrent AIHA | mPSL pulse |
AIHA autoimmune hemolytic anemia, PRCA pure red cell aplasia, M man, ACTH adenocorticotropic hormone, F female, M month, n.a not available, PSL prednisolone, W week, Tb tubercle bacillus, mPSL methylprednisolone, SLE systemic lupus erythematosus, VCR vincristine sulfate, AZP azathioprine, RA rheumatoid arthritis, COP cyclophosphamide, vincristine, and prednisolone, B-CLL B cell-type chronic lymphocytic leukemia, HPV B19 human parvovirus B19, Y year, CPA cyclophosphamide, HA hepatitis A. IVIG intravenous immunoglobulin, MACOPB methotrexate, doxorubicin, cyclophosphamide, vincristine, prednisolone, and bleomycin, CsA cyclosporine A, CHOP cyclophosphamide, doxorubicin, vincristine, and prednisolone, MPGN membrano proliferative glomerulonephritis, THP-COP pirarubicin vincristine, and prednisolone, Auto autologous peripheral blood stem cell transplantation, R-CHOP rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone, DM diabetes mellitus, D day, MZB mizoribine.
Table 3.
Characteristics of patients with AIHA associated with PRCA
| Median age | 51.5 (0.5-76) | N = 36, unknown: 2 |
| Male/female ratio | 11:21 | N = 32, unknown: 6 |
| Treatment of AIHA | PSL, 22; Cortisone, 6; mPSL, 1; mPSL pulse, 3; MZB, 1; IVIG, 4; Plasmapheresis, 2; R-CHOP, 1; CHOP, 2; THP-COP, 1; COP, 2; VCR, 1; MACOPB, 1; | N = 33, unknown: 5 |
| Nitrogen mustard, 1; Auto, 2; Rituximab, 1; AZP, 2; CsA, 3; CPA, 2; Anabolic hormone, 1; ACTH, 2; Splenectomy, 5; Immunoadsorption, 1; Anti-lymphocyte antibodies, 1; Unknown, 5 | ||
| Complication | Congenital chromosomal abnormality/balanced reciprocal translocation, 1; SLE, 3; Rheumatoid arthritis, 2; Evan’s syndrome, 3; DM, 1; Hashimoto’s disease, 1; Retinal detachment, 1; Lymphoma, 11; MPGN, 1; Acute hepatitis A, 2; β-thalassemia, 1; Chronic lymphocytic leukemia, 1; None, 11 | N = 33, unknown: 5 |
| Interval between the onset of AIHA and the onset of PRCA (months) and the number of patients | Simultaneous, N = 18 | N = 32, unknown: 6 |
| AIHA first and PRCA later: N = 8, median interval 28 (0.75 to 96) | ||
| PRCA first and AIHA later: N = 5, median interval 6 (72 to 0.8) | ||
| Cause of PRCA | HPBV 19, 10; Thymoma, 2; WDLL, 1; Lymphoma, 1; Acute hepatitis A, 1; SLE, 1; Contrast media? 1; None, 14 | N = 37, unknown: 1 |
| Treatment of PRCA | PSL, 20; Cortisone, 6; mPSL, 2; mPSL pulse, 3; MZB, 1; IVIG, 7; Plasmapheresis, 2; R-CHOP, 1; CHOP, 2; THP-COP, 1; COP, 2; MACOPB, 1; VCR, 1; Nitrogen mustard, 1; Auto, 2; AZP, 3; CsA, 3; CPA, 1; Anabolic hormone, 1; ACTH, 3; Splenectomy, 3; Immunoadsorption, 1; Anti-lymphocyte antibodies, 1; Thymectomy, 1; None, 1; Unknown, 4 | N = 34, unknown: 4 |
| Other characteristics | Hypergammaglobulinemia, 9; Hypogammaglobulinemia, 3; Normal γ-globulin, 1; Cold agglutinins, 1 | N = 13, unknown: 25 |
mPSL methylprednisolone, MZB mizoribine, IVIG intravenous immunoglobulin, R-CHOP rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone, CHOP cyclophosphamide, doxorubicin, vincristine, and prednisolone, THP-COP pirarubicin vincristine, and prednisolone, COP cyclophosphamide, vincristine, and prednisolone, VCR vincristine sulfate, MACOPB methotrexate, doxorubicin, cyclophosphamide, vincristine, prednisolone, and bleomycin, Auto autologous peripheral blood stem cell transplantation, AZP azathioprine, CsA cyclosporine A, CPA cyclophosphamide, ACTH adenocorticotropic hormone, DM diabetes mellitus, MPGN membrano proliferative glomerulonephritis.
Although it has been reported that the pathogenetic mechanism of AIHA is the same as that of PRCA [19,22,26,34], some reports suggest that the pathogenetic mechanisms of the two conditions differ [20] and that the actual mechanism is still unknown [25]. It is highly likely that the same mechanisms underlie the development of both AIHA and PRCA in cases where the various symptoms occur at the same time. In cases where AIHA and PRCA occur at different times, different mechanisms may be involved, and there are various possible causes, although the precise underlying mechanisms are still unknown. The present patient did not have PRCA at the first onset of AIHA, but simultaneously developed recurrent AIHA with PRCA caused by HPV B19 infection, suggesting that the same mechanism may be responsible for both the recurrent AIHA and PRCA. However, interestingly, the absence of PRCA at the first onset of AIHA may suggest that different mechanisms were involved in the development of the initial and recurrent AIHA.
It was considered that in the present patient, antibodies against mature erythrocytes (direct and indirect Coombs tests) caused the AIHA, and that anti-erythropoietin and anti-erythropoietin receptor antibodies inhibited the differentiation and maturation of erythroblasts, causing PRCA. There have been reports of patients showing positive test results for anti-erythropoietin antibodies [21,33] and anti-erythropoietin receptor antibodies [35], whereas ours is the only reported case in which both anti-erythropoietin and anti-erythropoietin receptor antibodies were found in the same patient, suggesting the importance of this case report in elucidating the etiology and developing treatment.
The present patient continues to show seropositivity for anti-HPV B19 IgM and IgG and HPV B19 DNA, suggesting persistent infection with HPV B19 (Figure 2B). In addition, the CD4 lymphocyte count is reduced (159.1 to 227.6/μL; Figure 2B). Therefore, he is at an elevated risk of recurrent PRCA [27] and bone marrow failure due to persistent infection with HPV B19 [15], and requires careful follow-up. The cause of the decreased CD4 lymphocyte count is unknown, however, Katori et al. pointed out that the high steroid doses may have exacerbated the PRCA [ref]. Ito et al. reported that in a patient with persistent HPV B19 infection treated with high-dose immunoglobulin (HDIVIG), the serum became positive for anti-HPV B19 IgG and the CD4 lymphocyte count increased to ≥300/μL, resulting in the elimination of HPV B19. These findings suggest that we should also probably consider HDIVIG treatment in case of recurrence in the present patient.
Disclosure of conflict of interest
None.
References
- 1.Davis LJ, Kennedy AC, Baikie AG, Brown A. Haemolytic anaemias of various types treated with ACTH and cortisone; report of ten cases, including one of acquired type in which erythropoietic arrest occurred during a crisis. Glasgow Med J. 1952;33:263–85. [PMC free article] [PubMed] [Google Scholar]
- 2.Eisemann G, Dameshek W. Splenectomy for pure red-cell hypoplastic (aregenerative) anemia associated with autoimmune hemolytic disease; report of a case. N Engl J Med. 1954;251:1044–1048. doi: 10.1056/NEJM195412232512603. [DOI] [PubMed] [Google Scholar]
- 3.Seip M. Aplastic crisis in a case of immuno-hemolytic anemia. Acta Med Scand. 1955;153:137–42. doi: 10.1111/j.0954-6820.1955.tb18212.x. [DOI] [PubMed] [Google Scholar]
- 4.Bove JR. Combined erythroid hypoplasia and symptomatic hemolytic anemia; report of a case. N Engl J Med. 1956;255:135–136. doi: 10.1056/NEJM195607192550307. [DOI] [PubMed] [Google Scholar]
- 5.Hennemann HH, Falck I. [Combinations of aplastic with hemolytic syndromes] . Acta Haematol. 1957;18:219–228. doi: 10.1159/000205326. German. [DOI] [PubMed] [Google Scholar]
- 6.Burston J, Husain OA, Hutt MS, Tanner EI. Two cases of auto-immune haemolysis and aplasia. Br Med J. 1959;1:83–86. doi: 10.1136/bmj.1.5114.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer LM, Bertcher RW. Acquired hemolytic anemia and transient erythroid hypoplasia of bone marrow. Am J Med. 1960;28:606–608. [Google Scholar]
- 8.Meyer RJ, Hoffman R, Zanjani ED. Autoimmune hemolytic anemia and periodic pure red cell aplasia in systemic lupus erythematosus. Am J Med. 1978;65:342–345. doi: 10.1016/0002-9343(78)90829-x. [DOI] [PubMed] [Google Scholar]
- 9.Mannoji M, Shimoda M, Koresawa S, Yamada O, Togawa A, Yawata Y, Umemura H, Kozuru M. [A case of angioimmunoblastic lymphadenopathy with dysproteinemia associated with autoimmune hemolytic anemia and pure red cell aplasia--with special references to its pathogenesis (author’s transl)] . Rinsho Ketsueki. 1981;22:1751–1758. Japanese. [PubMed] [Google Scholar]
- 10.Hirosawa S, Kamiyama R, Dan K, Kuriya S, Nomura T. [A case malignant lymphoma associated with pure red cell aplasia] . Rinsho Ketsueki. 1982;23:1463–1467. Japanese. [PubMed] [Google Scholar]
- 11.Mangan KF, Besa EC, Shadduck RK, Tedrow H, Ray PK. Demonstration of two distinct antibodies in autoimmune hemolytic anemia with reticulocytopenia and red cell aplasia. Exp Hematol. 1984;12:788–793. [PubMed] [Google Scholar]
- 12.Bertrand Y, Lefrere JJ, Leverger G, Courouce AM, Feo C, Clark M, Schaison G, Soulier JP. Autoimmune haemolytic anaemia revealed by human parvovirus linked erythroblastopenia. Lancet. 1985;2:382–383. doi: 10.1016/s0140-6736(85)92509-7. [DOI] [PubMed] [Google Scholar]
- 13.Lefrère JJ, Couroucé AM, Bertrand Y, Girot R, Soulier JP. Human parvovirus and aplastic crisis in chronic hemolytic anemias: a study of 24 observations. Am J Hematol. 1986;23:271–275. doi: 10.1002/ajh.2830230311. [DOI] [PubMed] [Google Scholar]
- 14.Taniguchi S, Shibuya T, Morioka E, Okamura T, Okamura S, Inaba S, Niho Y. Demonstration of three distinct immunological disorders on erythropoiesis in a patient with pure red cell aplasia and autoimmune haemolytic anaemia associated with thymoma. Br J Haematol. 1988;68:473–477. doi: 10.1111/j.1365-2141.1988.tb04239.x. [DOI] [PubMed] [Google Scholar]
- 15.Tomiyama J, Adachi Y, Hanada T, Matsunaga Y. Human parvovirus B19-induced aplastic crisis in autoimmune haemolytic anaemia. Br J Haematol. 1988;69:288–289. doi: 10.1111/j.1365-2141.1988.tb07637.x. [DOI] [PubMed] [Google Scholar]
- 16.Gundersen SG, Bjoerneklett A, Bruun JN. Severe erythroblastopenia and hemolytic anemia during a hepatitis A infection. Scand J Infect Dis. 1989;21:225–228. doi: 10.3109/00365548909039973. [DOI] [PubMed] [Google Scholar]
- 17.Vukelja SJ, Krishnan J, Link CM, Salvado AJ, Knight RD. Resolution of pure red cell aplasia and lymphoma: response to intravenous gammaglobulin and combination chemotherapy. Am J Hematol. 1989;32:129–133. doi: 10.1002/ajh.2830320210. [DOI] [PubMed] [Google Scholar]
- 18.Chitnavis VN, Patou G, Makar YF, Kendra JR. B19 parvovirus induced red cell aplasia complicating acute cold antibody mediated haemolytic anaemia. Br J Haematol. 1990;76:433–434. doi: 10.1111/j.1365-2141.1990.tb06380.x. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki A, Takahashi T, Taniguchi A, Kotake C, Seo T, Toda T, Kobayashi K, Tsukamoto N, Fukumoto M. Pure red cell aplasia associated with non-Hodgkin’s lymphoma and hemolytic anemia. Jpn J Clin Oncol. 1991;21:384–387. [PubMed] [Google Scholar]
- 20.Tohda S, Nara N, Tanikawa S, Imai Y, Murakami N, Aoki N. Pure red cell aplasia following autoimmune haemolytic anaemia. Cell-mediated suppression of erythropoiesis as a possible pathogenesis of pure red cell aplasia. Acta Haematol. 1992;87:98–102. doi: 10.1159/000204728. [DOI] [PubMed] [Google Scholar]
- 21.Linardaki GD, Boki KA, Fertakis A, Tzioufas AG. Pure red cell aplasia as presentation of systemic lupus erythematosus: antibodies to erythropoietin. Scand J Rheumatol. 1999;28:189–191. doi: 10.1080/03009749950154293. [DOI] [PubMed] [Google Scholar]
- 22.Zeidman A, Fradin Z, Barac Y, Bendayan D, Mittelman M, Orlin J. Splenic lymphoma presenting as warm autoimmune hemolytic anemia associated with pure red cell aplasia. Vox Sang. 2000;78:126–129. doi: 10.1159/000031163. [DOI] [PubMed] [Google Scholar]
- 23.Katayama H, Takeuchi M, Yoshino T, Munemasa M, Tada A, Soda R, Takahashi K. Epstein-Barr virus associated diffuse large B-cell lymphoma complicated by autoimmune hemolytic anemia and pure red cell aplasia. Leuk Lymphoma. 2001;42:539–542. doi: 10.3109/10428190109064614. [DOI] [PubMed] [Google Scholar]
- 24.Chehal A, Sharara AI, Haidar HA, Haidar J, Bazarbachi A. Acute viral hepatitis A and parvovirus B19 infections complicated by pure red cell aplasia and autoimmune hemolytic anemia. J Hepatol. 2002;37:163–165. doi: 10.1016/s0168-8278(02)00090-9. [DOI] [PubMed] [Google Scholar]
- 25.Toyota S, Nakamura N, Dan K. [Coexistence of pure red cell aplasia and autoimmune hemolytic anemia occurring during remission of malignant lymphoma] . Rinsho Ketsueki. 2002;43:493–495. Japanese. [PubMed] [Google Scholar]
- 26.Nakashima Y, Abe Y, Ohtsuka R, Tachikawa Y, Nagasawa E, Nishimura J, Ohshima K, Nawata H, Muta K. [Follicular lymphoma complicated with autoimmune hemolytic anemia and pure red cell aplasia] . Rinsho Ketsueki. 2004;45:1208–1210. Japanese. [PubMed] [Google Scholar]
- 27.Ito S, Oyake T, Uchiyama T, Sugawara T, Murai K, Ishida Y. Successful treatment with cyclosporine and high-dose gamma immunoglobulin for persistent parvovirus B19 infection in a patient with refractory autoimmune hemolytic anemia. Int J Hematol. 2004;80:250–253. doi: 10.1532/ijh97.04017. [DOI] [PubMed] [Google Scholar]
- 28.Katori H, Hoshino J, Sawa N, Tagami T, Ubara Y, Takemoto F, Hara S, Hara S, Takaichi N. [A case of membranoproliferative glomerulonephritis type I associated with pure red cell aplasia due to parvovirus B19 infection] . Jin-en Shorei Kenkyu. 2005;21:120. Japanese. [Google Scholar]
- 29.Mizobe T, Tsukada J, Higashi T, Iwashige A, Ota T, Kawano I, Kubota A, Matsuura A, Morimoto H, Ogawa R, Toda Y, Tanaka Y. [Angioimmunoblastic T-cell lymphoma accompanied by pure red cell aplasia] . Rinsho Ketsueki. 2005;46:211–216. Japanese. [PubMed] [Google Scholar]
- 30.Kuroda H, Matsunaga T, Iyama S, Takimoto R, Shirao S, Kida M, Watanabe H, Konuma Y, Hirayama Y, Kohda K, Niitsu Y. [De novo CD5-positive diffuse large B-cell lymphoma associated with autoimmune hemolytic anemia presenting as erythroid hypoplasia] . Rinsho Ketsueki. 2006;47:633–638. Japanese. [PubMed] [Google Scholar]
- 31.Suzuki J, Goto H, Usui M, Sakai J. Serous retinal detachment in a patient with aplastic anemia associated with parvovirus B19 infection. Graefes Arch Clin Exp Ophthalmol. 2007;245:324–326. doi: 10.1007/s00417-006-0315-5. [DOI] [PubMed] [Google Scholar]
- 32.Toyokawa Y, Kingetsu I, Yasuda C, Yasuda J, Yoshida K, Kurosaka D, Yamada A. A case of pure red cell aplasia complicated by Evans syndrome. Mod Rheumatol. 2007;17:333–337. doi: 10.1007/s10165-007-0584-9. [DOI] [PubMed] [Google Scholar]
- 33.Hara A, Wada T, Kitajima S, Toyama T, Okumura T, Kitagawa K, Iwata Y, Sakai N, Furuichi K, Higuchi M, Kaneko S. Combined pure red cell aplasia and autoimmune hemolytic anemia in systemic lupus erythematosus with anti-erythropoietin autoantibodies. Am J Hematol. 2008;83:750–752. doi: 10.1002/ajh.21241. [DOI] [PubMed] [Google Scholar]
- 34.Saha M, Ray S, Kundu S, Chakrabarti P. Pure red cell aplasia following autoimmune hemolytic anemia: an enigma. J Postgrad Med. 2013;59:51–53. doi: 10.4103/0022-3859.109495. [DOI] [PubMed] [Google Scholar]
- 35.Mladenovic J, Farber N, Burton JD, Zanjani ED, Jacob HS. Antibody to the erythropoietin receptor in pure red cell aplasia. Blood. 1985;66(Suppl 1):122. [Google Scholar]