Abstract
Spinal cord injury (SCI) is a serious medical problem with high mortality and disability rates. Hyperbaric oxygen (HBO) treatment is beneficial for neurological recovery after SCI, but the underlying mechanisms await characterization. This study examined whether HBO treatment following SCI in rats exerts a neuroprotective effect through activation of the toll-like receptor (TLR) 2/nuclear factor (NF)-κB signaling pathway. The SC of rats was injured via T10 laminectomy. Experimental animals (n = 144) were divided into four groups: sham-operated (SH), SH + HBO, SCI, and SCI + HBO. Each group was subdivided into six subgroups (n = 6 per group) that were examined at 12 h, and 1, 2, 3, 7, and 14 days post-injury. Functional recovery in the hind limb was evaluated using the Basso, Beattie, and Bresnahan (BBB) scoring system. The expression of TLR2 and NF-кB was assessed by real-time polymerase chain reaction and Western blotting, while interleukin-1 (IL)-1β and tumor necrosis factor (TNF)-α levels were measured by enzyme-linked immunosorbent assay. TLR2 and NF-кB levels and histological scores were higher in the SCI than in the SH and SH + HBO groups at various time points. HBO treatment decreased TLR2 and NF-кB expression and histological scores as well as IL-1β and TNF-α levels compared to the SCI group at early post-injury stages. In addition, BBB scores were improved in the SCI + HBO relative to the SCI group at 7 and 14 days. HBO treatment may mitigate secondary injury to the SC by inhibiting inflammatory responses induced by TLR2/NF-кB signaling, thereby promoting functional recovery and improving neurological outcome.
Keywords: Hyperbaric oxygen, spinal cord injury, toll-like receptor 2, nuclear factor κB
Introduction
Spinal cord injury (SCI) is a serious medical problem with high costs to healthcare, as well as social and psychological burdens. Trauma to the SC can cause irrecoverable primary damage by destroying tissue as well as secondary injury including hemorrhage, edema, inflammation, excitotoxicity, and oxidative cell damage, which can lead to permanent loss of neurological function [1,2]. Treatments that mitigate secondary injury and promote SC tissue survival can preserve the anatomic substrate necessary for functional recovery to take place.
Toll-like receptors (TLRs) are a family of signal transduction molecules that play a critical role in innate immunity and the inflammatory response via nuclear factor (NF)-κB-mediated signaling [3,4]. Of the 13 TLRs that have been identified in mouse, TLR2 has been associated with SCI-induced inflammation [5]. NF-κB is composed of a p65/p50 heterodimer in most cell types; once activated, the p65 subunit dissociates from its inhibitor IκB-α and translocates from the cytoplasm to the nucleus to activate the transcription of target genes, including pro-inflammatory cytokines such as interleukin (IL)-1β and tumor necrosis factor (TNF)-α [6,7].
Hyperbaric oxygen (HBO) therapy is a medical treatment that administers 100% O2 at a controlled pressure (greater than sea level) for a prescribed period of time (60–90 min). A growing number of studies have shown that HBO treatment can reduce SCI and promote the recovery of neurological function [8-11]. However, the mechanism underlying the neuroprotective effects of HBO has yet to be characterized.
The present study examined whether HBO treatment exerts protective effects by altering NF-κB signaling, by measuring changes in the expression of TLR2 and NF-κB, as well as IL-1β and TNF-α levels in rats with acute SCI.
Materials and methods
Animals
Healthy adult male Sprague-Dawley rats, weighing 220-250 g, were purchased from the Center of Experimental Animals of Capital Medical University (Beijing, China). The study protocol was approved by the Committee for the Purpose of Control and Supervision of Experiments on Animals at the university. Animals were housed in a temperature-controlled environment under a 12:12 h light/dark cycle with access to a standard laboratory diet and water ad libitum. Each rat was determined to have normal motor function prior to surgery.
Animal surgery
SCI was induced using a weight drop device as previously described [12]. Rats were anaesthetized by intraperitoneal injection of 10% chloral hydrate at a dose of 350 mg/kg. A longitudinal incision was made extending from the midto low thoracic region, followed by a laminectomy that included all T10, to expose the SC without damaging the dura. SCI was induced by a Multicenter Animal Spinal Cord Injury Study impactor using a 10-g rod from a distance of 25 mm, and its successful application was assessed by the presence of edema and hemorrhage in SC tissue; purple but intact dura; wagging tail reflex; bilateral lower limb flutter/retraction; and flaccid paralysis. The injuried muscle and skin were sutured in layers and animals were allowed to recover. Manual bladder expression was performed twice a day until rats were able to urinate unaided, and a single dose of penicillin (0.8 mg/g) was administered daily by subcutaneous injection until the hematuria ceased.
Experimental groups
Rats (n = 144) were randomly assigned to one of four groups: sham-operated (SH), SH + HBO, SCI, and SCI + HBO. Each group was subdivided into six subgroups (n = 6 rats per group) corresponding to the following post-operative time points: 12 h, and 1, 2, 3, 7, and 14 days. Animals in the SH and SH + HBO groups underwent a T10 laminectomy without SCI.
HBO treatment
After flushing CO2 from a hyperbaric chamber (701 Space Research Institute, Beijing, China) with 100% O2, rats in the SH + HBO and SCI + HBO groups were placed inside 6 h after surgery for a 60-min exposure to HBO (100% O2) at 2.0 atmospheres absolute (ATA) once a day. The chamber pressure was increased to 2.0 ATA over 10 min and gradually decompressed over 5 min to normobaric air (21% O2) at the end of the session. Rats in SH and SCI groups were treated with normobaric air at 1.0 ATA.
Behavioral tests
A motor function recovery test was performed at each time point after surgery using the scoring system of Basso, Beattie, and Bresnahan (BBB) [13], which ranges from complete paralysis to complete mobility and comprises 21 criteria for lower limb movement including limb movement; weight-bearing capability; coordinated and proper gait; movement at all hind limb joints; full weight support; and appropriate limb, body, and tail positions. The animals were scored by two investigators who were not part of this study and were blinded with respect to the treatment undergone by each animal.
Tissue sample collection
Animals were anesthetized at the appropriate time point, and transcardial perfusion was performed with saline followed by ice-cold 4% paraformaldehyde. The SC (4-6 mm length) containing the injury site was dissected, and divided into two parts: one was fixed with 10% formaldehyde solution at 4°C for 1 week for histological evaluation, and the other was stored at -80°C and used for molecular analyses.
Histology
The SC tissue was embedded in paraffin and sectioned at a thickness of 5 μm. Sections were stained with hematoxylin and eosin, and the injury was examined by microscopy and scored for edema, neutrophil infiltration, and hemorrhage on a scale of 0 to 4 as follows: 0 = none or minor; 1 = modest or limited; 2 = intermediate; 3 = widespread or prominent; and 4 = widespread and most prominent [14]. Samples were analyzed by a pathologist who was blinded to the experimental procedure and sampled region.
Enzyme-linked immunosorbent assay (ELISA)
IL-1β and TNF-α levels in SC tissue homogenates were measured by ELISA using commercial kits (DuoSet ELISA Development Systems; R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions and an ELISA analyzer (Coda, Bio-Rad, Hercules, CA, USA). Results are expressed as pg/mg of SC tissue.
Western blotting
SC tissue was homogenized in ice-cold isolation solution containing 250 mmol/L sucrose, 10 mmol/L triethanolamine, 1 mg/mL leupeptin, and 0.1 mg/mL phenylmethylsulfonyl fluoride. Homogenates were centrifuged at 15,000 g for 10 min at 4°C, and the protein concentration in the supernatant was measured using a protein assay kit (Sunbio, Beijing, China). Total protein (50 μg) was resolved by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes, which were incubated overnight at 4°C with primary antibodies against TLR2 (1:10 00), NF-κB (1:1000), and β-actin (1:2,000) (all from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). After washing, membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology, Inc.) diluted at 1:5000 for 1 h at 37°C, and signals were detected using an enhanced chemiluminescence kit (BestBio Inc., Shanghai, China). The film was scanned (Konica Minolta Medical Imaging USA, Inc., Wayne, NJ, USA) and protein expression was quantified with Adobe Photoshop (Adobe, Mountain View, CA, USA). and LabWorks software (UVP, Upland, CA, USA).
Real-time polymerase chain reaction (RT-PCR)
Total RNA was isolated from frozen SC tissue using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and an RNA extraction kit (Biomed, Beijing, China). RNA was reverse-transcribed to obtain cDNA, and RT-PCR was performed using the BioEasy SYBR Green I Real Time PCR Kit and Line-Gene sequence detector (Bioer, Hangzhou, China). The following primer sets were used: rat glyceraldehyde 3-phosphate-dehydrogenase (GAPDH), 5′-GGT GAA GGT CGG TGT GAA CG-3′ (forward) and 5′-CTC GCT CCT GGA AGA TGG TG-3′ (reverse); TLR2, 5′-TGA TGC TGC CAT TCT CAT TC-3′ (forward) and 5′-CGC AGC TCT CAG ATT TAC CC-3′ (reverse); and NF-κBp65, 5′-TCA CGG GAC CTG GCT GGG AG-3′ (forward) and 5′-CCG CCG AAG CTG CAT GGA CA-3′ (reverse). The cycling conditions were as follows: 95°C for 2 min; and 45 cycles of 95°C for 20 s, 59°C for 25 s, and 72°C for 30 s. Data were analyzed using the Line-Gene software. The mRNA expression levels were calculated with the 2−ΔΔCT method and normalized to GAPDH levels.
Statistical analysis
All quantitative data are presented as mean ± standard deviation. Data were analyzed by one-way analysis of variance using SPSS v. 15.0 software (SPSS Inc., Chicago, IL, USA). Statistical significance was set at P < 0.05.
Results
HBO promotes motor function recovery and reduces tissue damage after SCI
The extent of motor function recovery was evaluated based on BBB locomotor scores. Immediately after the surgery, rats in the SH and SH + HBO groups had scores of 20-21 and were able to walk normally, while those in the SCI and SCI + HBO groups had complete hind limb paralysis with scores between 0 and 1. The BBB score of the SCI + HBO group increased starting from day 3, and on post-operative days 7 and 14, was significantly higher than for the SCI group (Figure 1). These results suggest that HBO treatment improves motor functioning in rats after SCI.
Figure 1.
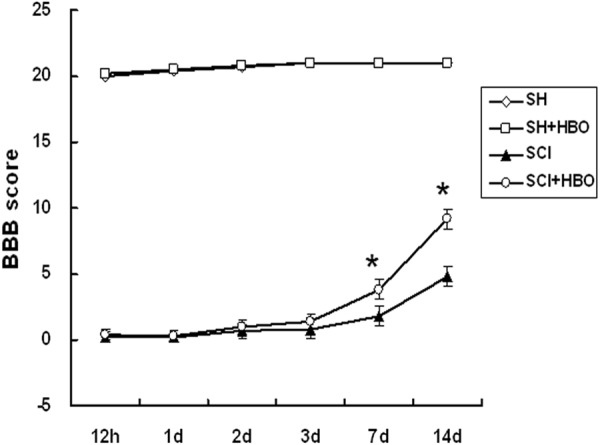
Effect of HBO on neurological recovery after SCI. The functional recovery of the hindlimb was evaluated and assigned a Basso, Beattie, and Bresnahan (BBB) score at the indicated time points following SCI. HBO, hyperbaric O2; SCI, spinal cord injury; SH, sham-operated. Data are shown as mean ± standard deviation. *P < 0.05.
SC tissue from the SH + HBO group had normal histological characteristics similar to those of the SH group (Figure 2A, 2B). In contrast, the SCI group had prominent hemorrhage, edema, neutrophil infiltration, and disordered tissue structure (Figure 2C). The pathological features of SC tissue from animals in the SCI + HBO group were markedly attenuated (Figure 2D); the histological scores confirmed that HBO treatment had a protective effect against SCI-induced tissue damage (Figure 3).
Figure 2.
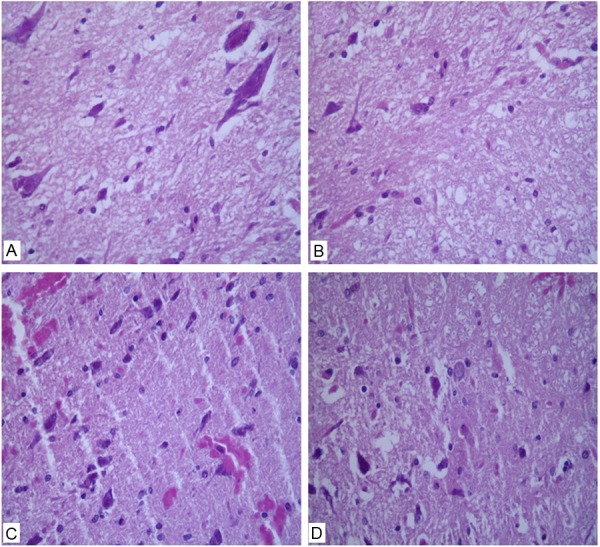
Effect of hyperbaric O2 (HBO) on spinal cord tissue after spinal cord injury (SCI). Hematoxylin and eosin-stained sections were examined 1 day after surgery in (A) sham-operated controls (SH); (B) SH animals receiving HBO treatment; (C) animals sustaining SCI; and (D) SCI animals receiving HBO treatment. Tissue damage consisting of edema, hemorrhage, and neutrophil infiltration was observed in the SCI group, which was improved by HBO treatment. Magnification: 400×.
Figure 3.
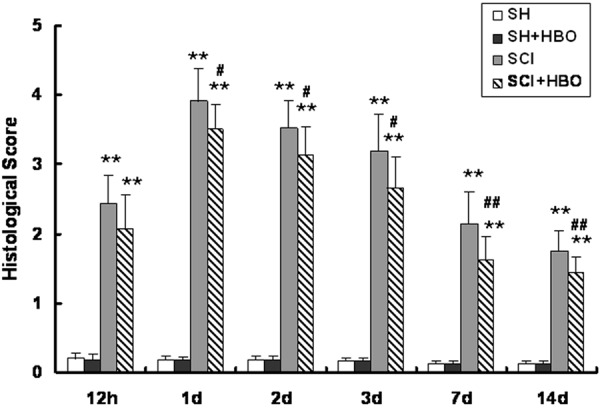
Effect of HBO on histological score after SCI. Scores were assigned based on the presence of edema, neutrophil infiltration, and hemorrhage on a scale of 0 to 4 at the indicated time points after SCI. HBO, hyperbaric O2; SCI, spinal cord injury; SH, sham-operated. Data are shown as mean ± standard deviation. **P < 0.01 vs. SH and SH + HBO groups; #P < 0.05, ##P < 0.01 vs. SCI group.
HBO treatment suppresses the upregulation of TLR2 and NF-κBp65 expression observed in SCI
Injury to the SC resulted in upregulation of TLR signaling pathway components. The expression levels of TLR2 and NF-κBp65 protein (Figures 4 and 5, respectively) and transcript (Figure 6) were higher in the SCI than in the SH and SH + HBO groups 12 h, and 1, 2, 3, and 7 days after injury (P < 0.01). On day 14, TLR2 mRNA and protein levels were higher in the SCI and SCI + HBO than in the SH and SH + HBO groups, but the differences were not statistically significant.
Figure 4.
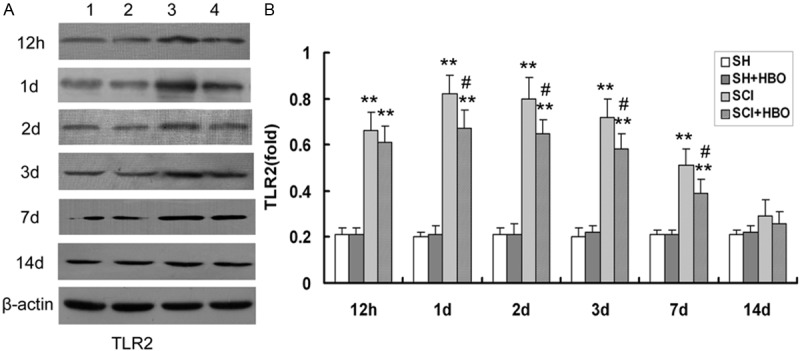
Effect of HBO on toll-like receptor (TLR) 2 protein expression in the spinal cord after SCI. A: A representative immunoblot is shown. Lane 1, SH; lane 2, SH + HBO; lane 3, SCI; lane 4, SCI + HBO. β-actin was used as a loading control. B: TLR2 expression was quantified from immunoblots at the indicated time points. HBO, hyperbaric O2; SCI, spinal cord injury; SH, sham-operated. Data are shown as mean ± standard deviation.*P < 0.05, **P < 0.01 vs. SH and SH + HBO groups; #P < 0.05 vs. SCI group.
Figure 5.
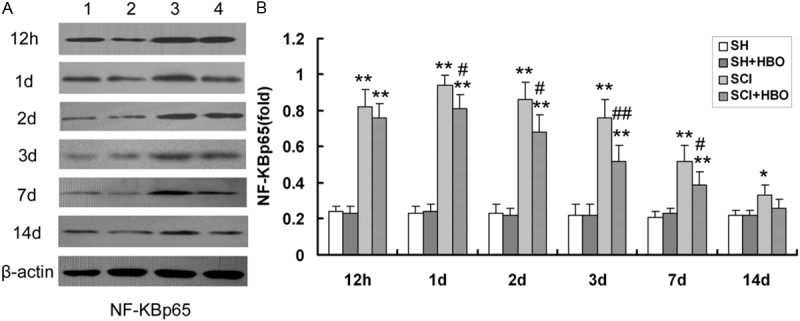
Effect of HBO on nuclear factor (NF)-κBp65 protein expression in the spinal cord after SCI. A: A representative immunoblot is shown. Lane 1, SH; lane 2, SH + HBO; lane 3, SCI; lane 4, SCI + HBO. β-actin was used as a loading control. B: NF-κBp65 expression was quantified from immunoblots at the indicated time points. HBO, hyperbaric O2; SCI, spinal cord injury; SH, sham-operated. Data are shown as mean ± standard deviation. *P < 0.05, **P < 0.01 vs. SH and SH + HBO groups; #P < 0.05, ##P < 0.01 vs. SCI group.
Figure 6.
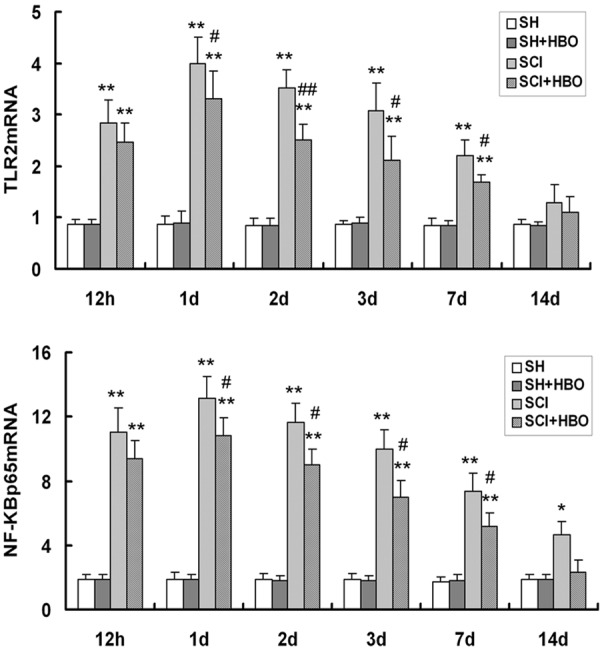
Effect of HBO on toll-like receptor (TLR) 2 and nuclear factor (NF)-κBp65 mRNA expression in the spinal cord after SCI. Transcript levels were measured by real-time quantitative PCR and normalized to the expression level of glyceraldehyde 3-phosphate-dehydrogenase. HBO, hyperbaric O2; SCI, spinal cord injury; SH, sham-operated. Data are shown as mean ± standard deviation. *P < 0.05, **P < 0.01 vs. SH and SH + HBO groups; #P < 0.05, ##P < 0.01 vs. SCI group.
NF-κBp65 protein and mRNA expression in the SCI group was increased compared to SH and SH + HBO groups on day 14 (P < 0.05), while the levels in the SCI + HBO group were comparable to those in the SH and SH + HBO groups. Moreover, the expression of both molecules was significantly lower in the SCI + HBO than in the SCI group on days 1, 2, 3, and 7, although there was no difference at 12 h or 14 days. Thus, upon injury to the SC, TLR2 and NF-κBp65 mRNA and protein expression reached peak values on post-operative day 1, but with HBO treatment the levels gradually decreased thereafter until day 14.
HBO treatment attenuates the expression of proinflammatory cytokines released upon SCI
The proinflammatory cytokines IL-1β and TNF-α were present at low levels in the SH and SH + HBO groups (Figure 7). Injured rats in the SCI group showed an increase in IL-1β level compared to SH and SH + HBO groups at 12 h, and 1, 2, and 3 days; however, compared to the SCI group, HBO-treated rats (SCI + HBO) had attenuated IL-1β level on days 1 and 2 (P < 0.05). Similarly, TNF-α was present at a higher level in the SCI than in the SH and SH + HBO groups at 12 h, and 1, 2, 3, and 7 days, but HBO treatment decreased TNF-α level relative to the SCI group 1, 2, and 3 days after surgery. Thus, IL-1β and TNF-α levels peaked on day 1 after SCI, and then gradually returned to baseline values. Collectively, these data indicate that HBO treatment can mitigate the histological damage wrought by SCI and promote functional recovery by suppressing the inflammatory response via attenuation of TLR/NF-κB signaling.
Figure 7.
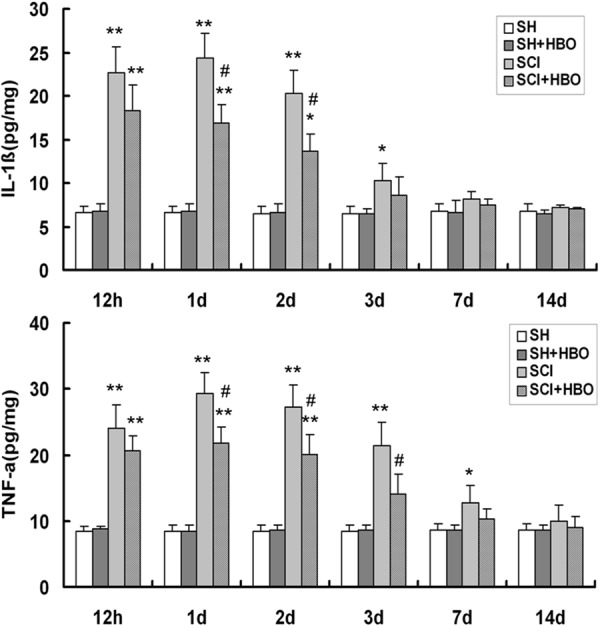
Effect of HBO on interleukin (IL)-1β and tumor necrosis factor (TNF)-α expression after SCI. Cytokine levels in the spinal cord were measured by enzyme-linked immunosorbent assay. HBO, hyperbaric O2; SCI, spinal cord injury; SH, sham-operated. Data are shown as mean ± standard deviation. *P < 0.05, **P < 0.01 vs. SH and SH + HBO groups; #P < 0.05 vs. SCI group.
Discussion
The results of this study showed that HBO treatment following SCI in rats exerts a protective effect by mitigating inflammation and resultant tissue damage, while promoting the recovery of locomotor function. This effect occurred through inhibition of the TLR2/NF-κB signaling pathway and consequent suppression of proinflammatory cytokine levels.
TLRs recognize pathogen-associated molecular patterns of microorganisms and are therefore key mediators of the innate immune response [15]. Cellular damage or stress stimulates the release of endogenous ligands collectively referred to as danger-/damage-associated molecular patterns that are recognized by TLRs [16], which elicit an inflammatory response in pathological states including CNS injury and various diseases [5,17,18]. Consistent with these reports, in the present study, TLR2 mRNA (Figure 6) and protein (Figure 4) expression were upregulated in response to SCI, which was accompanied by the activation of an inflammatory response.
Activated TLRs bind to the adaptor molecule MyD88, which associates with IL-1 receptor-associated kinase 4, leading to the sequential activation of tumor necrosis factor-associated factor 6 and the inhibitor of κB complex. This leads to the degradation of IκB and activation of NF-κB, which regulates a host of target genes including the proinflammatory cytokines TNF-α and IL-1β [3,7,19,20]. In the CNS, aberrant activation of NF-κB has been observed in neurons and glia following SCI [21-23], and inhibiting its activation may attenuate SCI-induced inflammation and apoptosis [24,25]. As for TLR2, in this study, NF-κBp65 mRNA (Figure 6) and protein (Figure 5) expression were increased in rats that sustained SCI compared to sham-operated animals.
The proinflammatory cytokines IL-1β and TNF-α mediate SCI-induced inflammation [26,27]. Indeed, elevated levels of IL-1β and TNF-α were observed in animals immediately after SCI (Figure 7), although the levels returned to baseline values after 2 weeks. These results provide evidence that the TLR2/NF-κB signaling pathway is activated upon SCI and induces an inflammatory response by increasing the levels of these cytokines. Interestingly, the activation of NF-κBp65 persisted for a longer period than TLR2 upregulation (i.e., up to 2 weeks vs. 1 week after SCI). This suggests that NF-κB activation may be regulated by other factors, such as extracellular signal-regulated kinase 1/2 signaling [28].
It is well-established that HBO treatment is beneficial in cases of acute and chronic SCI. Exposure to HBO decreased expression levels of superoxide dismutase, glutathione peroxidase, nitric oxide synthase, and NO in SC tissue, and improved neuroregeneration after SCI [10]. HBO also suppressed SCI-induced edema, stabilized the blood-spinal cord barrier, and promoted recovery of neurological function by inhibiting the expression of IL-6, matrix metalloproteinase-2 (MMP-2), and MMP-9 [29]. Our previous study showed that HBO alters the expression of vascular endothelial growth factor and connexin 43 after SCI, and promotes the functional recovery of the limb [30]; another report demonstrated that apoptosis was also inhibited [31]. In a model of multiple organ failure, exposure to HBO suppressed the zymosan-induced expression of TLR2 and 4, NF-κB activation, and cytokine production; these effects were associated with reduced injury to the lungs, liver, and intestine [32]. However, the present study is the first report of the effects of HBO treatment on the TLRs/NF-κB pathway-mediated inflammatory response in SCI.
In rats with SCI treated with HBO, the downregulation of TLR2, NF-κB, IL-1β, and TNF-α corresponded to lower histological scores compared to injured animals that did not receive treatment, which exhibited reduced inflammatory responses and extensive cell necrosis (Figures 2 and 3). Moreover, locomotor behavior scores were also improved in rats exposed to HBO after SCI (Figure 1). Thus, the suppression of inflammation and cytokine release induced by HBO treatment resulted in less damage to the tissue and greater functional recovery. Results from the neurological examination also indicate that a better outcome can be achieved by repeated HBO treatments.
In conclusion, exposure to HBO following SCI protects the SC from secondary injury by inhibiting the TLR2/NF-κB-mediated inflammatory response, resulting in significant recovery of neurological function. Future studies will focus on elucidating the underlying mechanisms, and determining the extent to which repeated exposure to HBO can benefit human patients that sustain SCI.
Acknowledgements
This study was supported by the grant from International Science and Technology Cooperation Program of Ministry of Science and Technology of China (2012DFA31240).
Disclosure of conflict of interest
None.
References
- 1.Young W. Secondary injury mechanisms in acute spinal cord injury. J Emerg Med. 1993;11(Suppl 1):13–22. [PubMed] [Google Scholar]
- 2.Norenberg MD, Smith J, Marcillo A. The pathology of human spinal cord injury: defining the problems. J Neurotrauma. 2004;21:429–440. doi: 10.1089/089771504323004575. [DOI] [PubMed] [Google Scholar]
- 3.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 4.Lee MS, Kim YJ. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu Rev Biochem. 2007;76:447–480. doi: 10.1146/annurev.biochem.76.060605.122847. [DOI] [PubMed] [Google Scholar]
- 5.Kigerl KA, Lai W, Rivest S, Hart RP, Satoskar AR, Popovich PG. Toll-like receptor (TLR)-2 and TLR-4 regulate inflammation, gliosis, and myelin sparing after spinal cord injury. J Neurochem. 2007;102:37–50. doi: 10.1111/j.1471-4159.2007.04524.x. [DOI] [PubMed] [Google Scholar]
- 6.Hoshino K, Kaisho T, Iwabe T, Takeuchi O, Akira S. Differential involvement of IFN-beta in Toll-like receptor-stimulated dendritic cell activation. Int Immunol. 2002;14:1225–1231. doi: 10.1093/intimm/dxf089. [DOI] [PubMed] [Google Scholar]
- 7.Lin MH, Yang YL, Chen YP, Hua KF, Lu CP, Sheu F, Lin GH, Tsay SS, Liang SM, Wu SH. A novel exopolysaccharide from the biofilm of thermus aquaticus YT-1 induces the immune response through toll-like receptor 2. J Biol Chem. 2011;286:17736–17745. doi: 10.1074/jbc.M110.200113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tai PA, Chang CK, Niu KC, Lin MT, Chiu WT, Lin CM. Attenuating experimental spinal cord injury by hyperbaric oxygen: stimulating production of vasculoendothelial and glial cell line-derived neurotrophic growth factors and interleukin-10. J Neurotrauma. 2010;27:1121–1127. doi: 10.1089/neu.2009.1162. [DOI] [PubMed] [Google Scholar]
- 9.Cristante AF, Damasceno ML, Barros Filho TE, de Oliveira RP, Marcon RM, da Rocha ID. Evaluation of the effects of hyperbaric oxygen therapy for spinal cord lesion in correlation with the moment of intervention. Spinal Cord. 2012;50:502–506. doi: 10.1038/sc.2012.16. [DOI] [PubMed] [Google Scholar]
- 10.Dayan K, Keser A, Konyalioglu S, Erturk M, Aydin F, Sengul G, Dagci T. The effect of hyperbaric oxygen on neuroregeneration following acute thoracic spinal cord injury. Life Sci. 2012;90:360–364. doi: 10.1016/j.lfs.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Topuz K, Colak A, Cemil B, Kutlay M, Demircan MN, Simsek H, Ipcioglu O, Kucukodaci Z, Uzun G. Combined hyperbaric oxygen and hypothermia treatment on oxidative stress parameters after spinal cord injury: an experimental study. Arch Med Res. 2010;41:506–512. doi: 10.1016/j.arcmed.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transaction. Exp Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 13.Xu J, Kim GM, Chen S, Yan P, Ahmed SH, Ku G, Beckman JS, Xu XM, Hsu CY. iNOS and nitrotyrosine expression after spinal cord injury. J Neurotrauma. 2001;18:523–532. doi: 10.1089/089771501300227323. [DOI] [PubMed] [Google Scholar]
- 14.Merrill JE, Benveniste EN. Cytokines in inflammatory brain lesions: helpful and harmful. Trends Neurosci. 1996;19:331–338. doi: 10.1016/0166-2236(96)10047-3. [DOI] [PubMed] [Google Scholar]
- 15.Kaisho T, Akira S. Critical roles of toll-like receptors in host defense. Crit Rev Immunol. 2000;20:393–405. [PubMed] [Google Scholar]
- 16.Krysko DV, Agostinis P, Krysko O, Garg AD, Bachert C, Lambrecht BN, Vandenabeele P. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 2011;32:157–164. doi: 10.1016/j.it.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 17.West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nat Rev Immunol. 2011;11:389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu YY, Huang M, Dong XQ, Xu QP, Yu WH, Zhang ZY. Ginkgolide B reduces neuronal cell apoptosis in the hemorrhagic rat brain: Possible involvement of Toll-like receptor 4/nuclear factor-kappa B pathway. J Ethnopharmacol. 2011;137:1462–1468. doi: 10.1016/j.jep.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 19.Yang FL, Yang YL, Liao PC, Chou JC, Tsai KC, Yang AS, Sheu F, Lin TL, Hsieh PF, Wang JT, Hua KF, Wu SH. Structure and immunological characterization of the capsular polysaccharide of a pyrogenic liver abscess caused by Klebsiella pneumoniae. J Biol Chem. 2011;286:21041–21051. doi: 10.1074/jbc.M111.222091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X, Yu G, Fan S, Bian M, Ma H, Lu J, Jin L. Sargassum fusiforme polysaccharide activates nuclear factor kappa-B (NF-κB) and induces cytokine production via Toll-like receptors. Carbohyd Polym. 2014;105:113–120. doi: 10.1016/j.carbpol.2014.01.056. [DOI] [PubMed] [Google Scholar]
- 21.Bethea JR, Castro M, Keane RW, Lee TT, Dietrich WD, Yezierski RP. Traumatic spinal cord injury induces nuclear factor-kappa B activation. J Neurosci. 1998;18:3251–3260. doi: 10.1523/JNEUROSCI.18-09-03251.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brambilla R, Bracchi-Ricard V, Hu WH, Frydel B, Bramwell A, Karmally S, Green EJ, Bethea JR. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med. 2005;202:145–156. doi: 10.1084/jem.20041918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rafati DS, Geissler K, Johnson K, Unabia G, Hulsebosch C, Nesic-Taylor O, Perez-Polo JR. Nuclear factor-kappaB decoy amelioration of spinal cord injury-induced inflammation and behavior outcomes. J Neurosci Res. 2008;86:566–580. doi: 10.1002/jnr.21508. [DOI] [PubMed] [Google Scholar]
- 24.Han X, Lu M, Wang S, Lv D, Liu H. Targeting IKK/NF-κB pathway reduces infiltration of inflammatory cells and apoptosis after spinal cord injury in rats. Neurosci Lett. 2012;511:28–32. doi: 10.1016/j.neulet.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 25.Yin X, Yin Y, Cao FL, Chen YF, Peng Y, Hou WG, Sun SK, Luo ZJ. Tanshinone IIA Attenuates the Inflammatory Response and Apoptosis after Traumatic Injury of the Spinal Cord in Adult Rats. PLoS One. 2012;7:e38381. doi: 10.1371/journal.pone.0038381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Z, Wang BR, Wang X, Kuang F, Duan XL, Jiao XY, Ju G. ERK1/2 and p38 mitogen-activated protein kinase mediate iNOS-induced spinal neuron degeneration after acute traumatic spinal cord injury. Life Sci. 2006;79:1895–1905. doi: 10.1016/j.lfs.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 27.Yang L, Jones NR, Blumbergs PC, Van Den Heuvel C, Moore EJ, Manavis J, Sarvestani GT, Ghabriel MN. Severity-dependent expression of pro-inflammatory cytokines in traumatic spinal cord injury in the rat. J Clin Neurosci. 2005;12:276–284. doi: 10.1016/j.jocn.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Yu CG, Yezierski RP. Activation of the ERK1/2 signaling cascade by excitotoxic spinal cord injury. Brain Res Mol Brain Res. 2005;138:244–255. doi: 10.1016/j.molbrainres.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 29.Yang J, Wang G, Gao C, Shao G, Kang N. Effects of hyperbaric oxygen on MMP-2 and MMP-9 expressions and spinal cord edema after spinal cord injury. Life Sci. 2013;93:1033–1038. [PubMed] [Google Scholar]
- 30.Liu X, Zhou Y, Wang Z, Yang J, Gao C, Su Q. Effect of VEGF and CX43 on the promotion of neurological recovery by hyperbaric oxygen treatment in, spinal cord-injured rats. Spine J. 2014;14:119–127. doi: 10.1016/j.spinee.2013.06.084. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Li W, Kang Z, Liu Y, Deng X, Tao H, Xu W, Li R, Sun X, Zhang JH. Hyperbaric oxygen preconditioning attenuates early apoptosis after spinal cord ischemia in rats. J Neurotrauma. 2009;26:55–66. doi: 10.1089/neu.2008.0538. [DOI] [PubMed] [Google Scholar]
- 32.Rinaldi B, Cuzzocrea S, Donniacuo M, Capuano A, Di Palma D, Imperatore F, Mazzon E, Di Paola R, Sodano L, Rossi F. Hyperbaric oxygen therapy reduces the toll-like receptor signaling pathway in multiple organ failures. Intensive Care Med. 2011;37:1110–1119. doi: 10.1007/s00134-011-2241-1. [DOI] [PubMed] [Google Scholar]


