Abstract
Leptin is a neuroendocrine peptide released by adipose tissue that enhances metabolism and acts on the hypothalamus to suppress appetite. Leptin also regulates aspects of cardiovascular function and low serum leptin has been associated with increased mortality in humans. We hypothesized that leptin deficiency alters the structure and function of the pulmonary vasculature. Methods: We examined two groups of C57BL/6 male mice aged 12 weeks: five ob/ob (B6.VLepob/ob) leptin-deficient and five wild type (WT) (C57BL/6) control mice. As expected, weight was significantly greater in ob/ob mice relative to WT mice [weight (g), Mean ± SD): ob/ob 52 ± 2.5 g, wild type 30 ± 2.5 g; p < 0.001]. The pulmonary vasculature of ob/ob mice and WT control animals was examined by histology, immunohistochemistry and immunofluorescence staining. Results: Pulmonary arterial wall thickness was significantly increased in ob/ob mice relative to WT littermates [median (interquartile range) distance in pixels: ob/ob 0.13 (0.05-0.18), wild type 0.03 (0.02-0.04); p = 0.001]. The ob/ob mice also exhibited significant right ventricular hypertrophy in comparison to control animals [RV thickness (Mean ± SD): ob/ob 0.75 ± 0.19, wild type; 0.58 ± 0.13 p < 0.001]. We observed substantial macrophage infiltration and abundant proliferation of myofibroblasts and fibroblasts in histological sections of pulmonary arterioles of ob/ob mice. In addition, we noted increased hyaluronan deposition, colocalizing with SMC-actin in the pulmonary vasculature of ob/ob mice relative to WT controls. Conclusions: The pulmonary pathology of leptin deficiency in ob/ob mice recapitulates many of the histological features of pulmonary vascular diseases, including pulmonary hypertension, suggesting that leptin deficiency is associated to the pathogenesis of pulmonary vascular disease.
Keywords: Leptin, vasculature, obesity, pulmonary hypertension
Introduction
Leptin is a 16-kDa neuroendocrine peptide released by adipose tissue that increases the metabolic rate and suppresses appetite through direct modulation of hypothalamic activity [1,2]. Mounting evidence suggests that in addition to playing a central role in metabolic homeostasis, leptin is an important mediator of cardiovascular processes including activation of the sympathetic nervous system [3-6], angiogenesis [7-9] and endothelial nitric oxide (NO) production [8,10,11]. While it has been demonstrated that endothelial cells express functional leptin receptors, the role of leptin in endothelial regulation remains controversial. Leptin-dependent endothelial NO production and subsequent vasodilation has been demonstrated in rats [11]. Leptin may also up-regulate inducible nitric oxide synthases (iNOS) to produce NO, potentially impairing endothelial function and promoting oxidative stress and atherogenesis [12].
Low serum leptin has been associated with increased incidence of cardiovascular events and mortality among patients with coronary artery disease, independent of related risk factors such as obesity [13]. Additional studies have reported an association between elevated serum leptin and decreased risk of cardiovascular mortality among patients with diabetes [14] and chronic kidney disease [15].
We have recently demonstrated low serum leptin concentration is independently associated with increased overall mortality in patients with pulmonary arterial hypertension (PAH) [16]. Pulmonary arterial hypertension is characterized by restricted flow through the pulmonary circulation, resulting in increased pulmonary vascular resistance and, ultimately, right heart failure and death [17]. Pathological evaluations of tissues collected from patients with PAH have demonstrated a positive correlation of median fractional thickness of the pulmonary arteries with perivascular inflammation and pulmonary hemodynamic measurements [18].
Leptin stimulates the proliferation and migration of vascular smooth muscle cells [19]. Related studies have demonstrated that leptin directs the synthesis and secretion of endothelin-1 in human umbilical vein endothelial cells [20], and promotes the expression of preproendothelin-1 and endothelin ETA receptor, angiotensinogen, and angiotensin type 1 receptor in rabbit portal vein smooth muscle cells [19]. Endothelin is a potent vasoconstrictor and mitogen and as a consequence, a dysregulation of the endothelin pathway mediates vascular abnormalities associated with PAH [21-23].
On the basis of these observations, we hypothesized that leptin deficiency alters the structure and function of the pulmonary vasculature. To test this hypothesis, we conducted a histopathological evaluation of the pulmonary vasculature in obese leptin-deficient mice (ob/ob) [24,25] and wild type control animals.
Material and methods
Animals
All experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the Cleveland Clinic. All efforts were made to minimize pain and distress during animal husbandry and experimental assessments. Male C57BL/6 Leptin-deficient (ob/ob) and wild type (WT) control mice (n = 5 in each group) aged 12 weeks were purchased from Jackson Laboratory (Bar Harbor, ME) and were fed ad libitum with regular chow diet for 3 weeks before euthanasia.
Histological studies
Lung tissues obtained from ob/ob and control mice were inflated and embedded in optimal cutting temperature (OCT) compound (R. A. Lamb; Eastbourne, UK) and 4-μm sections were prepared from frozen sections. The sections were stained with hematoxylin and eosin (H&E) for morphological examination. We measured the pixel intensity from microscopic images of each pulmonary arterial section using Image J software (NIH, Bethesda, MD), along ten radial line scans, each beginning outside the artery and extending across the artery wall. A total of 10 sections representing 10 arteries were quantified as described from both the ob/ob and control groups. Care was taken when staining sections and when obtaining and quantifying images to ensure identical procedures were applied to both groups.
Cardiac tissue from both the ob/ob and control animals were fixed in paraffin after the heart was transversely cut at the level of the left ventricular papillary muscles. Right ventricular (RV) hypertrophy was assessed by measuring the thickness of the RV wall at 20 different sectors per slide using Image J software in the manner described above.
Collagen staining
In a separate series of experiments, lungs were removed, inflated/embedded in OCT (R.A. Lamb; Eastbourne, UK), and prepared in 4-μm sections from the frozen tissue. After the removal of OCT in water, collagen was stained for 30 min using picrosirius red [0.1% (wt/vol) Sirius red 3FB (Aldrich; Dorset, UK) in saturated aqueous picric acid] with gentle agitation. Images were collected using an HCX PL APO x 40 / numerical aperture (NA) 1.25 oil-immersion objective lens on a Leica DMR upright microscope (Leica Microsystems, Wetzlar, Germany) equipped with a Retiga EXi Cooled CCD Camera (QImaging, Burnaby, British Columbia, Canada) and Image Pro Plus software (Media Cybernetics, Silver Spring, MD).
Immunostaining
In lung tissues, smooth muscles cells (SMCs) were stained with rabbit polyclonal anti-smooth muscle actin antibody (Abcam, cat. no. ab15267-7), hyaluronan (HA) was detected by HA binding protein probe (Calbiochem, cat. no. 385911). Lung macrophages (F4/80+ cells) were detected with rat anti-mouse F4/80 Ab (Serotec, cat. no. MCA497G) and fibroblasts were stained with polyclonal anti-FSP1/S100A4 (Millipore, cat. no. 07-2274). Slides were pre-incubated in Hank’s Balanced Salt Solution (HBSS) containing 2% Fetal Bovine Serum (FBS) for 30 min at room temperature. After discarding the medium, tissue slides were incubated in HBSS with biotinylated HA binding protein (1:100 dilution), anti-FS1/S100A4 (1:100 dilution), and anti-smooth muscle actin (1:100 dilution) overnight at 4°C in a humidified chamber to prevent drying. After overnight incubation, the tissue slides were washed three times with HBSS without FBS and then incubated with fluorescein-tagged secondary antibody (1:1000 dilutions) in HBSS with 2% FBS for 60 min at room temperature. The tissue slides were washed three times with HBSS without FBS and mounted with Vectashield mounting medium containing 4,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA). The slides were sealed with nail polish and the slides stored at -20°C. Fluorescence images were collected as described above.
Statistical analysis
All reported p values were reported as two-tailed. Continuous variables were compared using an independent, two-tailed Student’s t-test. A p value of < 0.05 was considered statistically significant. The statistical analyses were performed using the statistical software package JMP (Cary, NC), and R version 2.13.0 (The R Foundation for Statistical Computing) [26].
Results
Evaluation of pulmonary arterial wall thickness
We examined five ob/ob (C57BL/B6.V Lepob/ob) and five lean wild type (C57BL/6J) mice. As expected, the mean (SD) weight was significantly greater among the ob/ob mice relative to the wild type mice [weight (g), Mean ± SD): ob/ob 52 ± 2.5, wild type 30 ± 2.5; p < 0.001]. Pulmonary arterial wall thickness, measured in H&E stained arterial sections, was significantly greater in the ob/ob mice relative to control mice (Figure 1). Arterial thickness (median (interquartile range)) was 0.13 pixels (0.05-0.18) and 0.03 pixels (0.02-0.04) for the ob/ob and the wild type mice group, respectively (mean difference (95% CI): 0.12 (0.09-0.14), p < 0.001).
Figure 1.
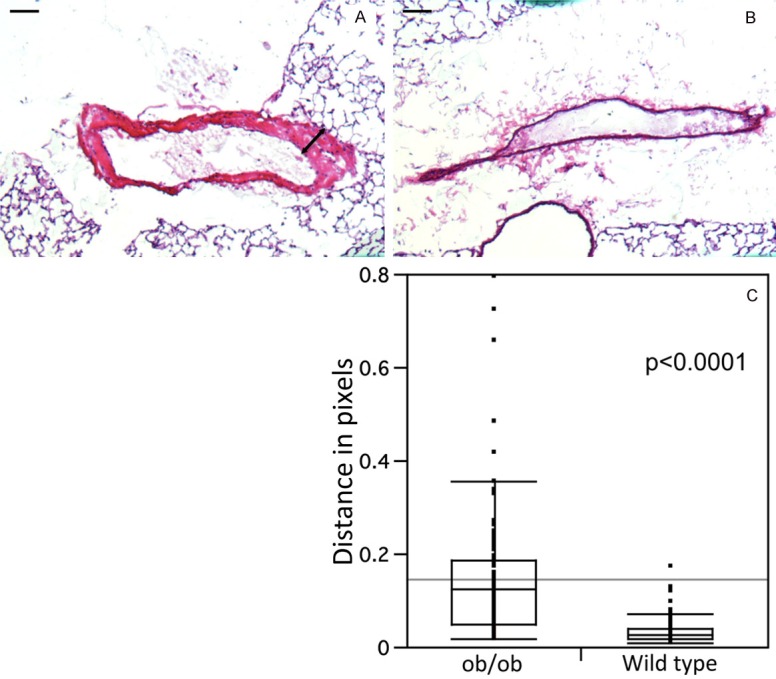
Lung tissues from ob/ob (A) and control (B) lungs were stained using hematoxylin and eosin (H&E). H&E staining demonstrates thickening smooth muscle cell wall and hyperplasia in the ob/ob arteriole (arrow in A) that was not observed in control animals. The p value for the difference in thickness of the arterial wall (distance in pixels) and standard errors were calculated from three different slides by using 10 arteries in each slide. Ten distinct segents of the arterial wall were measured (C). Bar is 100 μm.
Immunohistochemical evaluation of pulmonary artery pathology
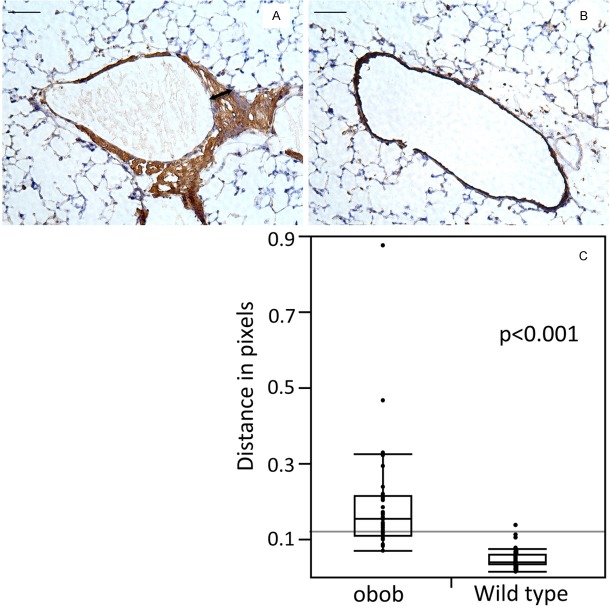
Immunohistochemical detection of the macrophage-specific antigen F4/80 Ab revealed increased macrophage infiltration in the pulmonary arterioles of ob/ob mice relative to control animals. The thickness of the arterial wall, measured as distance in pixels (mean ± SE), was significantly greater in ob/ob mice when compared with wild type animals in the macrophage-specific antigen F4/80 Ab stained arterial sections (ob/ob: 1.9 ± 0.02 px and wild type: 0.05 ± 0.004 px, p < 0.001)] (Figure 2).
Figure 2.
Immunohistochemical staining of frozen lung sections ffor the macrophage marker F4/80 in the lung of ob/ob mice (A) and control mice (B). F4/80 staining demonstrates accumulations of macrophages in the ob/ob arteriole (arrow in A) that was not observed in control animals. The p value for the difference in thickness of the arterial wall (distance in pixels) and standard errors were calculated from three different slides by using 10 arteries in each slide. Ten distinct segents of the arterial wall were measured (C). Bar is 100 μm.
Similarly, collagen deposition measured as distance in pixels (stained with picrosirius red) was more extensive in the pulmonary arterioles of ob/ob mice relative to wild type control mice, with Mean ± SE of 0.15 ± 0.07 in ob/ob and 0.06 ± 0.02 in the wild type, p < 0.001 (Figure 3). Fibroblasts (stained with anti-FSP1/S100A4) were also more prevalent in arterioles of ob/ob mice than in the control animals (Figure 4). Immunofluorescence staining for smooth muscle cell actin and hyaluronan, an extracellular component, indicated greater staining intensity and prevalence of both SMA and HA in the pulmonary arteries of ob/ob mice in comparison to control animals (Figure 5).
Figure 3.
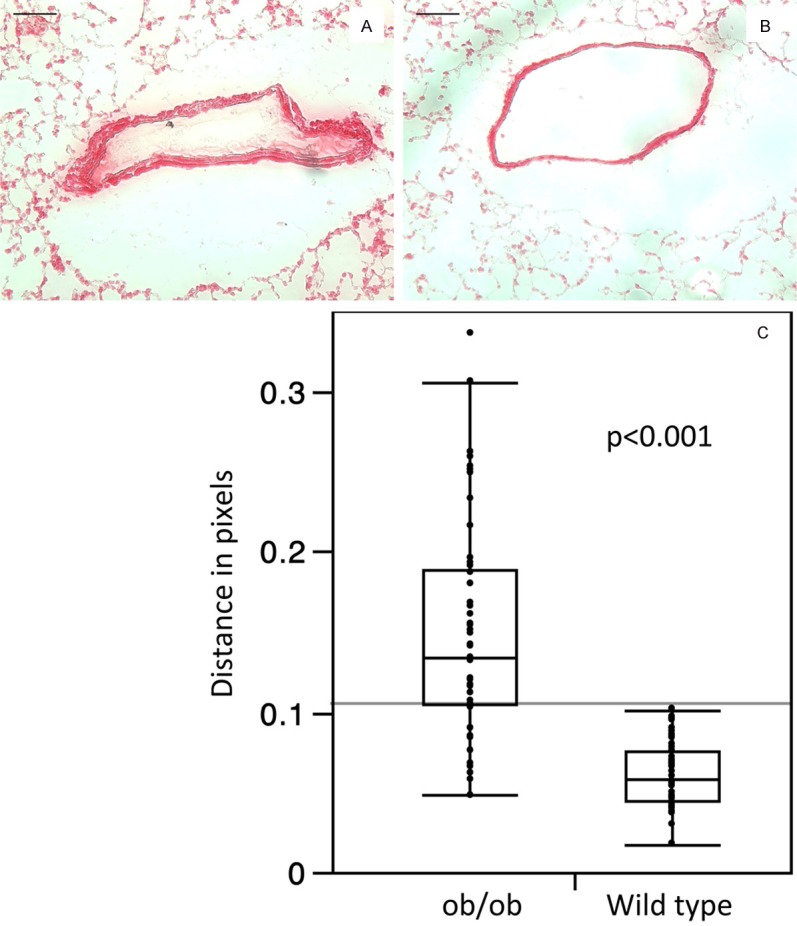
Lung tissues from ob/ob (A) and control (B) animals were stained for collagen with picrosirius red. Baris 100 μm.
Figure 4.
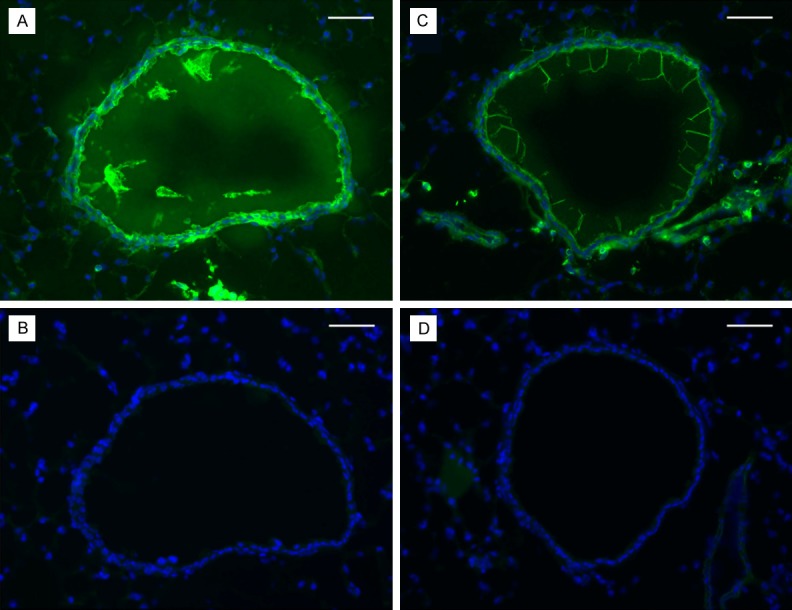
Lung tissues from ob/ob (left) and control (right) mice were stained for FSP1/S100A4 (fibroblasts). There is more intense fibroblast staining (green) in ob/ob arterioles (A) compared with the control (C). B and D are negative controls of the same sections stained by the secondary antibody only. Blue staining represents the 4,6-diamidino-2-phenylindole (DAPI) staining of the nuclei. Bar is 100 μm.
Figure 5.
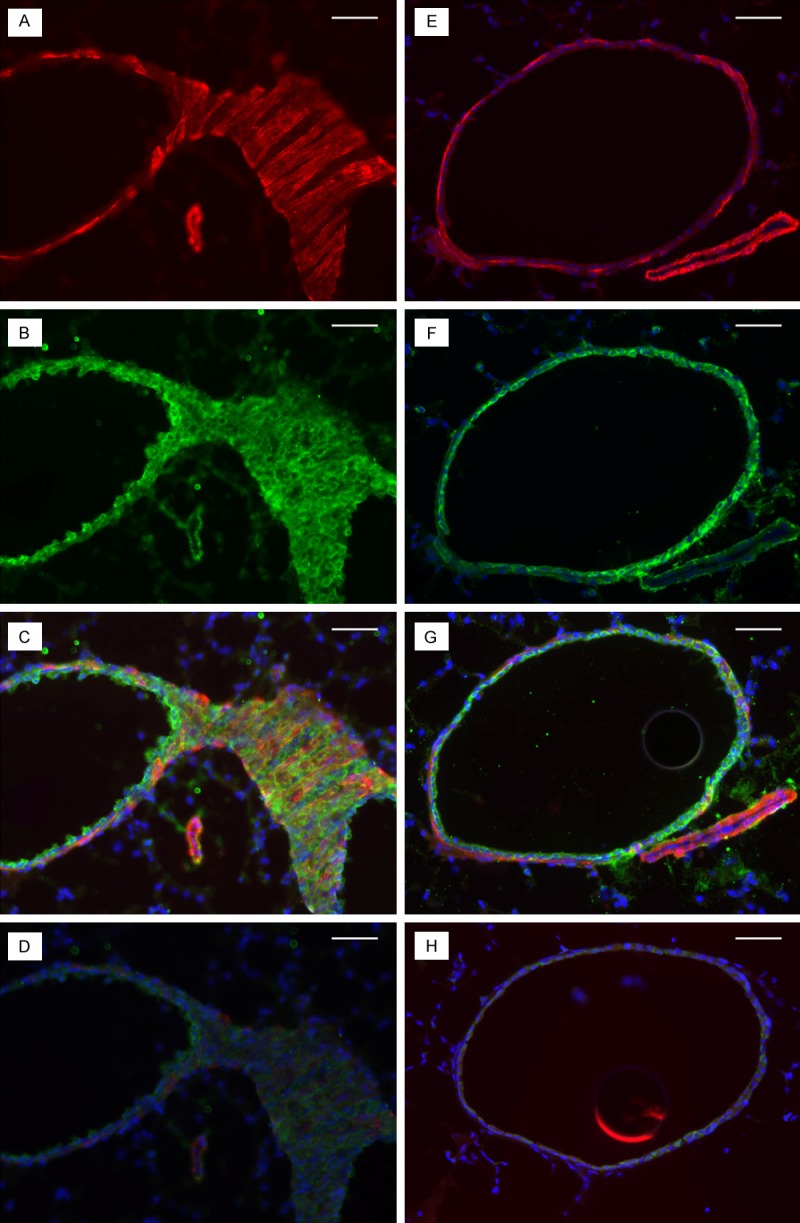
Lung tissues from ob/ob (left) and control (right) lungs were stained for smooth muscle cell actin (SMCs; smooth muscle actin) and HA (HA binding protein). The intensity of SMC actin (red) and HA (green) staining surrounding the ob/ob arteriole (A and B) is greater compared with the control (E and F). C and G are composite pictures of HA and SMC actin for the same sections of ob/ob and control lung tissues, respectively. D and H are negative controls of the same sections stained by the secondary antibody only. The minimal green staining here represents autofluorescence by the elastica, and the blue staining represents the 4,6-diamidino-2-phenylindole (DAPI) staining of the nuclei. Bar indicates 100 μm.
Comparison of thickness of right ventricular wall
We examined cardiac tissue from ob/ob mice and wild type control animals and found evidence of right ventricular hypertrophy in the ob/ob group. Measurement of right ventricular wall thickness as distance in pixels in 20 different sectors obtained from the five ob/ob mice and five wild type mice revealed a mean (SD) thickness of 0.58 (0.13) in the wild type mice and 0.75 (0.19) in the ob/ob mice (p < 0.001) (Figure 6, scale bar 600 μm).
Figure 6.
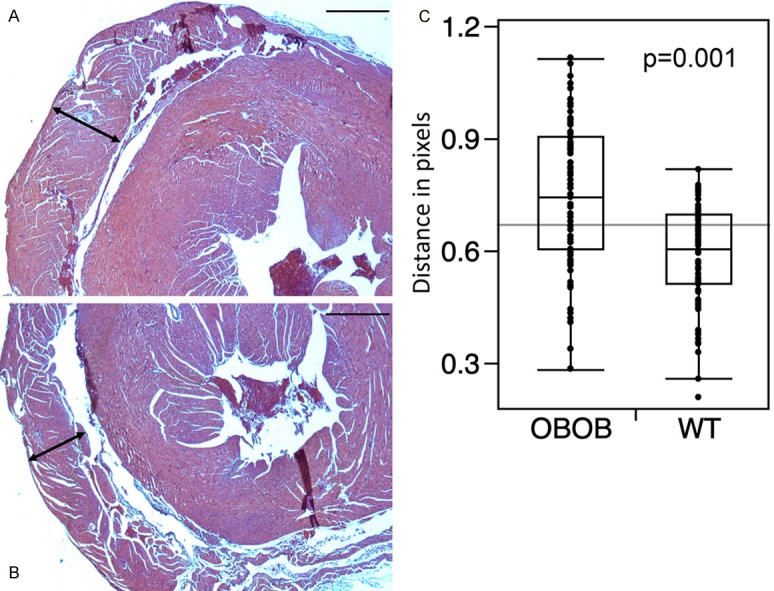
Transverse heart sections at the level of the left ventricular papillary muscles, showing right ventricle thickness (left-right arrows) in ob/ob (panel A) and wild type (panel B) mice. Panel C is a boxplot of the RV thickness measured in pixels, in 20 different sectors, on each slide for leptin deficient (ob/ob) and wild type (WT) mice. Bar indicates 100 μm.
Discussion
Leptin increases the metabolic rate and suppresses appetite through direct modulation of hypothalamic activity [1,2]. Recent evidence suggests that in addition to playing a central role in metabolic homeostasis, leptin is an important mediator of cardiovascular processes including activation of the sympathetic nervous system [3-6], angiogenesis [7-9] and endothelial NO production [8,10,11].
The present study demonstrates including increased pulmonary arterial wall thickness, right ventricular hypertrophy, proliferation of inflammatory and fibrotic cell types, and altered extracellular matrix deposition in the pulmonary vasculature of mice with a spontaneous deletion of the gene encoding the neuroendocrine peptide leptin (ob/ob). Decreased serum leptin concentration has been previously associated with increased mortality among patients with pulmonary arterial hypertension independent of other risk factors including BMI [16]; the present build on previous findings and suggest a potential mechanistic connection between decreased leptin secretion and the pathogenesis of pulmonary disease involving increased infiltration of inflammatory cells, expansion of fibrotic tissue, and altered extracellular matrix deposition.
Plasma leptin concentrations are inversely proportional to body fat mass in both humans and mice [27-29]. Elevation of circulating leptin has been associated with vascular calcification, increased blood pressure, and vascular compliance; these conditions have been attributed to acquired leptin resistance rather than the direct effects of excess leptin [30-32]. We observed that leptin deficient mice (ob/ob) developed the characteristic pulmonary vascular pathology of PAH, including increased pulmonary artery thickness due to the expansion of smooth muscle cells and right ventricular hypertrophy. Two possible explanations may account our observations: direct modulation of vascular function through endothelial and vascular smooth muscle cell leptin receptors, or the indirect effects of the absence of leptin on angiogenesis, sympathetic nerve activity, ectopic lipid deposition, insulin sensitivity or other related metabolic pathways [9-11,33]. Leptin is required for the maintenance of vascular compliance through the regulation of vascular function. The activation of the endothelial NO synthase (eNOS) phosphorylation pathway and the release of endothelin-1 by leptin in response to increases in nitric oxide (NO) production [10,34,35], may be an important counterbalance to the vasodilation mediated by NO [20].
In ob/ob mice we observed an increase in pulmonary artery collagen and hyaluronic acid deposition similar to the previously reported pathology of human PAH arterioles [36]. We also observed increased prevalence of smooth muscle cells, fibroblasts, and macrophages. Macrophages play an important role in the inflammatory and immune response and they are also major sources of inflammatory mediators. Elevated macrophage numbers are observed in obesity and are known to produce growth factors, including vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) that are important mediators of PAH pathogenesis [37,38]. Macrophages are present in pulmonary vascular lesions in PAH [39,40] and dysregulated immunity and inflammation are increasingly recognized as key pathologic features of PAH [41,42]. Inflammatory cells, including macrophages, accumulate in the small- to medium-sized pulmonary arteries of PAH patients, a finding that suggests a role for these cells in the pathogenesis of the disease [43]. The observation of inflammatory macrophage infiltration in the pulmonary arteries of leptin-deficient ob/ob mice is strikingly similar to the reported pathology of PAH. Together with the association between leptin deficiency and human PAH [16], our results suggest that leptin deficiency may plays a critical role in the pathogenesis of PAH-like macrophage infiltration of the pulmonary vasculature.
Leptin is known to promote the synthesis and secretion of endothelin-1 in human umbilical vein endothelial cells [20], and promotes the expression of preproendothelin-1 and endothelin ETA receptor, angiotensinogen, and angiotensin type 1 receptor in rabbit portal vein smooth muscle cells [19]. Macrophages, smooth muscle cells, and fibroblasts are all potential sources endothelin-1 (ET-1) [44], a very potent vasoconstrictor acting through two subtypes of smooth muscle endothelin receptor, ETA and ETB. Increased plasma ET-1 is associated with PAH [45] and is correlated with elevated right atrial pressure, elevated pulmonary vascular resistance, and increased mortality [46,47]. Future studies will be necessary to examine the potential mechanistic link between leptin deficiency and the synthesis of ET-1 in ob/ob mice and in human PAH.
In ob/ob mice we also observed right ventricle hypertrophy relative to wild type control animals. It is well known that right ventricular dysfunction and failure are strong indicators of poor prognosis in PAH. Decreased cardiac index and RV ejection fraction are associated with elevated mortality rates even in the presence of aggressive medical therapy [48-50].
There are important challenges in studying leptin. Obesity is associated with hyperleptinemia, which in turn increases leptin resistance [27,28,51,52] and further enhances the tendency towards obesity, making leptin resistance both a consequence and cause of obesity [52]. Thus the level of leptin resistance determines whether leptin signaling is adequate or inappropriately low [31,52,53]. Increased circulating leptin, an indicator of leptin insensitivity, may be directly associated with insulin resistance [54] and cardiovascular disease [55,56]. In mice it is difficult to clearly distinguish between the effects of obesity and the specific effects of leptin deficiency. Leptin-deficient ob/ob mice are characterized by severe obesity resulting from increased energy intake and decreased energy expenditure [25], while wild type obese mice exhibit elevated plasma leptin concentrations as a result of acquired leptin resistance [28,57]. A future study with hemodynamic and echocardiographic determinations in ob/ob mice, under normoxia/ hypoxia and receiving/not receiving replacement of leptin would be important to help clarify the leptin role in the pathogenesis of PAH.
Conclusions
The precise mechanistic role of leptin in the pathogenesis of human PAH remains unresolved and may be addressed by future investigations. However, the results of the present study demonstrate that leptin-deficient mice exhibit many of the characteristic pulmonary vascular pathology of human PAH, including increased pulmonary arterial wall thickness, right ventricular hypertrophy, proliferation of inflammatory and fibrotic cell types, and altered extracellular matrix deposition. Thus, leptin deficiency or leptin resistance may be essential components of the pathogenesis of human PAH.
Acknowledgements
We thank Michael Berk for help in harvesting mouse heart and lungs, and Dr Christine Moravec for help with evaluation of mouse hearts. Dr Adriano Tonelli is supported by CTSA KL2 Grant # RR024990 (A.R.T.) from the National Center for Research Resources (NCRR). Dr Dweik is supported by the following grants: HL081064, HL107147, from the National Institutes of Health (NIH), and BRCP 08-049 Third Frontier Program grant from the Ohio Department of Development (ODOD). Dr Metin Aytekin is supported by 0826095H from American Heart Association (AHA).
Disclosure of conflict of interest
The authors have no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
References
- 1.Breslow MJ, Min-Lee K, Brown DR, Chacko VP, Palmer D, Berkowitz DE. Effect of leptin deficiency on metabolic rate in ob/ob mice. Am J Physiol. 1999;276:E443–449. doi: 10.1152/ajpendo.1999.276.3.E443. [DOI] [PubMed] [Google Scholar]
- 2.Campfield LA, Smith FJ, Burn P. The OB protein (leptin) pathway--a link between adipose tissue mass and central neural networks. Horm Metab Res. 1996;28:619–632. doi: 10.1055/s-2007-979867. [DOI] [PubMed] [Google Scholar]
- 3.Mark AL, Correia M, Morgan DA, Shaffer RA, Haynes WG. State-of-the-art-lecture: Obesity-induced hypertension: new concepts from the emerging biology of obesity. Hypertension. 1999;33:537–541. doi: 10.1161/01.hyp.33.1.537. [DOI] [PubMed] [Google Scholar]
- 4.Haynes WG, Sivitz WI, Morgan DA, Walsh SA, Mark AL. Sympathetic and cardiorenal actions of leptin. Hypertension. 1997;30:619–623. doi: 10.1161/01.hyp.30.3.619. [DOI] [PubMed] [Google Scholar]
- 5.Morgan DA, Thedens DR, Weiss R, Rahmouni K. Mechanisms mediating renal sympathetic activation to leptin in obesity. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1730–1736. doi: 10.1152/ajpregu.90324.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang R, Barouch LA. Leptin signaling and obesity: cardiovascular consequences. Circ Res. 2007;101:545–559. doi: 10.1161/CIRCRESAHA.107.156596. [DOI] [PubMed] [Google Scholar]
- 7.Anagnostoulis S, Karayiannakis AJ, Lambropoulou M, Efthimiadou A, Polychronidis A, Simopoulos C. Human leptin induces angiogenesis in vivo. Cytokine. 2008;42:353–357. doi: 10.1016/j.cyto.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Bouloumie A, Drexler HC, Lafontan M, Busse R. Leptin, the product of Ob gene, promotes angiogenesis. Circ Res. 1998;83:1059–1066. doi: 10.1161/01.res.83.10.1059. [DOI] [PubMed] [Google Scholar]
- 9.Sierra-Honigmann MR, Nath AK, Murakami C, Garcia-Cardena G, Papapetropoulos A, Sessa WC, Madge LA, Schechner JS, Schwabb MB, Polverini PJ, Flores-Riveros JR. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–1686. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 10.Winters B, Mo Z, Brooks-Asplund E, Kim S, Shoukas A, Li D, Nyhan D, Berkowitz DE. Reduction of obesity, as induced by leptin, reverses endothelial dysfunction in obese (Lep(ob)) mice. J Appl Physiol. 2000;89:2382–2390. doi: 10.1152/jappl.2000.89.6.2382. [DOI] [PubMed] [Google Scholar]
- 11.Lembo G, Vecchione C, Fratta L, Marino G, Trimarco V, d’Amati G, Trimarco B. Leptin induces direct vasodilation through distinct endothelial mechanisms. Diabetes. 2000;49:293–297. doi: 10.2337/diabetes.49.2.293. [DOI] [PubMed] [Google Scholar]
- 12.Naseem KM. The role of nitric oxide in cardiovascular diseases. Mol Aspects Med. 2005;26:33–65. doi: 10.1016/j.mam.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Ku IA, Farzaneh-Far R, Vittinghoff E, Zhang MH, Na B, Whooley MA. Association of low leptin with cardiovascular events and mortality in patients with stable coronary artery disease: The Heart and Soul Study. Atherosclerosis. 2011;217:503–8. doi: 10.1016/j.atherosclerosis.2010.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piemonti L, Calori G, Mercalli A, Lattuada G, Monti P, Garancini MP, Costantino F, Ruotolo G, Luzi L, Perseghin G. Fasting plasma leptin, tumor necrosis factor-alpha receptor 2, and monocyte chemoattracting protein 1 concentration in a population of glucose-tolerant and glucose-intolerant women: impact on cardiovascular mortality. Diabetes Care. 2003;26:2883–2889. doi: 10.2337/diacare.26.10.2883. [DOI] [PubMed] [Google Scholar]
- 15.Scholze A, Rattensperger D, Zidek W, Tepel M. Low serum leptin predicts mortality in patients with chronic kidney disease stage 5. Obesity (Silver Spring) 2007;15:1617–1622. doi: 10.1038/oby.2007.191. [DOI] [PubMed] [Google Scholar]
- 16.Tonelli AR, Aytekin M, Feldstein AE, Dweik RA. Leptin levels predict survival in pulmonary arterial hypertension. Pulm Circ. 2012;2:214–219. doi: 10.4103/2045-8932.97607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J, Harrington RA, Anderson JL, Bates ER, Bridges CR, Eisenberg MJ, Ferrari VA, Grines CL, Hlatky MA, Jacobs AK, Kaul S, Lichtenberg RC, Moliterno DJ, Mukherjee D, Pohost GM, Schofield RS, Shubrooks SJ, Stein JH, Tracy CM, Weitz HH, Wesley DJ. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc. , and the Pulmonary Hypertension Association. Circulation. 2009;119:2250–2294. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 18.Stacher E, Graham BB, Hunt JM, Gandjeva A, Groshong SD, McLaughlin VV, Jessup M, Grizzle WE, Aldred MA, Cool CD, Tuder RM. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:261–272. doi: 10.1164/rccm.201201-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Mamputu JC, Wiernsperger N, Renier G. Signaling pathways involved in human vascular smooth muscle cell proliferation and matrix metalloproteinase-2 expression induced by leptin: inhibitory effect of metformin. Diabetes. 2005;54:2227–2234. doi: 10.2337/diabetes.54.7.2227. [DOI] [PubMed] [Google Scholar]
- 20.Quehenberger P, Exner M, Sunder-Plassmann R, Ruzicka K, Bieglmayer C, Endler G, Muellner C, Speiser W, Wagner O. Leptin induces endothelin-1 in endothelial cells in vitro. Circ Res. 2002;90:711–718. doi: 10.1161/01.res.0000014226.74709.90. [DOI] [PubMed] [Google Scholar]
- 21.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:13S–24S. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 22.Giaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shennib H, Kimura S, Masaki T, Duguid WP, Stewart DJ. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993;328:1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 23.Shao D, Park JE, Wort SJ. The role of endothelin-1 in the pathogenesis of pulmonary arterial hypertension. Pharmacol Res. 2011;63:504–511. doi: 10.1016/j.phrs.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 25.Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered. 1950;41:317–318. doi: 10.1093/oxfordjournals.jhered.a106073. [DOI] [PubMed] [Google Scholar]
- 26.Hmisc: Harrell Miscellaneous, 2010. Available from: http://cran.r-project.org/web/packages/Hmisc/index.html [Last accessed on 2011 Sep]
- 27.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 28.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 29.Ip MS, Lam KS, Ho C, Tsang KW, Lam W. Serum leptin and vascular risk factors in obstructive sleep apnea. Chest. 2000;118:580–586. doi: 10.1378/chest.118.3.580. [DOI] [PubMed] [Google Scholar]
- 30.Sikka G, Yang R, Reid S, Benjo A, Koitabashi N, Camara A, Baraban E, O’Donnell CP, Berkowitz DE, Barouch LA. Leptin is essential in maintaining normal vascular compliance independent of body weight. Int J Obes (Lond) 2010;34:203–206. doi: 10.1038/ijo.2009.208. [DOI] [PubMed] [Google Scholar]
- 31.Singhal A, Farooqi IS, Cole TJ, O’Rahilly S, Fewtrell M, Kattenhorn M, Lucas A, Deanfield J. Influence of leptin on arterial distensibility: a novel link between obesity and cardiovascular disease? Circulation. 2002;106:1919–1924. doi: 10.1161/01.cir.0000033219.24717.52. [DOI] [PubMed] [Google Scholar]
- 32.Martin SS, Qasim A, Reilly MP. Leptin resistance: a possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J Am Coll Cardiol. 2008;52:1201–1210. doi: 10.1016/j.jacc.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Unger RH. Hyperleptinemia: protecting the heart from lipid overload. Hypertension. 2005;45:1031–1034. doi: 10.1161/01.HYP.0000165683.09053.02. [DOI] [PubMed] [Google Scholar]
- 34.Vecchione C, Maffei A, Colella S, Aretini A, Poulet R, Frati G, Gentile MT, Fratta L, Trimarco V, Trimarco B, Lembo G. Leptin effect on endothelial nitric oxide is mediated through Akt-endothelial nitric oxide synthase phosphorylation pathway. Diabetes. 2002;51:168–173. doi: 10.2337/diabetes.51.1.168. [DOI] [PubMed] [Google Scholar]
- 35.Fruhbeck G. Pivotal role of nitric oxide in the control of blood pressure after leptin administration. Diabetes. 1999;48:903–908. doi: 10.2337/diabetes.48.4.903. [DOI] [PubMed] [Google Scholar]
- 36.Aytekin M, Comhair SA, de la Motte C, Bandyopadhyay SK, Farver CF, Hascall VC, Erzurum SC, Dweik RA. High levels of hyaluronan in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;295:L789–799. doi: 10.1152/ajplung.90306.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voelkel NF, Tuder RM. Cellular and molecular mechanisms in the pathogenesis of severe pulmonary hypertension. Eur Respir J. 1995;8:2129–2138. doi: 10.1183/09031936.95.08122129. [DOI] [PubMed] [Google Scholar]
- 39.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol. 1994;144:275–285. [PMC free article] [PubMed] [Google Scholar]
- 40.Gerasimovskaya E, Kratzer A, Sidiakova A, Salys J, Zamora M, Taraseviciene-Stewart L. Interplay of macrophages and T cells in the lung vasculature. American journal of physiology. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1014–1022. doi: 10.1152/ajplung.00357.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El Chami H, Hassoun PM. Immune and inflammatory mechanisms in pulmonary arterial hypertension. Prog Cardiovasc Dis. 2012;55:218–228. doi: 10.1016/j.pcad.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kherbeck N, Tamby MC, Bussone G, Dib H, Perros F, Humbert M, Mouthon L. The role of inflammation and autoimmunity in the pathophysiology of pulmonary arterial hypertension. Clin Rev Allergy Immunol. 2013;44:31–38. doi: 10.1007/s12016-011-8265-z. [DOI] [PubMed] [Google Scholar]
- 43.Savai R, Pullamsetti SS, Kolbe J, Bieniek E, Voswinckel R, Fink L, Scheed A, Ritter C, Dahal BK, Vater A, Klussmann S, Ghofrani HA, Weissmann N, Klepetko W, Banat GA, Seeger W, Grimminger F, Schermuly RT. Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:897–908. doi: 10.1164/rccm.201202-0335OC. [DOI] [PubMed] [Google Scholar]
- 44.Shao D, Park JE, Wort SJ. The role of endothelin-1 in the pathogenesis of pulmonary arterial hypertension. Pharmacol Res. 2011;63:504–511. doi: 10.1016/j.phrs.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Stewart DJ, Levy RD, Cernacek P, Langleben D. Increased plasma endothelin-1 in pulmonary hypertension: marker or mediator of disease? Ann Intern Med. 1991;114:464–469. doi: 10.7326/0003-4819-114-6-464. [DOI] [PubMed] [Google Scholar]
- 46.Cacoub P, Dorent R, Nataf P, Carayon A. Endothelin-1 in pulmonary hypertension. N Engl J Med. 1993;329:1967–1968. doi: 10.1056/NEJM199312233292618. [DOI] [PubMed] [Google Scholar]
- 47.Cacoub P, Dorent R, Nataf P, Carayon A, Riquet M, Noe E, Piette JC, Godeau P, Gandjbakhch I. Endothelin-1 in the lungs of patients with pulmonary hypertension. Cardiovasc Res. 1997;33:196–200. doi: 10.1016/s0008-6363(96)00189-7. [DOI] [PubMed] [Google Scholar]
- 48.van Wolferen SA, Boonstra A, Marcus JT, Marques KM, Bronzwaer JG, Postmus PE, Vonk-Noordegraaf A. Right ventricular reverse remodelling after sildenafil in pulmonary arterial hypertension. Heart. 2006;92:1860–1861. doi: 10.1136/hrt.2005.085118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawut SM, Horn EM, Berekashvili KK, Garofano RP, Goldsmith RL, Widlitz AC, Rosenzweig EB, Kerstein D, Barst RJ. New predictors of outcome in idiopathic pulmonary arterial hypertension. Am J Cardiol. 2005;95:199–203. doi: 10.1016/j.amjcard.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 50.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Cottin V, Degano B, Jais X, Montani D, Souza R, Simonneau G. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122:156–163. doi: 10.1161/CIRCULATIONAHA.109.911818. [DOI] [PubMed] [Google Scholar]
- 51.Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, McCamish M. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 52.Martin SS, Qasim A, Reilly MP. Leptin resistance: a possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J Am Coll Cardiol. 2008;52:1201–1210. doi: 10.1016/j.jacc.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sikka G, Yang R, Reid S, Benjo A, Koitabashi N, Camara A, Baraban E, O’Donnell CP, Berkowitz DE, Barouch LA. Leptin is essential in maintaining normal vascular compliance independent of body weight. Int J Obes (Lond) 2010;34:203–206. doi: 10.1038/ijo.2009.208. [DOI] [PubMed] [Google Scholar]
- 54.Mantzoros CS, Liolios AD, Tritos NA, Kaklamani VG, Doulgerakis DE, Griveas I, Moses AC, Flier JS. Circulating insulin concentrations, smoking, and alcohol intake are important independent predictors of leptin in young healthy men. Obes Res. 1998;6:179–186. doi: 10.1002/j.1550-8528.1998.tb00335.x. [DOI] [PubMed] [Google Scholar]
- 55.Wallace AM, McMahon AD, Packard CJ, Kelly A, Shepherd J, Gaw A, Sattar N. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS) Circulation. 2001;104:3052–3056. doi: 10.1161/hc5001.101061. [DOI] [PubMed] [Google Scholar]
- 56.Wolk R, Berger P, Lennon RJ, Brilakis ES, Johnson BD, Somers VK. Plasma leptin and prognosis in patients with established coronary atherosclerosis. J Am Coll Cardiol. 2004;44:1819–1824. doi: 10.1016/j.jacc.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 57.Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1:1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]