Abstract
Special AT-rich sequence-binding protein 1 (SATB1) has been identified as a key factor in the progression of some cancers, functioning as a global genome organizer and chromatin regulator. We examined the levels of SATB1 mRNA expression in NPC cell lines 5-8F (high metastasis) and 6-10B (low metastasis) and immortalized human nasopharyngeal epithelial cells NP69-SV40T by quantitative real-time PCR. We also examined the protein expression levels of SATB1 in 72 cases of nasopharyngeal carcinoma (NPC) tissues and 30 cases of normal nasopharyngeal (NNP) tissues by immunohistochemistry, and then assessed the correlations between SATB1 expression and clinicopathological factors. The expression level of SATB1 mRNA in 5-8F was much higher than those in 6-10B and NP69-SV40T (P < 0.05). The expression level of SATB1 mRNA in 6-10B was higher than in NP69-SV40T, but the difference was not statistically significant (P > 0.05). The positive expression rates of SATB1 protein in NPC (38/72, 52.8%) were significantly higher than in NNP (4/30, 13.3%) (P < 0.05). SATB1 protein levels in NPC were not associated with gender, age, and T stage (P > 0.05), but positively correlated with the titers of EBVCA-IgA, metastasis (N and M stage), recurrence, and survival (P < 0.05). Multivariate analysis showed that the overexpression of SATB1 protein is an independent prognostic factor for NPC. The expression levels of SATB1 were obviously upregulated in primary NPC tissues and human NPC cell lines. Therefore, SATB1 may be a valuable predictor in assessing the metastasis, recurrence, and prognosis of NPC.
Keywords: Nasopharyngeal carcinoma, SATB1, immunohistochemistry, quantitative real-time PCR, prognosis
Introduction
Nasopharyngeal carcinoma (NPC) is an epithelial malignancy and endemic in southern China including Fujian Province. It has strongly aggressive and metastatic features and tends to develop lymphatic or distant metastasis in early stage. The high incidence of metastasis and high relapse rate inevitably lead to a poor clinical outcome for NPC patients. The Epstein-Barr virus and molecular pathogenic processes are involved in the tumorigenesis and progression of NPC. However, the principal molecular mechanisms in terms of how genes facilitate tumor development and metastasis were not good elucidated.
Special AT-rich sequence-binding protein 1 (SATB1) is a nuclear matrix associated protein, as a ‘genome organizer’ and ‘global regulator’ that orchestrates the function of multiple genes [1-3]. It has been reported that SATB1 abnormally expressed in many epithelial tumors, such as breast, gastric, bladder, prostate, laryngeal, and colorectal cancer [4-9], which suggest a crucial role in promoting tumor growth, invasion and metastasis, and may also is an independent factor for several cancers prognosis [3,5,7,10]. However, to our knowledge, very few reports have been looked for the abnormal SATB1 expression in clinical evaluation of NPC. In order to explore the potential role of SATB1 in assessing the risk of tumor metastasis and recurrence as well as predicting prognosis, we investigated the levels of STAB1 expression in the human nasopharyngeal cell lines, normal nasopharyngeal (NNP) tissues and primary NPC tissues, and then determined the correlations between SATB1 expression, clinicopathological factors and survival of patients with NPC.
Materials and methods
Patients and tissues
A total of 102 pathological specimens were collected from January 2006 to July 2008 at Zhongshan Hospital of Xiamen University in Xiamen, Fujian Province (NPC endemic region in China), including 72 cases of primary NPC tissues and 30 cases of NNP. All specimens were confirmed by histopathological examination. No patients had received radiotherapy and chemotherapy before biopsy. Seventy-two patients with NPC comprised 54 men and 18 women with age from 18 to 70 years (median, 45.6 years). The tumor-node-metastasis (TNM) classification was defined according to the World Health Organization (WHO) 2005 NPC staging system [11]. Patients with NPC underwent radical radiotherapy and concurrent chemotherapy after histopathological diagnosis and were followed up. The end of follow-up periods of this study was defined as 8 August 2013. The clinicopathological data of NPC patients are shown in Table 1. In addition, specimens of NNP were obtained from 30 healthy persons suspected of having cancer at above hospital. Thirty cases of NNP included 18 men and 12 women with age range between 20 and 61 years (mean age 40.8 years). Prior consents of the patients and ethical approval for this study from the Medical Ethics Committee at Zhongshan Hospital of Xiamen University were obtained.
Table 1.
Relationship between STAB1 protein expression and clinicopathological factors of NPC patients
| Parameters | Cases | SATB1 expression | P | |
|---|---|---|---|---|
|
| ||||
| Negative (n) | Positive (n) | |||
| Gender | 0.785 | |||
| Male | 54 | 26 | 28 | |
| Female | 18 | 8 | 10 | |
| Age (yrs) | 0.958 | |||
| < 50 | 40 | 19 | 21 | |
| ≥ 50 | 32 | 15 | 17 | |
| T stage | 0.380 | |||
| T1-T2 | 30 | 16 | 14 | |
| T3-T4 | 42 | 18 | 24 | |
| N stage | 0.021 | |||
| N0 | 30 | 19 | 11 | |
| N1-N3 | 42 | 15 | 27 | |
| M stage | 0.030 | |||
| M0 | 63 | 33 | 30 | |
| M1 | 9 | 1 | 8 | |
| EBVCA-IgA titer | 0.020 | |||
| < 1:40 | 26 | 17 | 9 | |
| ≥ 1:40 | 46 | 17 | 29 | |
| Recurrence | 0.000 | |||
| Yes | 24 | 4 | 20 | |
| No | 48 | 30 | 18 | |
| Survival time (yrs) | 0.001 | |||
| ≥ 5 | 40 | 26 | 14 | |
| < 5 | 32 | 8 | 24 | |
Cell lines and culture
Human immortalized nasopharyngeal epithelial cell line (NP69-SV40T) and NPC cell lines 5-8F (high metastasis) and 6-10B (low metastasis) were purchased from Xiangya Central Experiment Laboratory of Central South University (Changsha, Hunan, China). 5-8F and 6-10B cells were cultured in RPMI-1640 (Gibco Life Technologies, Paisley, Scotland, UK) supplemented with 10% fetal bovine serum, 100 μg/mL penicillin, and 100 μg/mL streptomycin. NP69-SV40T cells were cultured in Serum-Free Keratinocyte Medium (Gibco Life Technologies, Grand Island, NY, USA).
Quantitative real-time polymerase chain reaction analysis
Total RNA was isolated from cell lines (NP69-SV40T, 5-8F, 6-10B) using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. RQ1 RNase-free DNase (Promega, Madison, WI, USA) was used to treat RNA samples to exclude contamination by genomic DNA. One microgram of total RNA extract was reverse transcribed with oligo (dT)18 primer (Fermentas, Burlington, Ontario, Canada) using RevertAidTM M-MuLV Reverse Transcriptase (Fermentas) according to the manufacturer’s protocol.
The levels of SATB1 mRNA expression in above cell lines were examined at five times using real-time quantitative polymerase chain reaction (qPCR). The sequences of the SATB1 qPCR primers were as follows: forward 5’-TGCAAAGGTTGCAGCAACCAAAAGC-3’ and reverse 5’-AACATGGATAATGTGGGGCGGCCT-3’. GAPDH was used as housekeeping gene for normalization, with GAPDH primers as follows: forward 5’-GTGGACCTGACCTGCCGTCT-3’ and reverse 5’-GGAGGAGTGGGTGTCGCTGT-3’. Blank samples in which the template was omitted were used controls. Real-time PCR was performed using the SYBR® Green with ROX™ (Applied Biosystems, Foster City, CA, USA) dye detection method on the ABI PRISM 7500 real-time PCR system (Applied Biosystems) under the following conditions: 95°C for 5 min and 40 cycles of 95°C for 15 s, 60°C for 1 min. Relative quantification of SATB1 expression was calculated using the comparative Ct (2−ΔΔCT) method [12].
Immunohistochemical analysis
Serial paraffin sections (4 μm) were dewaxed and rehydrated. Antigen retrieval was performed in boiling citrate buffer (PH = 6.0) for 2 min. After blocking of endogenous peroxidase, the slides were incubated with rabbit anti-human SATB1 monoclonal antibody (diluted 1:100; Epitomics, Burlingame, CA, USA) at 4°C overnight and then washed three times with PBS before being incubated with the secondary antibody (Santa Cruz, CA, USA) at room temperature for 10 min. After washing, sections were incubated with immunoglobulins conjugated with horseradish peroxidase (HRP) for 10 min. Finally, the reaction was developed with 3, 3’-diaminobenzidine substrate. Tissue sections were counterstained with hematoxylin-eosin, returned blue using lithium carbonate, dehydrated in gradient ethanol, mounted with resinene, and subsequently analyzed using a bright field microscope. Negative controls were performed using an isotype IgG instead of the primary antibody. A known positive slide (colon carcinoma specimen) was used as a positive control.
The total SATB1 immunostaining was scored based on the intensity and percentage of cells with SATB1 nuclear staining on the following scale: score 0, negative nuclear staining for all tumor cells; score 1, weak nuclear staining representing all positive staining other than score 2; score 2, moderate nuclear staining > 50% or strong nuclear staining in > 5% of the tumor cells. The staining of immunohistochemistry was assessed independently by two experienced pathologists (D-N Zhou and P Yin). The scoring process repeated more than 3 times. For statistical analysis, final staining scores of 0 and 1-2 were respectively classified as negative and positive SATB1 expression.
Statistical analysis
All statistical calculations were performed using IBM SPSS statistics version 19 (SPSS Inc., Chicago, IL, USA). Correlation between SATB1 expression in primary NPC tissues and clinicopathological factors was analyzed using the Chi-square or Fisher’s exact test. Quantitative values of real-time PCR were presented as mean ± SD. The Student-Newman-Keuls (SNK) q test was used to determine the statistical differences in the relative mRNA expression levels of SATB1 between different cell lines. Overall survival (OS) and disease-free survival (DFS) curves were illustrated using the Kaplan–Meier analysis and compared by log-rank test according to negative and positive SATB1 expression. Multivariate survival analysis with Cox’s proportional hazard regression model was used to evaluate the independent prognostic factors. A P value < 0.05 was considered statistically significant.
Results
Relative expression of SATB1 mRNA in cell lines
SATB1 transcript levels were determined using qPCR and normalized against GAPDH expression, standard curves showed that the amplification efficiency of SATB1 was consistent with housekeeping gene GAPDH. The relative expression levels of SATB1 mRNA (Δ CT) using qPCR test in cell lines NP69-SV40T, 6-10B and 5-8F were 11.98 ± 0.17, 12.14 ± 0.44 and 14.18 ± 0.56, respectively. The relative expression levels of SATB1 mRNA (2−ΔΔCT) in 5-8F and 6-10B were higher than in NP69-SV40T. It exhibited 4.93-fold increase in 5-8F compared to NP69-SV40T, and so did 1.16-fold increase between 6-10B and NP69-SV40T. The fold differences (2−ΔΔCT) between 5-8F and NP69-SV40T showed statistically significant (q = 20.76; P < 0.05), but the fold differences between 6-10B and NP69-SV40T were not statistically significant (q = 1.94; P > 0.05). The relative levels of SATB1 mRNA (2−ΔΔCT) in 5-8F cell line demonstrated 4.09-fold increase compared to 6-10B cell line, and the fold differences were statistically significant (q = 18.82; P < 0.05) (Figure 1).
Figure 1.
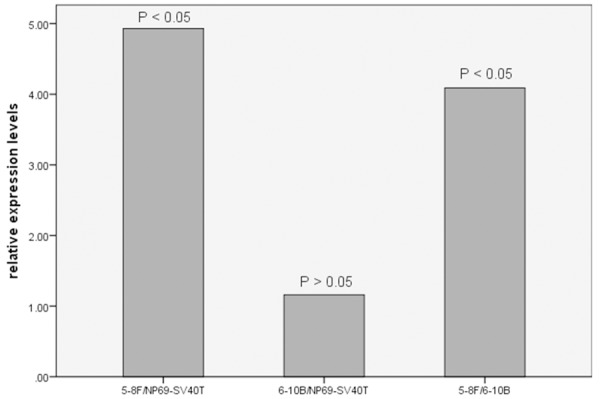
The fold differences of relative SATB1 mRNA expression among different cell lines.
Expression of SATB1 protein in NPC and NNP specimens
Representative examples of SATB1 staining in NPC and NNP were shown in Figure 2A-F. SATB1 staining was mainly localized in the nucleus. The percentages of NPC and NNP samples with positive expression of SATB1 were 52.8% (38/72) and 13.3% (4/30), respectively. The expression level of SATB1 was significantly upregulated in NPC compared with NNP (P < 0.05).
Figure 2.
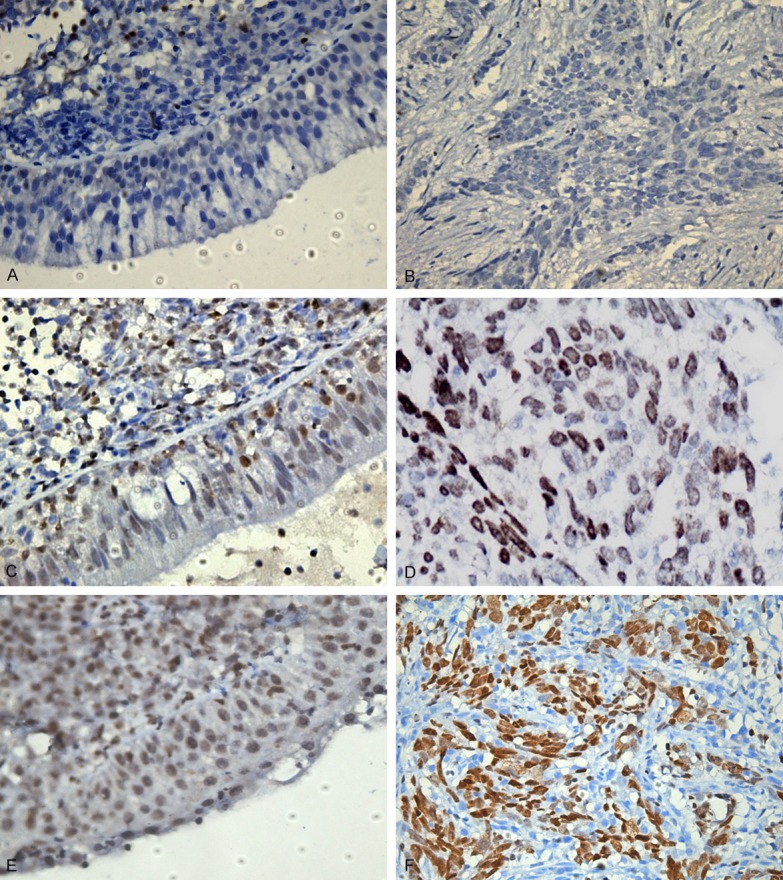
Immunohistochemical images of SATB1 staining in different tissues. A. Negative staining in NNP (score 0). B. Negative staining in NPC (score 0). C. Positive staining in NNP (score 1). D. Positive staining in NPC (score 1). E. Positive staining in NNP (score 2). F. Positive staining in NPC (score 2). Magnification: 400 ×.
Clinicopathological relevance of SATB1 protein expression
The relationships between clinicopathological factors and SATB1 protein expression level in NPC patients were summarized in Table 1. SATB1 expression had no positive correlation with gender, age, and T stage (P > 0.05), whereas it had a significant correlation with the titers of EBVCA-IgA, N stage, M stage, tumor recurrence and the post-treatment 5-year overall survival time of NPC patients (P < 0.05). The percentages of positive STAB1 expression in groups with lymph node metastasis, distant metastasis, tumor recurrence, higher titers of EBVCA-IgA, and shorter survival time were significantly higher than that in groups without lymph node metastasis, distant metastasis, tumor recurrence, and with lower titers of EBVCA-IgA and prolonged survival time.
Relevance of SATB1 protein expression with NPC patient’s survival
We examined the post-treatment OS and DFS of 72 NPC patients according to the expression levels of SATB1 protein. Figures 3A, 3B showed the survival curves of the patients according to positive or negative SATB1 expression. Patients with SATB1-positive tumors exhibited lower OS and DFS than patients with SATB1-negative tumors (P = 0.000; P = 0.001). We next performed multivariate analysis including age, gender, T stage, metastasis (N and M stage), the titers of EBVCA-IgA, and SATB1 expression for OS and DFS. The results revealed that SATB1 expression level and metastasis were independent prognostic factors for NPC (Table 2). SATB1 expression may play a useful role in predicting OS and DFS of NPC patients.
Figure 3.
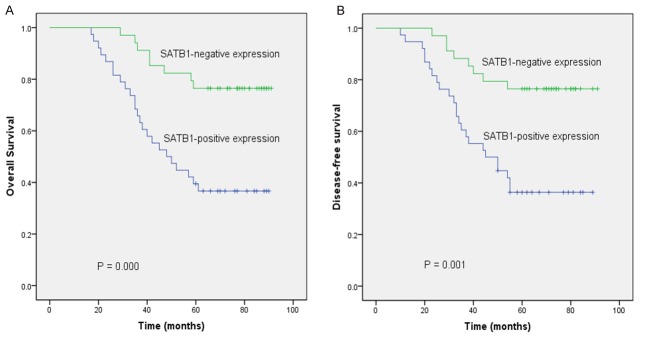
Kaplan-Meier analysis of overall survival and disease-free survival in 72 NPC patients according to the expressions level of SATB1 protein (A and B). P values were determined by the log-rank test.
Table 2.
Multivariate survival analysis of prognostic factors in patients with NPC
| Variables | Overall survival | Disease-free survival | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P | HR | 95% CI | P | |
| Nodal metastasis | ||||||
| Yes vs. No | 2.939 | 1.193-7.242 | 0.019 | 3.104 | 1.290-7.471 | 0.011 |
| Distant metastasis | ||||||
| Yes vs. No | 5.822 | 1.989-17.039 | 0.001 | 5.367 | 2.540-11.341 | 0.000 |
| SATB1 expression | ||||||
| Positive vs. Negative | 0.394 | 0.165-0.942 | 0.036 | 2.662 | 1.080-6.560 | 0.033 |
HR, hazard ratio. CI, confidence interval.
Discussion
SATB1 gene is located on chromosome 3p23 and codes a matrix attachment region (MAR)-binding protein. This nuclear protein functions as a global genome organizer and epigenetic regulator. Prior study showed SATB1 has been suggested to make tumor cells more aggressive and promote a metastatic phenotype by reprogramming gene expression. Overexpression of SATB1 in human breast cancer can alter the expression of hundreds of genes [4,13]. Recently, abnormal expression of SATB1 has been investigated in some tumors, including human breast cancer, laryngeal cancer, and carcinomas of the stomach, colon, lung, ovary, bladder, and liver [14]. High SATB1 expression level has also been associated with unfavorable clinicopathological characteristics and poor prognosis in gastric, ovarian, colorectal cancer, and lung cancer. Recent study has shown that SATB1 silencing by siRNA inhibits the proliferation and invasion of lung cancer cells and human osteosarcoma cells [15,16].
To date, little is known about the relationship between SATB1 and NPC, especially on prognosis of NPC. Endo et al. [17] found that EBV latent membrane protein 1 (LMP1) upregulated SATB1 RNA and protein expression in human nasopharyngeal cell lines and found that the elevation of SATB1 protein levels in tissues from patients with EBV-positive NPC are directly correlated with the expression of LMP1. Shen et al. [18] revealed that EBV LMP1-mediated overexpression of SATB1 was associated with NPC progression. Similarly, our present study found that increased SATB1 expression was correlated with higher titers of EBVCA-IgA. These implicated that EBV LMP1 induced upregulation of SATB1 expression in NPC, which was a critical step for EBV-induced tumorigenesis.
In this report, we found that SATB1 mRNA expression level was sequentially increased in NP69-SV40T, 6-10B, and 5-8F cell lines. We revealed that the SATB1 mRNA expression levels were significantly upregulated in NPC cell lines compared to immortalized nasopharyngeal epithelial cell. We also revealed that SATB1 mRNA expression levels were significantly upregulated in the high metastatic NPC cell line 5-8F compared to the low metastatic NPC cell line 6-10B. Above results suggest that upregulation of SATB1 mRNA expression correlates to the metastatic capacity of NPC cell.
In this study, we revealed that SATB1 protein expression level was significantly upregulated in primary NPC samples compared to NNP samples, suggesting that SATB1 might be connected with the tumorigenesis of NPC. We also revealed that the upregulated expression of SATB1 was closely linked to lymphatic and distant metastasis in NPC, indicating that SATB1 might play an important role in metastasis of NPC as a metastasis promoter. This investigation was the first to discover that abnormal SATB1 protein expression was correlated with the clinical outcome of NPC patients. NPC patients with tumor recurrence and post-treatment survival time below five years had a higher level of SATB1 positive expression, indicating that SATB1 protein could act as a useful molecular marker in assessment of tumor recurrence and surveillance of treatment for NPC.
This study first demonstrated the potential influence of increased expression of SATB1 protein on the survival rate of NPC patients. SATB1 expression level in NPC was inversely correlated with patient’s OS and DFS. Furthermore, SATB1 protein expression level was an independent prognostic factor for NPC. These present the evidence that elevated expression of SATB1 may predict a higher risk of metastasis, relapse and shortened survival in NPC. The current findings suggest that SATB1 may be a valuable prognostic marker for NPC.
In conclusion, SATB1 was overexpressed in primary NPC tissues and NPC cell lines, especially in high metastatic cell line. The expression level of SATB1 was significantly correlated with the titer of EBVCA-IgA, N stage, M stage, recurrence, and survival of NPC patients. SATB1 expression level was also associated with the metastatic potentials of NPC cells. These results all show aberrant expression of SATB1 plays a significantly important role in NPC development and progression. SATB1 may be a good biomarker for clinical evaluation including metastasis, relapse, and survival of NPC. SATB1 may be a promising molecular target for NPC therapy in the future. Our next work will focus on the functional analysis and RNAi experiment for SATB1 in NPC.
Acknowledgements
This work was supported by the Medical Innovation Program Fund of Fujian Province (Grant Number 2011-CXB-35), the Science and Technology Project of Xiamen Science and Technology Bureau (Grant Number 3502Z20134019) and the Natural Science Fund of Fujian Province (Grant Number 2013D003), China.
Disclosure of conflict of interest
None.
References
- 1.Mir R, Pradhan SJ, Galande S. Chromatin organizer SATB1 as a novel molecular target for cancer therapy. Curr Drug Targets. 2012;13:1603–15. doi: 10.2174/138945012803530008. [DOI] [PubMed] [Google Scholar]
- 2.Kohwi-Shigematsu T, Poterlowicz K, Ordinario E, Han HJ, Botchkarev VA, Kohwi Y. Genome organizing function of SATB1 in tumor progression. Semin Cancer Biol. 2013;23:72–9. doi: 10.1016/j.semcancer.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nodin B, Hedner C, Uhlén M, Jirström K. Expression of the global regulator SATB1 is an independent factor of poor prognosis in high grade epithelial ovarian cancer. J Ovarian Res. 2012;5:24. doi: 10.1186/1757-2215-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han HJ, Russo J, Kohwi Y, Kohwi-Shigematsu T. SATB1 reprogrammes gene expression to promote breast tumour growth and metastasis. Nature. 2008;452:187–93. doi: 10.1038/nature06781. [DOI] [PubMed] [Google Scholar]
- 5.Nodin B, Johannesson H, Wangefjord S, O’Connor DP, Lindquist KE, Uhlén M, Jirström K, Eberhard J. Molecular correlates and prognostic significance of SATB1 expression in colorectal cancer. Diagn Pathol. 2012;7:115. doi: 10.1186/1746-1596-7-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao XD, Ji WY, Zhang W, He LX, Yang J, Liang HJ, Wang LL. Overexpression of SATB1 in laryngeal squamous cell carcinoma. ORL J Otorhinolaryngol Relat Spec. 2010;72:1–5. doi: 10.1159/000264777. [DOI] [PubMed] [Google Scholar]
- 7.Lu X, Cheng C, Zhu S, Yang Y, Zheng L, Wang G, Shu X, Wu K, Liu K, Tong Q. SATB1 is an independent prognostic marker for gastric cancer in a Chinese population. Oncol Rep. 2010;24:981–7. doi: 10.3892/or.2010.981. [DOI] [PubMed] [Google Scholar]
- 8.Mao L, Yang C, Wang J, Li W, Wen R, Chen J, Zheng J. SATB1 is overexpressed in metastatic prostate cancer and promotes prostate cancer cell growth and invasion. J Transl Med. 2013;11:111. doi: 10.1186/1479-5876-11-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han B, Luan L, Xu Z, Wu B. Expression and biological roles of SATB1 in human bladder cancer. Tumour Biol. 2013;34:2943–9. doi: 10.1007/s13277-013-0857-1. [DOI] [PubMed] [Google Scholar]
- 10.Selinger CI, Cooper WA, Al-Sohaily S, Mladenova DN, Pangon L, Kennedy CW, McCaughan BC, Stirzaker C, Kohonen-Corish MR. Loss of special AT-rich binding protein 1 expression is a marker of poor survival in lung cancer. J Thorac Oncol. 2011;6:1179–89. doi: 10.1097/JTO.0b013e31821b4ce0. [DOI] [PubMed] [Google Scholar]
- 11.Barnes L, Eveson JW, Reichart P, Sidransky D. World Health Organization classification of tumors: pathology and genetics of head and neck tumors. Lyon: IARC Press; 2005. pp. 81–106. [Google Scholar]
- 12.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 13.Hanker LC, Karn T, Mavrova-Risteska L, Ruckhäberle E, Gaetje R, Holtrich U, Kaufmann M, Rody A, Wiegratz I. SATB1 gene expression and breast cancer prognosis. Breast. 2011;20:309–13. doi: 10.1016/j.breast.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Tu W, Luo M, Wang Z, Yan W, Xia Y, Deng H, He J, Han P, Tian D. Upregulation of SATB1 promotes tumor growth and metastasis in liver cancer. Liver Int. 2012;32:1064–78. doi: 10.1111/j.1478-3231.2012.02815.x. [DOI] [PubMed] [Google Scholar]
- 15.Huang B, Zhou H, Wang X, Liu Z. Silencing SATB1 with siRNA inhibits the proliferation and invasion of small cell lung cancer cells. Cancer Cell Int. 2013;13:8. doi: 10.1186/1475-2867-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Qu S, Li S, Wang Y, Li Y, Wang Y, Wang Z, Li R. Silencing SATB1 inhibits proliferation of human osteosarcoma U2OS cells. Mol Cell Biochem. 2013;378:39–45. doi: 10.1007/s11010-013-1591-0. [DOI] [PubMed] [Google Scholar]
- 17.Endo K, Shackelford J, Aga M, Yoshizaki T, Pagano JS. Upregulation of special AT-rich-binding protein 1 by Epstein-Barr virus latent membrane protein 1 in human nasopharyngeal cells and nasopharyngeal cancer. J Gen Virol. 2013;94:507–13. doi: 10.1099/vir.0.046243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen Z, Zeng Y, Guo J, Wu Y, Jiang X, Ding R, Wu C, Li R, Luo B, Zeng C, Jiang H, Jie W. Over-expression of the special AT rich sequence binding protein 1 (SATB1) promotes the progression of nasopharyngeal carcinoma: association with EBV LMP-1 expression. J Transl Med. 2013;11:217. doi: 10.1186/1479-5876-11-217. [DOI] [PMC free article] [PubMed] [Google Scholar]


