Abstract
Aberrant angiogenesis is essential to the development and progression of leukemia. Ginsenoside Rg3 has been commonly used in anti-angiogenic therapy of solid tumors. This study aimed to investigate the anti-angiogenic effects of Rg3 in patients with acute leukemia. Bone marrow stromal cells derived from patients with acute leukemia were treated with Rg3 and the expression of vascular endothelial growth factor (VEGF) and hypoxia-inducible factor 1α (HIF-1α) was detected by RT-PCR and western blot analysis. The results showed that Rg3 inhibited VEGF and HIF-1α expression at both mRNA and protein levels in bone marrow stromal cells. In addition, Rg3 treatment led to reduced serum levels of HIF-1α and VEGF in patients with acute leukemia. Mechanistically, we demonstrated that Rg3 downregulated the phosphorylation of Akt and ERK1/2 in BMSCs. In conclusion, Rg3 exhibits anti-leukemia effect in part due to its anti-angiogenic activity via inhibiting PI3K/Akt and ERK1/2 pathways, which act to regulate the expression of HIF-1α and VEGF.
Keywords: Acute leukemia, Rg3, angiogenesis, hypoxia-inducible factor 1, bone marrow stromal cells, vascular endothelial growth factor
Introduction
Acute leukemia is the most common malignancy originated from malignant transformation of hematopoietic stem cells. Aberrant angiogenesis in the microenvironment of bone marrow is essential to the development and progression of acute leukemia [1]. Increased vascularization has been found in acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL), as well as various preleukemic disorders including myelodysplastic syndromes (MDS) and myeloproliferative neoplasms (MPN) [2,3]. Both the leukemic blasts and the bone marrow microenvironment contribute to neoangiogenesis by secreting angiogenic growth factors and mediators, such as vascular endothelial growth factor (VEGF). Therefore, anti-angiogenic therapy is an important approach to the treatment of hematologic malignancy [4]. Ginsenoside Rg3, a saponin extracted from ginseng, has demonstrated anti-cancer activity in vitro and in vivo with relatively low toxicity, especially on vessels or angiogenesis in tumors [5].
It is known that hypoxia-inducible factor (HIF-1α) activates the transcription of VEGF and other factors involved in tumor development [6]. Growth factors, cytokines, and other signaling molecules stimulate the synthesis of HIF-1α protein via activating phosphatidylinositol 3-kinase (PI3K)/AKT and mitogen-activated protein kinase (MAPK) [7]. Although Rg3 has demonstrated anti-tumor activity in several types of solid malignancy, the efficacy of Rg3 in patient with acute leukemia and the underlying mechanism are still not clear.
In this study we evaluated the efficacy of Rg3 on angiogenesis and cell growth using bone marrow stromal cells (BMSCs) derived from acute leukemia patients, and examined the effects of Rg3 on the expression of HIF-1α and VEGF in vivo using the serum obtained from patients with acute leukemia. In addition, we explored the role of PI3K/Akt and MAPK pathways in mediating Rg3 induced regulation of HIF-1α and VEGF expression in BMSCs.
Materials and methods
Reagents
Ginsenoside Rg3 was extracted from northeast China’s ginseng with purity no less than 99.5% from Jilin Yatai Pharmaceuticals CO., LTD (China). Rg3 were prepared as 800 mg/L diluted in dimethyl sulfoxide (DMSO, with the concentration no more than 0.01%).
Cell culture
BMSCs were obtained from thirty-one randomly selected patients who had been newly diagnosed with acute leukemia, including sixteen patients with ALL and fifteen patients with AML, at Xinqiao Hospital (Chongqing, China) in 2008. Twenty-eight healthy subjects were recruited as the control group. Approval from the Ethics Committee of Xinqiao Hospital and informed consent from all subjects were obtained before this study was started.
Heparinised bone marrow aspirates of 2-3 ml were collected from each subject with informed consent and diluted at the ratio of 1:1 using phosphate buffered saline (PBS) for centrifugation at 550 g. The supernatants were discarded and the cell pellets were resuspended in 4 ml PBS. Mononuclear cells (MNCs) in the bone marrow were separated by Percoll density-gradient centrifugation (1.073 g/l, Pharmacia) and washed twice using PBS. MNCs were seeded at a density of 1×105 cells/ml in T25 flasks with RPMI 1640 medium supplemented with 10% fetal bovine serum, and placed in a humidified incubator at 37°C with 5% CO2. The medium was changed every 24 h. The cells were ready for use after two passages.
Patients
Twenty patients with AML were randomly selected, who were 20-60 years of age with expected survival time longer than 3 months, normal function in the liver, kidney and heart, and without other severe complications. These AML patients underwent two courses of DA chemotherapy as described previously [8], and were randomly assigned to one of the two groups: group A (control) took two placebo tablets twice a day, whereas group B (treatment) took two Rg3 tablets twice a day for 2 months. Peripheral blood plasma was collected from all patients both before and after Rg3 treatment. The anti-angiogenesis effect was evaluated at the end of the second cycle, when improvement or stable complete remission was expected to appear if not terminated due to cure or change to other treatments.
Cell viability assay
The viability of BMSCs was detected by MTT assay. The cells were seeded into 96-well plates at 1×104 cells/well, and then exposed to 20, 40, 60, 80, and 100 mg/L Rg3 for 6, 12, 24, and 48 h, respectively. The absorbance at 540 nm was recorded using OPTImax microplate reader (Molecular Devices; Sunnyvale, CA). The cell survival percentages were calculated by dividing the mean OD of cells exposed to Rg3 by that of cells exposed to DMSO.
RT-PCR assay
Total RNA was extracted from BMSCs using TRIzol reagent (Invitrogen) and reverse Transcripted into cDNA using Superscribe First-Strand Synthesis System (Invitrogen). Aliquots of cDNA were used as the template for PCR. The following oligonucleotides were used as primers: HIF-1α 5’-TCCATGGTACCATGAGGAAA-3’ and 5’-ACCAAGCAGGTCATAGGTGG-3’; VEGF 5’-GAGCGGAGCCGCGAGAAGTG-3’ and 5’-TCCAT GAGCCCGGCTTCCGA-3’; and β-actin 5’-TTGTAACCAACTGGGACGATATGG-3’ and 5’-GATCTTGATCTTCATGGTGGTAGG-3’. PCR conditions were as follows: 30 s at 95°C, 30 s at 55°C, and 30 s at 72°C for 27 cycles. PCR products were visualized on 1.0% agarose gel containing 5 μg/ml of ethidium bromide.
Western blot analysis
The cells were harvested and lysed on ice for 30 min in the lysis buffer (10 mM Tris pH 8.0, 1 mM EDTA, 400 mM NaCl, 10% glycerol, 0.5% NP-40, 5 mM sodium fluoride, 0.1 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol). An equal amount of protein (25 μg) was separated by 8-12% SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked overnight in the blocking buffer (250 mM NaCl, 0.02% Tween 20, 5% goat serum, and 3% BSA), and incubated with primary antibody against Akt, phosphorylated Akt (Ser473), ERK1/2, phosphorylated ERK1/2 (Thr202/Tyr204) (Cell Signaling, Beverly, MA, USA), VEGF or HIF-1α (Santa Cruz Biotech, Santa Cruz, CA, USA). The membranes were washed and then incubated with peroxidase-conjugated secondary antibody. Immuno-reactive bands were detected by ECL (Amersham).
Immunofluorescence
The cells were cultured on glass cover slips and fixed with 4% paraformaldehyde. The cells were then incubated with primary antibody against HIF-1α, VEGF, phosphorylated ERK and Akt. After thorough washing, the cells were incubated with secondary antibodies conjugated with FITC or Cy3. The nuclei were stained with 4’,6-diamino-2-phenylindole (DAPI) (Vector Laboratories). Fluorescent images were processed using Leica TCS-NT confocal laser scanning microscope (Leica, Bensheim, Germany).
Enzyme-linked immunosorbent assay (ELISA)
Serum VEGF and HIF-1α levels in the patients before and after Rg3 treatments were detected by ELISA kit following the manufacturer’s recommendations.
Statistical analysis
Data were presented as means ± SD with triplicate determinations and analyzed by using SPSS13.0 software (SPSS Inc, USA). Examined data were assessed using the t-test, and P value < 0.05 was considered statistically significant.
Results
Rg3 inhibits the viability of BMSCs
First we evaluated the effects of Rg3 on the viability of BMSCs derived from acute leukemia patients. MTT assay showed that Rg3 inhibited the viability of BMSCs derived from acute leukemia patients in a dose and time dependent manner (Figure 1).
Figure 1.
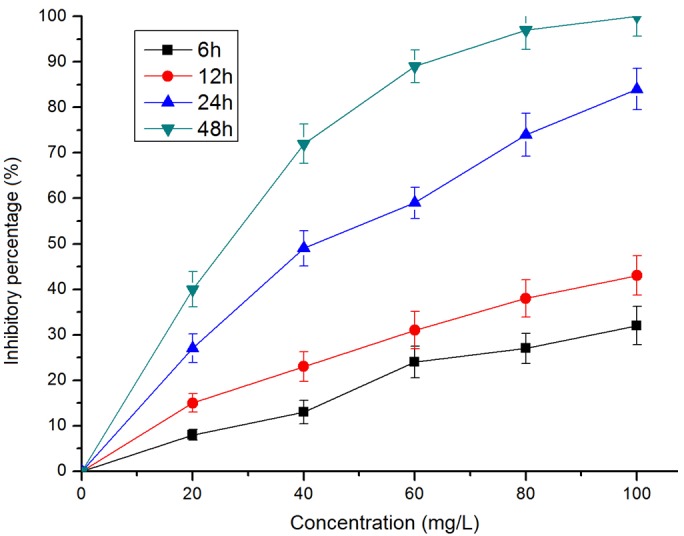
Rg3 inhibits the viability of BMSCs. BMSCs derived from acute leukemia patients were exposed to Rg3 at the indicated concentration and time. Te cell viability was evaluated by MTT assay and the inhibitory effect was expressed as the percentage of viable cells (means ± SD, n=3).
Rg3 inhibits VEGF and HIF-1α expression in BMSCs
RT–PCR assay showed that the expression of VEGF and HIF-1α at mRNA level was increased in BMSCs derived from AML and ALL patients compared to those from control subjects. However, Rg3 inhibited the expression of VEGF and HIF-1α at mRNA level in BMSCs derived from control subjects, AML and ALL patients (Figure 2A, 2B).
Figure 2.
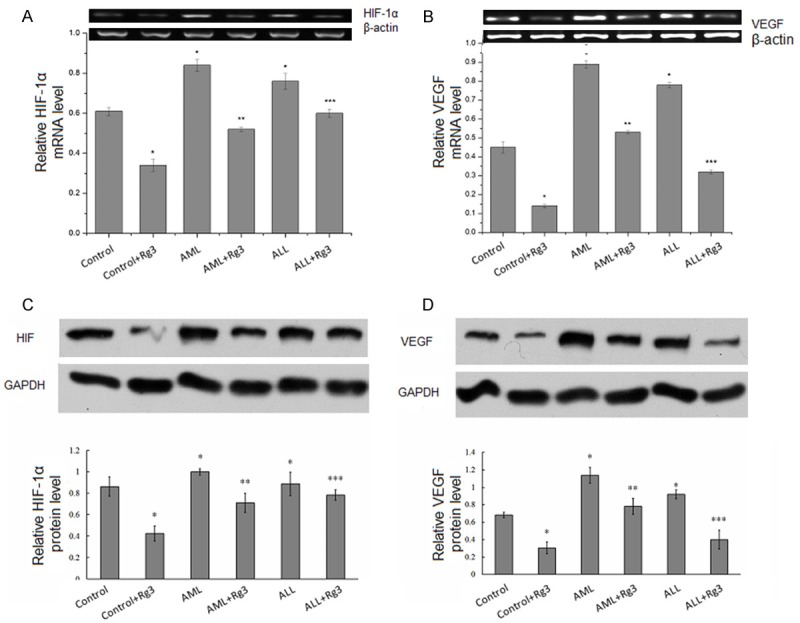
Rg3 inhibits VEGF and HIF-1α expression in BMSCs. RT–PCR assay of VEGF mRNA level (A) and HIF-1α mRNA level (B) in BMSCs derived from control subjects, AML and ALL patients. Western blot analysis of VEGF protein level (C) and HIF-1α protein level (D) in BMSCs derived from control subjects, AML and ALL patients. Results were presented are mean ± S.E. from three independent experiments performed in duplicate. *P < 0.05, compared with control; **P < 0.05, compared with AML; ***P < 0.05, compared with ALL. Actin served as internal control.
Western blot analysis showed that the expression of VEGF and HIF-1α at protein level was increased in BMSCs derived from AML and ALL patients compared to those from control subjects. However, Rg3 inhibited the expression of VEGF and HIF-1α at protein level in BMSCs derived from control subjects, AML and ALL patients (Figure 2C, 2D).
Rg3 inhibits the activation AKT and ERK1/2 in BMSCs
PI3K/AKT and ERK1/2 pathways are involved in regulating the stability and synthesis of HIF-1α protein [8]. In order to understand whether Rg3 inhibits HIF-1α expression via these pathways, we detected the phosphorylation of AKT and ERK1/2 in BMSCs exposed to Rg3. Western blot analysis showed that the phosphorylation levels of AKT and ERK1/2 declined in BMSCs derived from control subjects, AML and ALL patients, after exposure to Rg3 (Figure 3). These data suggest that Rg3 inhibits the activation of AKT and ERK1/2 in BMSCs.
Figure 3.
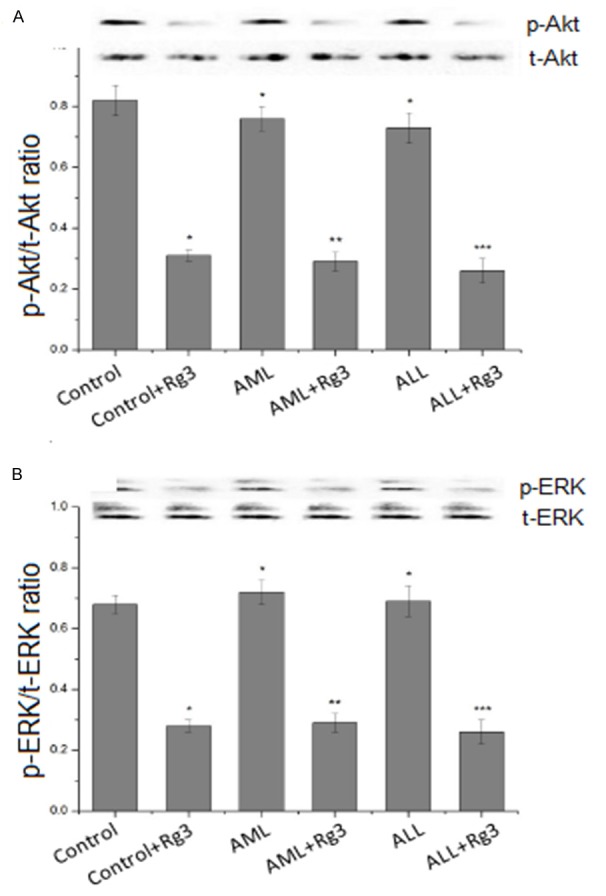
Rg3 inhibits the activation of Akt and ERK1/2 in BMSCs. BMSCs were seeded in 60-mm dishes, cultured to 80~90% confluence, and treated with 40 mg/L Rg3 for 24 h. The activation of Akt and ERK1/2 was detected by antibody against phospho-AKT (Ser473) and phospho-ERK (Thr202/Tyr204). *P < 0.05, compared with control; **P < 0.05, compared with AML; ***P < 0.05, compared with ALL.
Localization of HIF-1α, VEGF, and phosphorylated ERK and Akt in BMSCs
Next we utilized immunofluorescence technique to detect the localization of HIF-1α, VEGF, and phosphorylated ERK and Akt in BMSCs derived from acute leukemia patients. HIF-1α was detected in the cytoplasm and the nuclei, whereas VEGF and phosphorylated ERK and Akt were apparently present in the cytoplasm. In addition, exposure to Rg3 reduced the levels of HIF-1α, VEGF, and phosphorylated ERK and Akt in BMSCs (Figure 4).
Figure 4.
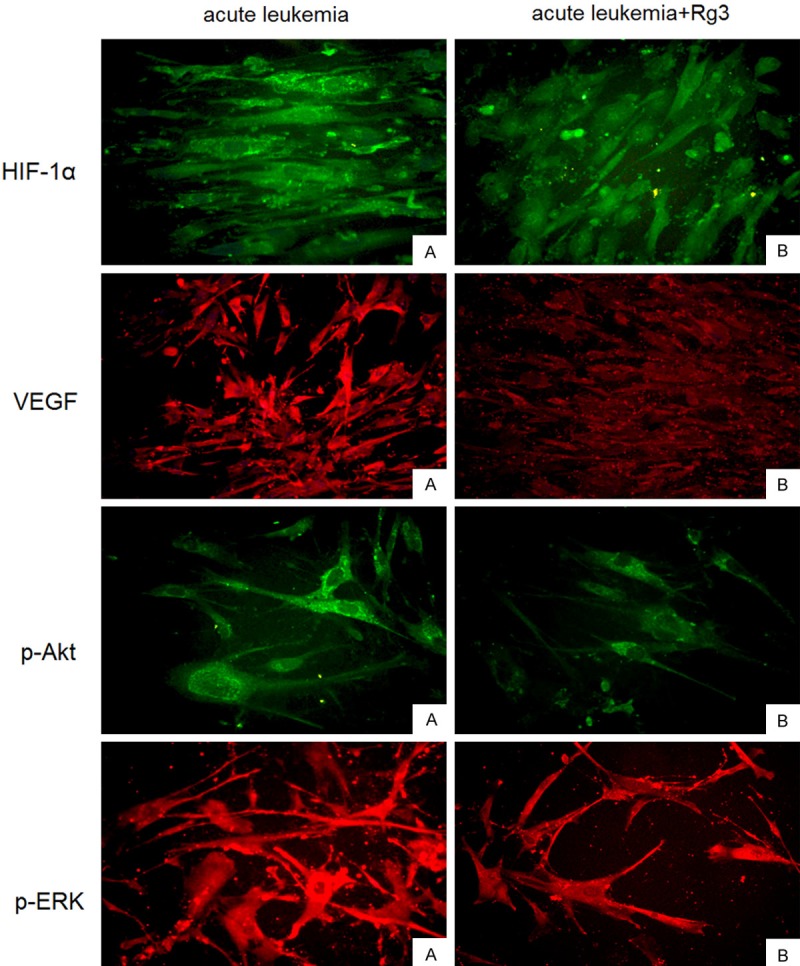
Localization of HIF-1α, VEGF, and phosphorylated ERK and Akt in BMSCs.Immunofluorescence images of the staining of HIF-1α, VEGF, and phosphorylated ERK and Akt in BMSCs derived from acute leukemia patients. HIF-1α was detected in the cytoplasm and the nuclei, whereas VEGF and phosphorylated ERK and Akt were apparently present in the cytoplasm.
Rg3 inhibits HIF-1α and VEGF expression in acute leukemia patients
Finally, we evaluated the in vivo effects of Rg3 in acute leukemia patients. Rg3 was administered to patients in the treatment group for two months, simultaneously with the routine chemotherapy. ELISA assay of the patient serum showed that serum level of HIF-1α and VEGF declined significantly in acute leukemia patients treated with Rg3, compared to patients treated with chemotherapy only (Figure 5).
Figure 5.
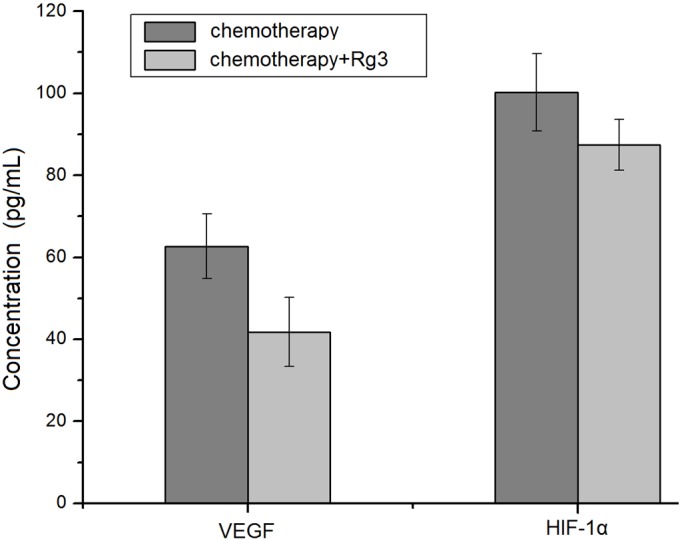
Rg3 inhibits HIF-1α and VEGF expression in acute leukemia patients. Acute leukemia patients were divided into two groups: chemotherapy alone, and chemotherapy plus Rg3 treatment. Serum levels of HIF-1α and VEGF were detected by ELISA. The results were expressed as means ± SD from at least twenty different donors.
Discussion
The importance of bone marrow angiogenesis to the progression of acute leukemia has been widely recognized recently, but the underlying mechanism remains unclear. The microenvironments of bone marrow and leukemic blasts both contribute to angiogenesis by secreting different angiogenic growth factors and mediators [9]. HIF-1α is an important factor that activates the transcription of multiple genes which encode angiogenic growth factors and cytokines which are involved in the angiogenesis in acute leukemia. The genes activated by HIF-1 include those encoding VEGF, stromal-derived factor 1, placental growth factor, angiopoietin 1 and 2, and platelet-derived growth factor [10]. These growth factors bind to cognate receptors on vascular endothelial, smooth muscle cells, as well as endothelial progenitor cells, mesenchymal stem cells, and other bone marrow-derived angiogenic cells [7].
Patients with haematological malignancies exhibit increased bone marrow MVD, and anti-angiogenic drugs are currently tested in these patients [11,12]. Ginsenoside Rg3 is the effective component extracted from ginseng with C42H72O13 framework and 784 Da molecular weight. The anti-cancer activity of Rg3 has been postulated to be related to the suppression of growth, invasion, and metastasis of various tumors, as well as the inhibition of neovascularization [13]. In this study, we evaluated the effects of Rg3 in vivo and our results showed that Rg3 treatment led to reduced serum levels of HIF-1α and VEGF in acute leukemia patients. These data complement our in vitro results and confirm the anti-angiogenesis effects of Rg3.
To further investigate the molecular mechanism of Rg3 induced anti-angiogenesis effect in acute leukemia, we detected HIF-1α and VEGF expression at mRNA and protein levels in BMSCs exposed to Rg3. Our results showed that Rg3 inhibited HIF-1α and VEGF protein synthesis in BMSCs derived from acute leukemia patients. Furthermore, we explored the signaling pathways by which Rg3 regulates HIF-1α and VEGF expression. Our results indicated that Rg3 blocked the activation of Akt and MAPK in BMSCs derived from acute leukemiam patients, suggesting that Rg3 regulates HIF-1α and VEGF expression via inhibiting Akt and ERK kinases. Future studies such as multi-centered clinical trials and long term follow-up are needed to confirm that Rg3 could be utilized for anti-angiogenesis therapy of haematological malignancies.
Acknowledgements
The study was supported in part by Military Natural Science fund of China (No. 06-MB238) and National Natural Science Foundation of China (No. 81000195).
Disclosure of conflict of interest
None.
References
- 1.Otrock ZK, Mahfouz RA, Makarem JA, Shamseddine AI. Understanding the biology of angiogenesis: review of the most important molecular mechanisms. Blood Cells Mol Dis. 2007;39:212–220. doi: 10.1016/j.bcmd.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Di Raimondo F, Palumbo GA, Azzaro MP, Giustolisi R. Angiogenesis in acute myeloid leukemia. Blood. 2000;96:3656–3657. [PubMed] [Google Scholar]
- 3.Aguayo A, Kantarjian H, Manshouri T, Gidel C, Estey E, Thomas D, Koller C, Estrov Z, O’Brien S, Keating M, Freireich E, Albitar M. Angiogenesis in acute and chronic leukemias and myelodysplastic syndromes. Blood. 2000;96:2240–2245. [PubMed] [Google Scholar]
- 4.Kruizinga RC, de Jonge HJ, Kampen KR, Walenkamp AM, de Bont ES. Vascular Endothelial Growth Factor A isoform mRNA expression in pediatric acute myeloid leukemia. Pediatr Blood Cancer. 2011;56:294–297. doi: 10.1002/pbc.22783. [DOI] [PubMed] [Google Scholar]
- 5.Lu JM, Yao Q, Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009;7:293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong CG, Wu WK, Feng SY, Yu J, Shao JF, He GM. Suppressing the malignant phenotypes of glioma cells by lentiviral delivery of small hairpin RNA targeting hypoxia-inducible factor-1α. Int J Clin Exp Pathol. 2013;6:2323–2332. [PMC free article] [PubMed] [Google Scholar]
- 7.Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 2009;24:97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- 8.Fang J, Cao Z, Chen YC, Reed E, Jiang BH. 9-beta-D-arabinofuranosyl-2-fluoroadenine inhibits expression of vascular endothelial growth factor through hypoxia-inducible factor-1 in human ovarian cancer cells. Mol Pharmacol. 2004;66:178–186. doi: 10.1124/mol.66.1.178. [DOI] [PubMed] [Google Scholar]
- 9.Ayala F, Dewar R, Kieran M, Kalluri R. Contribution of bone microenvironment to leukemogenesis and leukemia progression. Leukemia. 2009;23:2233–2241. doi: 10.1038/leu.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsirakis G, Pappa CA, Kanellou P, Stratinaki MA, Xekalou A, Psarakis FE, Sakellaris G, Alegakis A, Stathopoulos EN, Alexandrakis MG. Role of platelet-derived growth factor-AB in tumour growth and angiogenesis in relation with other angiogenic cytokines in multiple myeloma. Hematol Oncol. 2012;30:131–136. doi: 10.1002/hon.1014. [DOI] [PubMed] [Google Scholar]
- 11.Antic D, Jovanovic MP, Fekete MD, Cokic V. Assessment of bone marrow microvessel density in chronic lymphocytic leukemia. Appl Immunohistochem Mol Morphol. 2010;18:353–356. doi: 10.1097/PAI.0b013e3181d18ae2. [DOI] [PubMed] [Google Scholar]
- 12.Li WW, Hutnik M, Gehr G. Antiangiogenesis in haematological malignancies. Br J Haematol. 2008;143:622–631. doi: 10.1111/j.1365-2141.2008.07372.x. [DOI] [PubMed] [Google Scholar]
- 13.Jia L, Zhao Y, Liang XJ. Current evaluation of the millennium phytomedicine-ginseng (II): Collected chemical entities, modern pharmacology, and clinical applications emanated from traditional Chinese medicine. Curr Med Chem. 2009;16:2924–2942. doi: 10.2174/092986709788803204. [DOI] [PMC free article] [PubMed] [Google Scholar]


