Abstract
The coexistence of mesothelioma and other primary malignancies has been previously reported in literature, but the finding of a pleural mesothelioma with a synchronous peritoneal mesothelioma has not been reported so far. We report a case of a 58-years-old woman that came to our attention for the incidental finding of an inguinal mass. Fine-needle biopsies of the mass and a thoracoscopy with pleural biopsies were performed, after imaging studies showed pleural thickenings suspicious for malignancy. Histological morphology and growth pattern were similar in both cases. Both tumors stained for calretinin, but only the pleural mesothelioma showed positivity for Wilms-Tumor 1 antibody. We tried to demonstrate with molecular biology techniques whether they were synchronous or one was the metastasis of the other, but our studies did not give informative results. The prognosis in this case is poor, and after 6 months the patient is still following a chemotherapy regimen, which is the only practicable approach given the extent of the disease.
Keywords: Mesothelioma, X-chromosome inactivation, EGFR, pleural malignancy, peritoneal malignancy
Introduction
Malignant mesothelioma is a rare neoplasm that develops from mesothelial cells of the serosal membranes, in particular pleura (75%), peritoneum (10-20%), pericardium (1%) or tunica vaginalis (1%). Mesothelioma is primarily a disease of adults and usually arises in the fifth to seventh decades, and 70-80% of cases occur in men. Worldwide, the main cause identified in pathogenesis of malignant mesothelioma is asbestos exposure. Unlike lung cancer, there seems to be no association between mesothelioma and tobacco smoking, but smoking greatly increases the risk of other asbestos-induced cancers. Signs and symptoms of pleural mesothelioma include shortness of breath due to pleural effusion, chest wall pain, and constitutional signs such as unexplained weight loss. Peritoneal mesothelioma usually presents with abdominal pain, nausea, vomiting, ascites and a more rapid weight loss. Medical examination can show the presence of an abdominal solid mass. The diagnosis may be suspected on chest X-ray and computerized tomography (CT) scan findings, but must be confirmed either by examining serous effusion cytology or with a biopsy. There are three histological types of malignant mesothelioma. Epithelioid mesothelioma is composed of epithelioid cells with large, eosinophilic cytoplasm arranged in glandular or pseudoacinar structures. Sarcomatoid histotype is characterized by the presence of markedly atypical spindle cells, in a storiform pattern, which forms whorls or beams. Biphasic (mixed) mesothelioma usually shows both epithelioid and sarcomatoid components. Mesothelioma usually carries a poor prognosis: overall survival is 50% at two years and 20% at five years. Young age at diagnosis, a good performance status and the absence of weight loss are predictive of a more favorable outcome. The histological subtype is also correlated with prognosis: epithelioid mesothelioma usually carries a better prognosis than the sarcomatoid or mixed histotype [1,2]. The coexistence of mesothelioma and other primary malignancies has been previously reported in literature, but the finding of a pleural mesothelioma with a synchronous peritoneal mesothelioma has not been reported so far [3-11].
We report a case of 58 years old woman with a radiological and histological diagnosis of pleural and peritoneal mesothelioma. We tried to demonstrate with molecular biology techniques whether they were synchronous or one was the metastasis of the other.
Case presentation
A 58-years-old non-smoker Caucasian female patient came to our attention for the incidental finding of a 2 cm inguinal mass. Clinical anamnesis revealed asbestos exposure during childhood. A positron emission tomography (PET) - computed tomography (CT) scan highlighted a large area of increased fixation of radiolabeled glucose in the right hemithorax. This area involved mainly the upper lung affecting the anterior, posterior, lateral and mediastinal pleura. A second area of increased fixation was also projected on the soft tissues of the right inguinal region. She underwent a fine-needle biopsy of the inguinal mass and thoracoscopy with pleural biopsies. Written informed consent was obtained from the patient for this study.
The samples were paraffin-embedded and 2-μm thick slides were stained with H&E. Immunohistochemistry according to standard protocols was performed on the most representative blocks of the lesions. The following antibodies were used: Calretinin (5A5, Novocastra, dilution 1:100) and Wilms Tumor-1 (6F-H2, Dako, dilution 1:50).
Molecular analyses
Genomic DNA was extracted from peripheral blood (PBL) and formalin fixed paraffin embedded (FFPE), tumor samples, using the QiAmp MiniKit (Qiagen) and the QIAamp DNA FFPE Tissue Kit (Qiagen), respectively. The X-chromosome inactivation (XCI) pattern was evaluated by the amplification (polymerase chain reaction) and capillary electrophoresis of the highly polymorphic short tandem repeat HUMARA (Androgen Receptor - AR - locus, Xq11.2-q12), flanked by methylation-sensitive restriction sites. The HUMARA assay [12] was performed on both PBL and tumor samples. FFPE samples, PCR was performed before and after enzymatic digestions of the methylation sensitive restriction sites, using the HpaII and HhaI enzymes (Boehringer Ingelheim, Mannheim, Germany) as previously reported [12]. All samples were tested in duplicate and one male DNA sample was included in the experiment as control for enzymatic digestion. XCI pattern was determined by the analysis of peak size resulting from capillary electrophoresis. Mutations of EGFR gene (exons 18, 19, 21) were investigated by means of pyrosequencing analysis using CE-IVD kit EGFR TKI response® (sensitivity) (Diatech Pharmacogenetics, Italy). Briefly, 50-100 ng of DNA were amplified using Rotor-Gene 6000 Q (Corbett Research, Australia) and 20 μl of each PCR product were analyzed by pyrosequencing using PyroMark Q96 ID instrument and Gold Q96 reagents (Qiagen), in accordance with the manufacturer’s instructions. The sequences were analyzed with PyroMark ID 1.0 software (Qiagen) according to the commercial kit handbooks.
Results
Histological slides of both samples showed a papillary and pseudoacinar malignant proliferation, with marked cytologic atypia with abundant and eosinophilic cytoplasm and with the invasion of the deep soft tissues. A spindle cell component was not identified, so a biphasic mesothelioma was excluded. Immunohistochemically, both the neoplasms showed reactivity for Calretinin; only the pleural mesothelioma showed immunoreactivity for Wilms Tumor-1 (Figures 1 and 2).
Figure 1.
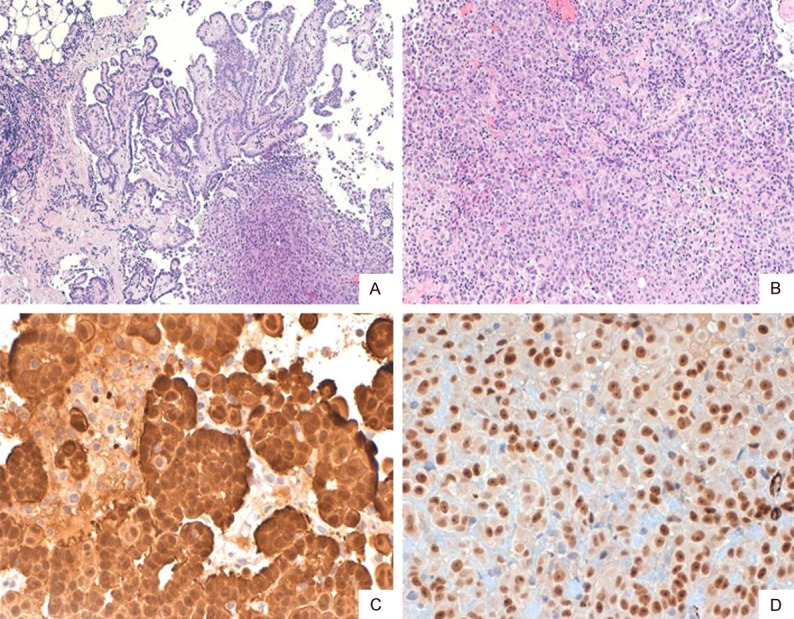
Pleural mesothelioma, showing papillary and solid growth pattern with soft tissue infiltration (A: H&E, ×40; B: H&E, ×100). Calretinin (C: ×400) and WT1 (D: ×400) stained the whole tumor.
Figure 2.
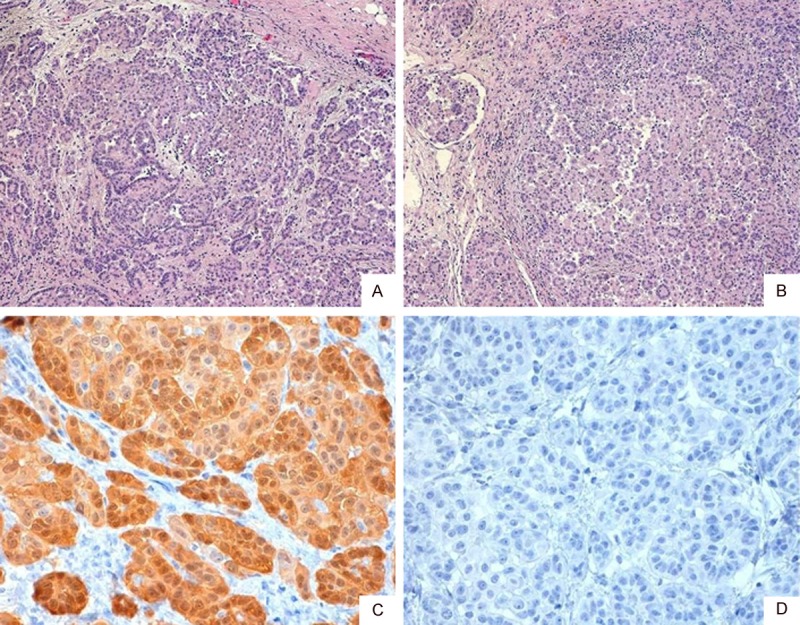
Peritoneal mesothelioma, also showing papillary and solid growth pattern with soft tissue infiltration (A: H&E, ×40; B: H&E, ×100). Immunohistochemistry for Calretinin (C: ×400) showed a strong positivity, but WT-1 (D: ×400) did not stain any tumoral cell.
In order to understand whether the two neoplasms were independent primaries or one the metastasis of the other, we performed X-chromosome inactivation pattern and we investigated mutations of the EGFR gene. As depicted in Figure 3, the analysis of DNA extracted from PBL indicated that patient was heterozygous at the AR locus. The evaluation of XCI of pleural mesothelioma and peritoneal mesothelioma after enzymatic digestion revealed the same profile in both samples, thus indicating that in both tumors the same X chromosome was inactivated. Pyrosequencing analyses of the two mesotheliomas identified a wild-type pattern of EGFR gene in both samples.
Figure 3.
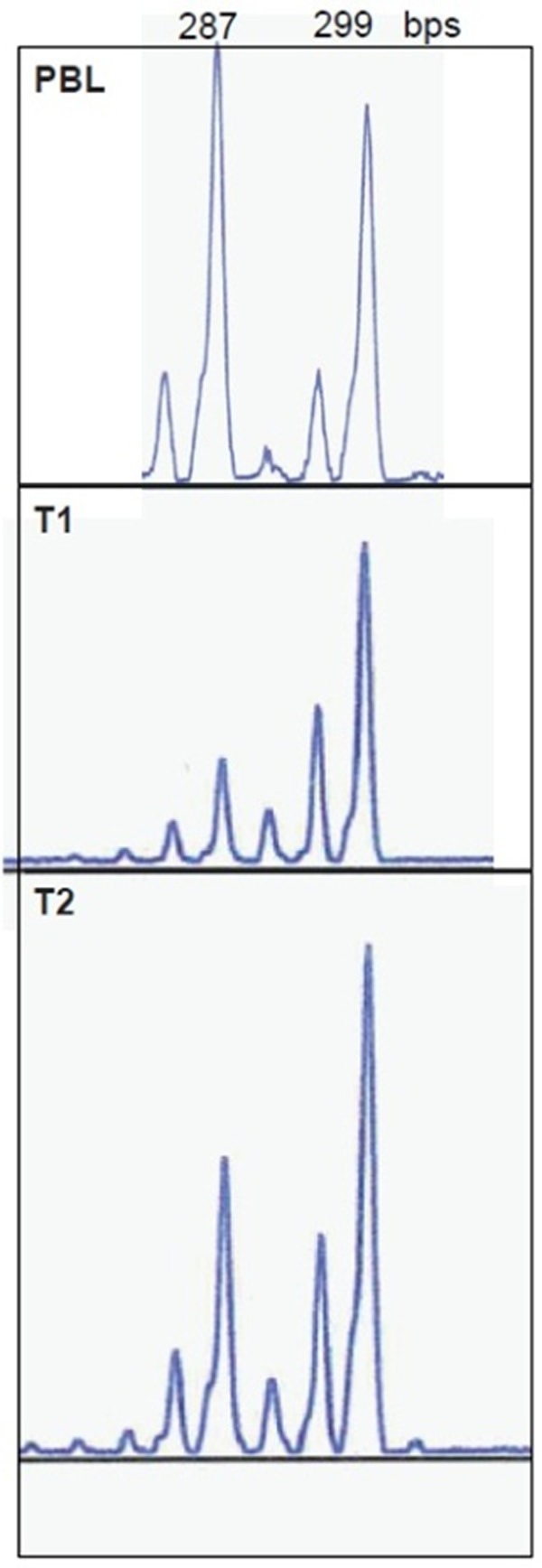
Allelic profile of PBL (top), pleural (middle) and peritoneal (bottom) with the corresponding allele sizing are depicted.
Discussion
The finding of synchronous pleural malignant mesothelioma and peritoneal mesothelioma has never been described in the literature. As showed in Table 1, pleural mesotheliomas have been described mainly in association with lung cancer although Bianchi et al showed in a large series [10] an association with prostatic, renal and bladder carcinomas, and hematologic malignancies.
Table 1.
Pleural, peritoneal and tunica vaginalis mesotheliomas in association with other malignancies published in literature
| Case # | Sex | Age | Tumor 1 | Tumor 2 |
|---|---|---|---|---|
| 1 [3] | F | 55 | pleural | Lung, adenocarcinoma |
| 2 | M | 62 | pleural | Lung, adenocarcinoma |
| 3 | M | 72 | pleural | Lung, adenocarcinoma |
| 4 | M | 64 | pleural | Lung, adenocarcinoma |
| 5 | M | 71 | pleural | Lung, adenocarcinoma |
| 6 | n/d | n/d | pleural | Lung, adenocarcinoma |
| 7 | n/d | n/d | pleural | Lung, adenosquamous |
| 8 | n/d | n/d | pleural | Lung, small cell |
| 9 [4] | M | 36 | Pleural | Inguinal lymph node metastasis |
| 10 [5] | M | 62 | Pleural | Lung, adenocarcinoma |
| 11 | M | 62 | Pleural | Lung, adenocarcinoma |
| 12 | M | 73 | Pleural | Lung, adenocarcinoma |
| 13 | M | 64 | Pleural | Lung, squamous cell |
| 14 | M | 70 | Pleural | Lung, squamous cell |
| 15 | M | 72 | Pleural | Lung, small cell |
| 16 | M | 60 | Pleural | Pancreas, adenocarcinoma |
| 17 | M | 75 | Pleural | Colon, adenocarcinoma |
| 18 | F | 75 | Pleural | Breast, adenocarcinoma |
| 19 [6] | M | 21 | Tunica vaginalis | Tunica vaginalis, contralateral |
| 20 [7] | n/d | n/d | Peritoneum | Colorectal, adenocarcinoma |
| 21 [8] | n/d | n/d | Pleural | Stomach, adenocarcinoma; bladder, TCC; peritoneum, metastatic mesothelioma |
| 22 [9] | M | n/d | Tunica vaginalis | Tunica vaginalis, contralateral |
| 23 [10] | n/d | n/d | Pleural | Prostate, adenocarcinoma |
| 24 | n/d | n/d | Pleural | Prostate, adenocarcinoma |
| 25 | n/d | n/d | Pleural | Prostate, adenocarcinoma |
| 26 | n/d | n/d | Pleural | Prostate, adenocarcinoma |
| 27 | n/d | n/d | Pleural | Prostate, adenocarcinoma |
| 28 | n/d | n/d | Pleural | Prostate, adenocarcinoma |
| 29 | n/d | n/d | Pleural | Prostate, adenocarcinoma |
| 30 | n/d | n/d | Pleural | CLL |
| 31 | n/d | n/d | Pleural | CLL |
| 32 | n/d | n/d | Pleural | CLL |
| 33 | n/d | n/d | Pleural | CLL |
| 34 | n/d | n/d | Pleural | CLL |
| 35 | n/d | n/d | Pleural | Bladder, TCC |
| 36 | n/d | n/d | Pleural | Bladder, TCC |
| 37 | n/d | n/d | Pleural | Bladder, TCC |
| 38 | n/d | n/d | Pleural | Bladder, TCC |
| 39 | n/d | n/d | Pleural | Kidney, carcinoma |
| 40 | n/d | n/d | Pleural | Kidney, carcinoma |
| 41 | n/d | n/d | Pleural | Kidney, carcinoma |
| 42 | n/d | n/d | Pleural | Kidney, carcinoma |
| 43 | n/d | n/d | Pleural | Colon, adenocarcinoma |
| 44 | n/d | n/d | Pleural | Colon, adenocarcinoma |
| 45 | n/d | n/d | Pleural | Colon, adenocarcinoma |
| 46 | n/d | n/d | Pleural | Colon, adenocarcinoma |
| 47 | n/d | n/d | Pleural | Liver, carcinoma |
| 48 | n/d | n/d | Pleural | Liver, carcinoma |
| 49 | n/d | n/d | Pleural | Liver, carcinoma |
| 50 | n/d | n/d | Pleural | Liver, carcinoma |
| 51 [11] | M | 72 | Pleural | Lung, carcinoid and B-cell lymphoma |
The simultaneous presence of two mesotheliomas has been identified only in the tunica vaginalis of the testis, bilaterally [9]. Only one case of pleural mesothelioma metastatic to an inguinal lymph node was described [4], but in our case the tissue from the inguinal mass did not show nodal parenchyma.
Given the monoclonal origin of tumors, it was conceivable that two different tumors were characterized by a different molecular pattern of XCI and/or a different spectrum of EGFR mutation. The finding of molecular differences between pleural mesothelioma and peritoneal mesothelioma would have allowed us to demonstrate a different origin of the two tumors. In investigated samples, the same molecular profile was found, which could indicate the same origin of the two tumors or could be a stochastic event. For this reason, the analyses did not give informative results.
Even in this case, the association with asbestos exposure has been highlighted by clinical anamnesis.
Asbestos fibers can deposit in the lung parenchyma and penetrate the visceral pleura, reaching the pleural surface and leading to the development of malignant mesothelial plaques.
The pathogenesis of peritoneal mesothelioma remains still unknown, although it has been proposed that asbestos fibers from the lung can be transported to the peritoneum and abdominal organs via the lymphatic system. Asbestos fibers, moreover, can be deposited in the gut after ingestion of sputum contaminated with asbestos fibers.
The clinical presentation of our case is peculiar because the patient, although affected by a pleural mesothelioma, presented to our observation for an inguinal mass without complaining any symptoms referable to the chest disease. The prognosis in this case is poor, according to the data related to the thoracic and abdominal disease separately; after 6 months of follow-up the patient is still alive and is following a chemotherapy regimen, which is the only practicable approach given the extent of disease.
Acknowledgements
The authors would like to thank Dr. Rossella Falcone, for helping in performing molecular biology analysis.
Disclosure of conflict of interest
None.
References
- 1.Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC Press; 2004. Chapter 2: Tumors of the pleura; pp. 25–144. [Google Scholar]
- 2.Churg A, Cagle PT, Roggli VL. Tumors of the serosal membranes, 4th series. American Registry of Pathology. 2006. Diffuse malignant tumors of the serosal membranes; pp. 33–72. [Google Scholar]
- 3.Allen TC, Moran C. Synchronous pulmonary carcinoma and pleural diffuse malignant mesothelioma. Arch Pathol Lab Med. 2006;130:721–724. doi: 10.5858/2006-130-721-SPCAPD. [DOI] [PubMed] [Google Scholar]
- 4.Raju B, Kotilingam K, Ravindra Babu G, Ramakoteswarao N. Pleural mesothelioma with inguinal lymphnode metastasis - a case report. Lung India. 1989;1:44–47. [Google Scholar]
- 5.Attanoos RL, Thomas DH, Gibbs AR. Synchronous diffuse malignant mesothelioma and carcinomas in asbestos-exposed individuals. Histopathology. 2003;43:387–392. doi: 10.1046/j.1365-2559.2003.01685.x. [DOI] [PubMed] [Google Scholar]
- 6.Pelzer A, Akkad T, Herwig R, Rogatsch H, Pinggera GM, Bartsch G, Rehder P. Synchronous bilateral malignant mesothelioma of tunica vaginalis testis: early diagnosis. Urology. 1996;64:1031. doi: 10.1016/j.urology.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Jatzko GR, Jester J. Simultaneous occurrence of a rectal carcinoma and a diffuse well differentiated papillary mesothelioma of the peritoneum. Int J Colorectal Dis. 1997;12:326–328. doi: 10.1007/s003840050117. [DOI] [PubMed] [Google Scholar]
- 8.Shikuwa S, Itoh M, Sekine I, Fujii H, Kitara M, Kobayasi M. A autopsy case of synchronous triple cancers including gastric cancer, early urinary bladder cancer and malignant pleural mesothelioma with metastatic malignant mesothelioma of the stomach. Nihon Shokakibyo Gakkai Zasshi. 1991;88:2871–2876. [PubMed] [Google Scholar]
- 9.Menut P, Hervé JM, Barbagelata M, Botto H. Bilateral malignant mesothelioma of the tunica vaginalis testis: Apropos of a case. Prog Urol. 1996;6:587–589. [PubMed] [Google Scholar]
- 10.Bianchi C, Bianchi T, Ramani L. Malignant mesothelioma of the pleura and other malignancies in the same patient. Tumori. 2007;93:19–22. doi: 10.1177/030089160709300104. [DOI] [PubMed] [Google Scholar]
- 11.Peros-Golubicic T, Smojver-Jezek S, Gorecan M, Gredelj N, Tekavec-Trkanjec J, Alilovic M. Multiple primary intrathoracic neoplasms: case report and a review of the literature. Mt Sinai J Med. 2005;72:274–278. [PubMed] [Google Scholar]
- 12.Allen RC, Zoghbi HY, Moseley AB, Rosenblatt HM, Belmont JW. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet. 1992;51:1229–1239. [PMC free article] [PubMed] [Google Scholar]


