Abstract
Aim: It is not clear how the podocyte damage manifests in different glomerulopathies. This study evaluated the podocyte-associated mRNA profiles in renal tissue and urine of patients with proliferative (PGs) or non-proliferative (NPGs) glomerulopathies. Methods: Messenger RNA levels of nephrin, podocin, podocalyxin, synaptopodin, and alpha-actinin-4 were measured in the kidney tissue and urinary cells by real-time polymerase chain reaction. Podocyte-associated mRNAs were correlated with proteinuria and renal function, and the effect of immunosuppressive treatment of PGs and NPGs on urine mRNAs was assessed up to one year of follow up. Results: Podocyte-associated mRNAs were expressed consistently less in kidney tissue from patients with NPGs, and urinary podocyte mRNA levels were significantly higher in the PG group. After six months of immunosuppressive therapy, patients with PGs showed a significant reduction in the expression of podocin, podocalyxin, and alpha-actinin-4 compared with baseline (p<0.001). In the NPG group, alpha-actinin-4 levels decreased (p=0.008), and there was also a trend toward reduced podocalyxin mRNA (p=0.08). Urine podocyte-associated mRNAs correlated with the level of proteinuria at baseline and at six months, and there was a trend toward an inverse correlation between urinary mRNAs and kidney function at one year of follow up. Conclusions: Podocyte-associated mRNAs were inhibited in kidney tissue concomitantly with their increase in urine in these patients with glomerulopathies. Different profiles of mRNA expression were seen, pointing to a higher degree of intra-renal podocytopenia in the NPGs and of podocyturia in the PGs. The immunosuppressive therapy effectively reduced the urinary levels of podocyte-associated mRNAs.
Keywords: Glomerulopathy, podocyte, proteinuria, nephrin, podocin, podocalyxin
Introduction
Podocytes are highly specialized cells that play a critical role in the function of the glomerular filtration barrier [1]. Any injury to the glomerular tuft can potentially alter the podocyte architecture and function, resulting in podocyte effacement and podocytopenia, which have been associated with progressive glomerulosclerosis, as described in experimental and clinical studies [2-4].
Podocyte-associated proteins are essential in maintaining a healthy glomerular filtration barrier. Slit diaphragm nephrin and podocin are closely linked to the cytoskeletal proteins alpha actinin-4 and synaptopodin, which promote dynamic rearrangements of the podocyte morphology; podocalyxin is a sialoglycoprotein of the luminal membrane that limits the passage of albumin to Bowman’s space [5]. Disarrangement of these proteins by diverse mechanisms, such as immune, infectious, ischemic, or toxic insults, results in damage to the filtration barrier and proteinuria [1,4,5].
The pathogenesis of podocyte lesions in proteinuric glomerulopathies is not yet clear, but it involves the fusion of podocyte foot processes without cellular proliferation, the deposition of immune complexes, the secretion of cytokines and growth factors, and inflammation [1,5-7]. Podocyte injury leads to podocyte detachment from the glomerular basement membrane (GBM) and urinary excretion of viable and/or apoptotic cells [8,9]. The type and severity of glomerular lesions probably differ in non-inflammatory and inflammatory glomerulonephritis, but consistently result in altered podocyte mRNAs in both the kidney tissue [10,11] and urinary cells [12,13].
Recently, the identification of podocyte markers in urinary cells by immunocytochemical or molecular analyses has become a noninvasive tool for determining the activity and progression of glomerular diseases [14,15]. Moreover, studies demonstrated that drugs potentially targeting the podocytes, such as immunosuppressants [16], anti-diabetics [17], and angiotensin inhibitors [18], can reduce the urinary excretion of podocytes.
Few studies have evaluated the expression of podocyte-associated mRNAs simultaneously in the kidney and urine of patients with acquired proteinuric diseases. In this study, we hypothesized that the expression of podocyte markers would differ following different podocyte insults that occur in non-proliferative/non-inflammatory glomerulopathies (NPGs) compared with proliferative/inflammatory glomerulopathies (PGs). The effect of immunosuppressive drugs used to treat these glomerular diseases on the urine podocyte-associated mRNAs over time was also assessed.
Methods
Study design
This was a cross-sectional study utilizing a prospective cohort.
Patients
This study included seventy-six adult patients undergoing kidney biopsies for clinical indications at the Division of Nephrology of Hospital de Clínicas de Porto Alegre (HCPA) from September 2009 to May 2012. They were grouped according to their histological diagnosis: nonproliferative glomerulopathy (NPG group, n=35) or proliferative glomerulopathy (PG group, n=41). All patients had urine and kidney tissue samples collected at baseline, and urine samples were also collected at 6 months (n=56) and 12 months (n=43) after the initial treatment for the glomerular disease. Controls were used for normal reference of mRNA expression in kidney tissue and urine. For control tissue samples, renal tissue free from neoplasia was collected from 11 patients undergoing nephrectomy for renal tumor with no other evidence of renal disease. For control urine samples, we collected urine from another set of 10 healthy individuals. Volunteers were defined as healthy when they reported no personal or familial history of kidney disease, when blood pressure levels were <140 x 90 mm Hg; when estimated GFR was higher than 90 mL/min/1.73 m2 and there was no hematuria or proteinuria in urinalysis. This study was approved by the Research Ethics Committee of the HCPA. All subjects agreed to participate in the study and signed an informed consent form.
The following demographic and clinical data were collected from the medical records: age, gender, ethnicity, disease duration, systolic blood pressure (SBP) and diastolic blood pressure (DBP), and drug use (immunosuppressants, angiotensin-converting enzyme inhibitors [ACEi], and angiotensin II receptor blockers [ARB-2]). The laboratory data included serum creatinine, serum albumin, proteinuria (protein-to-creatinine ratio [Pru/Cru] in urine samples), and the estimated glomerular filtration rate (eGFR), as calculated using the Chronic Kidney Disease Epidemiology Collaboration equation. Serum creatinine, eGFR, and Pru/Cru were determined at baseline for all patients and at 6 and 12 months in patients treated with immunosuppressive therapy to measure the urinary gene expression over time.
Immunosuppression and treatment response
Immunosuppression protocols for the treatment of glomerulopathies followed the Brazilian Society of Nephrology guidelines [19], which were standardized in our unit. Treatment responses were defined as follows: a) complete response: reduction in Pru/Cru to <0.35 and increase in serum albumin to >3.5 g/dL; b) partial response: reduction in Pru/Cru between 0.35 and 3.5 g/dL or at least 50% reduction in baseline proteinuria; and c) no response: Pru/Cru remained >3.5 g/dL at month 6 of immunosuppressive therapy [20]. Seven patients with type 2 diabetes mellitus and diabetic nephropathy were treated with anti-diabetic agents in addition to ACEi or ARB-2. Four patients with IgA nephropathy were receiving only ACEi. Thus, these eleven patients were not included in the analysis of the effect of immunosuppressive therapy on the urinary mRNA of podocyte proteins.
Histopathological diagnosis
Conventional histological staining techniques were used, and the histopathological diagnosis was established by a renal pathologist. The percentage of interstitial fibrosis and tubular atrophy (IF/TA) in the biopsy samples was estimated through a semi-quantitative method in the Masson’s Trichrome staining. The histological diagnoses in the NPG group included the following: focal segmental glomerulosclerosis (FSGS) (primary, n=22), membranous glomerulonephritis (MGN) (n=4), minimal change disease (MCD) (n=2), and diabetic nephropathy (DN) (n=7). In the PG group, the histology was as follows: lupus nephritis (LN, n=19; class IV (n=11), class III (n=5), and class II (n=3)), IgA nephropathy (n=14), membranoproliferative glomerulonephritis (MPGN) (n=6), and crescentic glomerulonephritis (CGN) (n=2).
Quantification of podocyte-associated mRNAs in kidney tissue and urine
Podocyte-associated mRNAs were quantified after being measured in the kidney tissue and in the urine sediment cells of morning urine samples (whole stream). The expression of nephrin, podocin, podocalyxin, alpha-actinin-4, and synaptopodin were measured using the cortex of the kidney biopsy. Messenger RNAs were quantified for the NPG and PG groups, as well as separately by the type of glomerulopathy. Podocyturia was assumed to be present when there was an increased amount of urinary mRNA with respect to the level found in healthy individuals.
Messenger RNA extraction and complementary DNA transcription
Messenger RNA was extracted from urine samples using the QIAamp® RNA Blood Mini Kit (Qiagen Inc. Chatsworth, CA, USA) according to the manufacturer’s instructions. Urine samples were centrifuged at 1,800 rpm for 10 minutes. The supernatant was discarded, and the pellet was resuspended with buffered saline and centrifuged at 10,000 rpm for 10 minutes before being stored at -80°C until use. Total RNA was quantified with the NanoDrop® 1000 Spectrophotometer v.3.7 (Thermo Fisher Scientific, Wilmington, DE, USA). The ratio of absorbance at 260/280 nM was used to assess RNA purity. Reverse transcription of total RNA was performed using the High-Capacity cDNA Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. The final volume of purified RNA was 20 μL, and the RNA was stored at -20°C.
Real-time polymerase chain reaction (RT-PCR)
Real-time polymerase chain reaction (RT-PCR) was performed using Taqman® Universal PCR Master Mix. Gene-specific primers of the following genes were used (all from Applied Bioystems, Foster City, CA, USA) according to the manufacturer’s instructions: NPSH1, nephrin [ID: Hs00190446_m1]; NPSH2, podocin [ID: Hs00387817_m1]; podocalyxin [ID: Hs01574644_m1]; synaptopodin [ID: Hs00326493_m1]; and alpha actinin-4 [ID: Hs00245168_m1]. In addition, 18s rRNA (Taqman® PDAR, Foster City, CA, USA) was used as an endogenous control to correct for variations in the samples. RT-PCR was performed in duplicate in 96-well plates containing 2 μL of cDNA. The thermal conditions of the cycles were as follows: 50°C for 2 minutes, 60°C for 30 minutes, and 95°C for 5 minutes, followed by 40 cycles at 94°C for 20 seconds and 62°C for 60 seconds. Data were collected in the ABI PRISM SDS 7000 thermal cycler (Applied Bioystems). The relative quantification of target gene expression was performed using the 2-ΔΔCt comparative method, in which the threshold cycle (CT) value was defined by the point at which there was a statistically significant detectable increase in fluorescence.
Statistical analysis
Descriptive statistics are presented as means ± SD or medians and percentiles. To compare demographic, clinical, and laboratory data, the chi-square or Fisher’s exact test, ANOVA, Kruskal-Wallis or the independent t test, were used as appropriate.
The Friedman test was used to compare the medians of mRNA values at three time points (at biopsy and 6 and 12 months). Messenger RNA values were log-transformed to reduce asymmetry. Spearman’s coefficient was used to assess the correlations of podocyte mRNAs with proteinuria and renal function. Sample size was calculated to search a difference of 30% in the levels of urine log10 mRNA between patients and controls, using the WINPEPI version 9.7 [21].
The change in urine mRNA levels over time was compared at the three time points using the generalized estimating equation (GEE) model with a log-gamma distribution. We assessed the mRNA levels by the patient group, time point, and group-time point interaction. The results were expressed as means and 95% confidence intervals (95% CIs). All analyses were performed using SPSS for Windows (version 18.0, SPSS Inc., Chicago, IL, USA). The level of significance was set at p<0.05.
Results
Table 1 shows the demographic and clinical characteristics of patients and controls. Seventy-six patients were evaluated at baseline. Of these patients, 56 (74%) were also evaluated at 6 months, and 43 (57%) completed the 12-month follow-up. The reasons for not completing the 12-month follow-up included the following: late inclusion in the study with sample collection at baseline only (n=16), loss to follow-up (n=8), renal transplantation or hemodialysis (n=5), and death (n=4). Excluding patients with diabetic nephropathy, the median disease durations were 4.5 (1.7-9.8) and 3 (1.5-5.0) months in the NPG and PG groups, respectively (p=0.197).
Table 1.
Demographic and clinical characteristics of patients with glomerulopathies and controls
| NPGs | PGs | Controls | p-value | ||
|---|---|---|---|---|---|
|
| |||||
| Urine | Kidney tissue | ||||
| N | 35 | 41 | 10 | 11 | |
| Age (years) | 41±17a | 35±11a | 44±12a | 59±11b | <0.001 |
| Gender (male) | 10 (29) | 19 (46) | 4 (40) | 6 (54) | 0.185 |
| Ethnicity (white) | 26 (74) | 37 (90) | 9 (90) | 9 (81) | 0.137 |
| Disease duration (months) | 3 (1.7-18)a | 5 (2.0-21)a | - | - | 0.256 |
| SBP (mm Hg) | 133±15a | 134±12a | 111±7.2b | 115±7.9b | <0.001 |
| DBP (mm Hg) | 81±10a | 85±10a | 71±7.3b | 72±6.5b | <0.001 |
| Baseline serum creatinine (mg/dL) | 2.11±1.44a | 2.33±1.72a | 0.83±0.17b | 0.88±0.14b | 0.046 |
| eGFR (mL/min/1.73 m2) | 58.4±41.6a | 62.3±41.8a | 92.1±9b | 85.2±11.3b | 0.008 |
| Baseline proteinuria (PCR) | 5.10±4.70a | 4.40±3.80a | 0.05±0.04b | 0.06±0.03b | 0.01 |
| Serum albumin | 2.61±0.95a | 3.08±0.84b | 4.51±0.32c | 4.28±0.38c | <0.001 |
| IF/TA (%) | 24±20a | 18±16a | - | 3±2b | 0.002 |
SBP: systolic blood pressure; DBP: diastolic blood pressure; eGFR: estimated glomerular filtration rate; PCR: protein-to-creatinine ratio; IF/TA: interstitial fibrosis/tubular atrophy; NPGs: non-proliferative glomerulopathies; PGs: proliferative glomerulopathies;
means or medians followed by different letters indicate a statistically significant difference between groups (p<0.05);
means or medians followed by different letters indicate a statistically significant difference between groups (p<0.05);
means or medians followed by different letters indicate a statistically significant difference between groups (p<0.05).
P-values were determined by ANOVA, chi-square or Fisher’s exact test, or the Mann-Whitney test.
At baseline, 92 and 71% of the patients in the NPG and PG groups were on ACEi (p=0.024) and 9 and 15% were on ARB-2, respectively (p=0.494); at 6 months, 63 and 54% remained on ACEi (p=0.418) and 6 and 15% were taking ARB-2 (p=0.275), respectively; and at 12 months, 57 and 48% were still taking ACEi (p=0.598) and 5 and 12% were still taking ARB-2 (p=0.06), respectively.
Sixty-five patients were treated with immunosuppressants (FSGS=22, MGN=4, MCD=2, LN=19, IgA=10, MPGN=6, and CGN=2); the remainder received only ACEi or ARB-2 (DN=7, IgA=4). Of the patients treated with immunosuppressants, 48 completed 6 months of follow up and showed a complete (n=9) or partial (n=26) response to treatment (n=35). Thirteen patients were resistant to therapy: FSGS=5; LN=4 (1 in class III and 3 in class IV); IgA=2; and MPGN=2. Overall, proteinuria levels at the time of biopsy and at 6 months were 4.80±3.78 and 1.30±1.01 in responders (p<0.001) and 5.60±5.41 and 4.86±2.61 in non-responders (p=0.630), respectively. The eGFR values at baseline and 6 months were 55.2±44.0 and 78.3±36.2 mL/min/1.73 m2 in responders (p=0.04) and 63.8±48.2 mg/dL and 62.2±39.8 mL/min/1.73 m2 in non-responders (p=0.985).
Podocyte-associated mRNAs in kidney tissue
Podocyte mRNAs (except of synaptopodin) expressed in the kidney biopsies were significantly decreased in the NPG group compared with controls (Figure 1). The PG group also showed lower intra-renal mRNA levels, with statistical significance for podocin (p=0.024 compared with controls) and alpha actinin-4 (p=0.049 compared with controls). There was also a trend toward a lower expression of nephrin and podocalyxin. There was also a trend toward a lower expression of nephrin and podocalyxin. Although mRNAs were also reduced in PGs, in the non-proliferative glomerulopathies this reduction was more pronounced (Figure 1).
Figure 1.
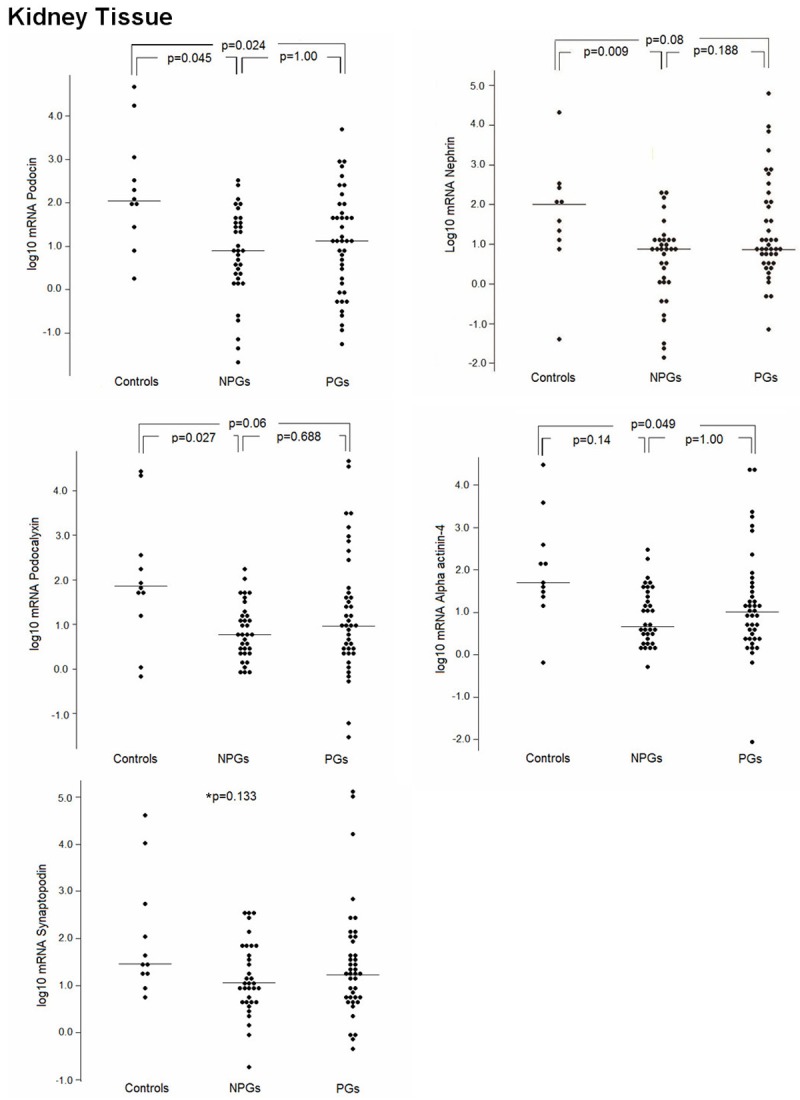
Kidney tissue expression of podocyte-associated mRNAs. Messenger RNA levels of nephrin, podocin, podocalyxin, synaptopodin, and alpha-actinin-4 in the kidney tissue of NPG and PG patients and controls. NPGs: non-proliferative glomerulopathies; PGs: proliferative glomerulopathies. *p=0.133: comparison among the three groups.
Podocyte-associated mRNAs in the urinary cells
The expression profiles of urine podocyte mRNAs contrasted with those found in kidney tissues, i.e., higher mRNA levels compared with controls. Higher urinary mRNA levels of nephrin, podocin, and podocalyxin were found in the PG group compared with the NPG group and healthy individuals, as shown in Figure 2. Both the PG and NPG groups showed a significantly higher mRNA expression of alpha actinin-4 than that was found in the controls. However, despite the higher mRNA levels of podocin and podocalyxin in the NPG group, there was no statistical difference compared with controls.
Figure 2.
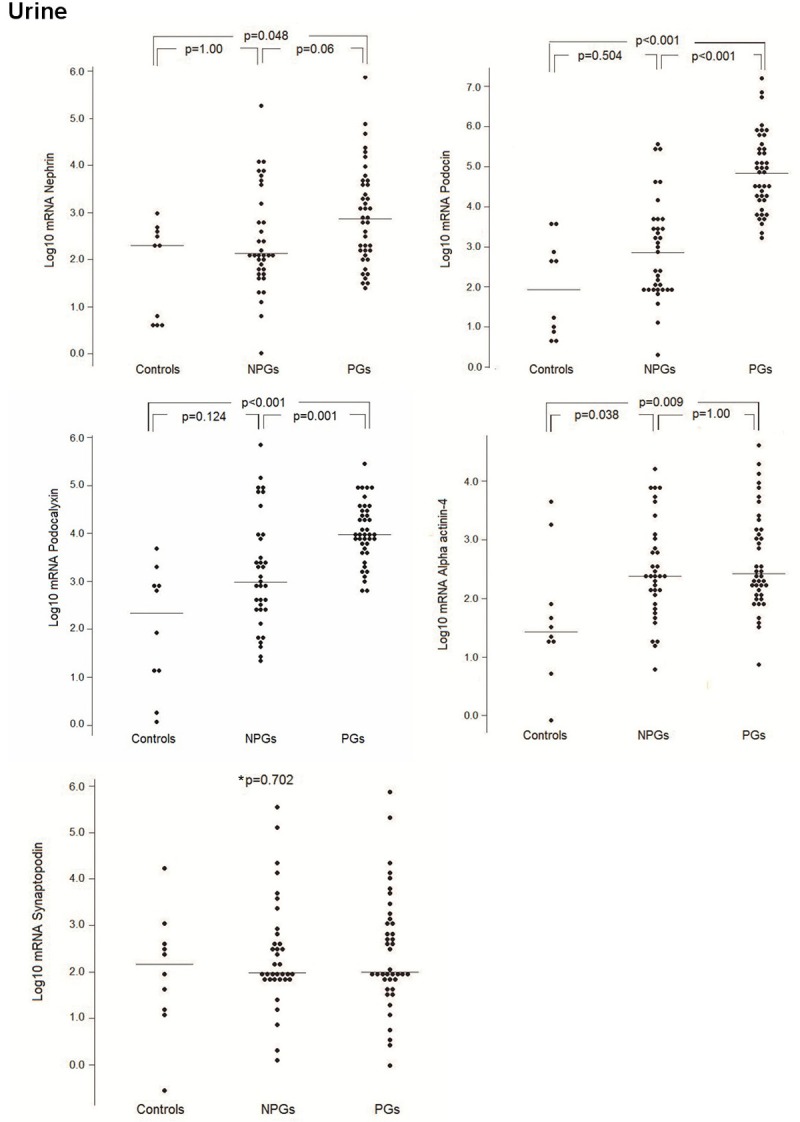
Expression of podocyte-associated mRNAs in the urinary cells. Messenger RNA levels of nephrin, podocin, podocalyxin, synaptopodin, and alpha-actinin-4 in the urinary sediment cells of NPG and PG patients and controls. NPGs: non-proliferative glomerulopathies; PGs: proliferative glomerulopathies. *p=0.702: comparison among the three groups.
Messenger RNA expression of podocin by type of glomerulopathy
Messenger RNA of podocin best reflected the patterns of expression when the different histological types of glomerulopathies were analyzed. Figure 3 shows the medians of the log10 mRNA values of podocin by compartment (renal tissue or urine cells) and type of disease (proliferative or non-proliferative) analyzing each histological type compared with the expression in healthy individuals. There were significantly lower tissue podocin mRNA in all patients with glomerulopathies, except in MNG and CG (p>0.05). Urine mRNA of podocin was significantly higher in all types of PGs, but not in the non-proliferative forms. Overall, neither the non-inflammatory nor the inflammatory glomerulopathies showed a specific pattern of podocyte mRNAs expression.
Figure 3.
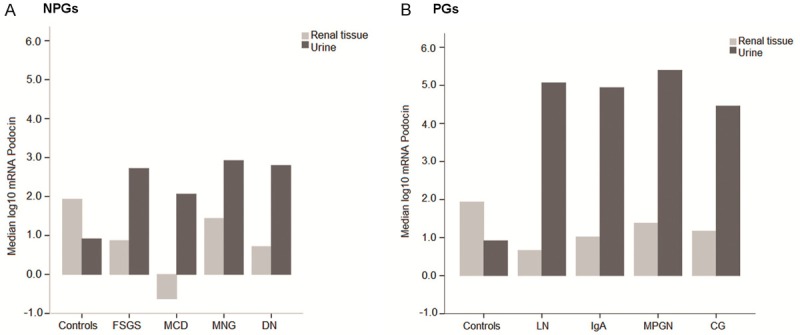
Messenger RNA of podocin in non-proliferative and proliferative glomerulopathies. Messenger RNA of podocin by the type of glomerulopathy and compartment (renal tissue or urinary cells) is presented separately in the NPG and PG groups; each mRNA expression is relative to that in healthy individuals. NPGs: renal tissue: FSGS (p=0.004), MCD (p=0.041), MGN (p=0.240), DN (p=0.014); urine: FSGS (p=0.095); MCD (p=1.000); MNG (p=0.257); DN (p=0.118). PGs: renal tissue: LN (p=0.027); IgA (p=0.044); MPGN (p=0.020); CG (p=0.236) and urine: LN, IgA, and MPGN (p<0.001); CG (p=0.031). NPGs: non-proliferative glomerulopathies; PGs: proliferative glomerulopathies; FSGS: Focal Segmental Glomerulosclerosis; MCD: Minimal Change Disease; MGN: Membranous Glomerulonephritis; DN: Diabetic Nephropathy; LN: Lupus Nephritis; IgA: IgA nephropathy; MPGN: membrano-proliferative glomerulonephritis; CG: Crescentic Nephritis.
Effect of immunosuppressive treatment on the podocyte-associated mRNAs in urine
After immunosuppressive treatment, urine mRNA of podocin, podocalyxin, and alpha actinin-4 significantly decreased in the PG group compared with baseline levels: podocin and podocalyxin (p<0.001, baseline vs. 6 and 12 months) and alpha actinin-4 (p=0.006, baseline vs. 6 months). There was a non-significant reduction in the urine mRNA of nephrin. The messenger RNA levels of synaptododin did not change over time in both groups. The reduction of mRNA in patients with proliferative nephritis persisted from the sixth to twelfth months for podocin and podocalyxin (6 vs. 12 months, p=1.000), as well as for alpha actinin-4 (6 vs. 12 months, p=0.774). In the NPG group, the reduction of urinary mRNA after treatment was not as marked, but there were significantly lower levels of alpha actinin-4 (baseline vs. 6 months, p=0.008) and podocalyxin (baseline vs. 6 months, p=0.039, and baseline vs. 12 months, p=0.026). Nephrin mRNA levels showed a slight but non-significant increase at 12 months.
Figure 4 shows the reduction of urine mRNA over time, as analyzed by the GEE model. The mRNA levels of nephrin (Wald chi-square test: 4.33, p=0.037) and podocalyxin (Wald chi-square test: 8.61, p=0.003) were higher in the PG group. The time point of measurement did not have any effect on nephrin mRNA expression, but this effect was seen for podocalyxin and alpha actinin-4 at the three time points, indicating a significant reduction in urine mRNA levels. There was an interaction of time point and group for the podocin gene, indicating that podocin expression depends on a combined effect of time point and measurement group (Wald chi-square test: 23.037, p<0.001). Thus, the PG group showed a more pronounced reduction in podocin than the NPG group from baseline to 6 months. Messenger RNA levels of synaptopodin were similar between the two groups and remained unchanged over time.
Figure 4.

Effect of immunosuppressive treatment on the urinary podocyte-associated mRNAs. Effect of immunosuppressive treatment of patients with NPGs and PGs on the podocyte-associated mRNAs in urine at 6 and 12 months of therapy, expressed as means and standard errors. Bars: error bars show the 95% confidence interval. NPGs: non-proliferative glomerulopathies; PGs: proliferative glomerulopathies. Synaptopodin: p>0.05 for all comparisons.
Urine podocyte-associated mRNAs were also analyzed according to the response to treatment, i.e., comparing responders (either total or partial response) with non-responders after six months of immunosuppressive therapy. Podocyte mRNAs at baseline was similar between the 2 groups, but the decrease in podocyte excretion over 12 months was more pronounced in responders; however, this difference was not statistically significant. A Cox regression analysis including the 48 patients who received immunosuppressants showed that the podocyte mRNAs did not predict a lack of response to therapy (Pru/Cru >3.5) at six months of observation (data not shown).
Correlations of the podocyte-associated mRNAs with proteinuria and renal function
Table 2 shows the correlations between the different podocyte mRNAs in kidney tissue and urine at baseline. These correlations in the kidney tissue were strong and significant (p<0.001), except with synaptopodin. Nephrin and podocin in baseline urine were significantly correlated with each other, as well as with podocalyxin and alpha actinin-4, but these correlations were weaker than those in the kidney tissue.
Table 2.
Correlations between the expression of podocyte-associated mRNAs in kidney tissue and urine of patients with glomerulopathies
| Spearman correlation coefficient (r, p-value) | Nephrin | Podocin | Podocalyxin | Synaptopodin | Alpha actinin-4 | |
|---|---|---|---|---|---|---|
| Kidney tissue | Nephrin | 1 | 0.655a | 0.768 | 0.224 | 0.722 |
| Podocin | <0.001b | 1 | 0.548 | 0.209 | 0.529 | |
| Podocalyxin | <0.001 | <0.001 | 1 | 0.072 | 0.867 | |
| Synaptopodin | 0.042 | 0.073 | 0.482 | 1 | 0.104 | |
| Alpha actinin-4 | <0.001 | <0.001 | <0.001 | 0.261 | 1 | |
| Urine sediment (baseline) | Nephrin | 1 | 0.497 | 0.393 | 0.233 | 0.376 |
| Podocin | <0.001 | 1 | 0.583 | 0.267 | 0.223 | |
| Podocalyxin | <0.001 | <0.001 | 1 | 0.168 | 0.193 | |
| Synaptopodin | 0.038 | 0.013 | 0.123 | 1 | 0.101 | |
| Alpha actinin-4 | <0.001 | 0.039 | 0.075 | 0.355 | 1 |
Spearman correlation coefficient;
p value.
Baseline urine mRNAs correlated with proteinuria as follows: nephrin (r=0.637, p<0.001), podocin (r=0.513, p=0.002), and alpha-actinin-4 (r=0.340, p=0.05); additionally, there was also a trend with podocalyxin (r=0.320, p=0.06). At six months (but not at twelve months), a significant correlation remained for nephrin (r=0.621, p=0.001), podocin (r=0.576, p=0.003), and podocalyxin (r=0.577, p=0.003), and a marginal correlation remained for alpha-actinin-4 (r=0.345, p=0.09). No correlations between proteinuria and kidney tissue mRNAs were found.
There was no correlation between the level of kidney function and urine mRNAs at baseline; at twelve months, there was a trend toward an inverse correlation between eGFR and the urine mRNA of podocin (r=-0.272, p=0.08), podocalyxin (r=-0.303, p=0.051), and alpha-actinin-4 (r=-0.320, p=0.06). Median of podocyte mRNAs from patients with a positive or a negative variation of eGFR after one year of follow up (eGFR at 12 months – baseline eGFR) did not differ, either in tissue or in urine.
Discussion
Recent evidence has shown that injuries to the podocytes play a critical role in the development of proteinuria and progression of glomerular disease [1,2,4,14,22]. The two major mechanisms of podocyte loss are apoptosis and the detachment of viable cells, but in situ apoptosis seems to be a rare event [23]. The extent to which apoptosis and/or detachment occur after podocyte injury is still controversial, but detached podocytes or their fragments traverse the tubules and are excreted in the urine, being detected by immunostaining techniques [13,24,25] or by mRNA quantification of podocyte-associated molecules [12,22,25-27]. Vogelmann et al [8] and others [25] have reported that, following the shedding of podocytes in urine, the majority of these cells are still viable in inflammatory glomerular diseases. Even in healthy individuals, viable podocytes can be encountered to a lesser degree.
As other groups did [11,12,22] we measured podocyte-associated mRNAs in diverse glomerulopathies presented in routine clinical practice. These diseases have different etiopathogenesis, i.e., mechanisms resulting in cellular proliferation and inflammation, or when focal and segmental glomerulosclerosis or GBM thickening predominate. Our study was not designed to address mechanistic pathways, but to investigate the amount of ‘podocytopenia’ and ‘podocyturia’ in proliferative and non- proliferative glomerular diseases, assuming that podocyturia follows podocyte detachment from the GBM that results in intra-renal podocytopenia. One study [27] compared the urine mRNA expression of NPHS1, NPHS2 and other genes in patients with proliferative and non-proliferative glomerulonephiritis. Only NPHS1 discriminated the non-proliferative from proliferative glomerular types mainly because the expression of NPHS1 in patients with FSGS was substantially higher. This result was not confirmed in our study regarding the urinary podocin mRNA expression in patients with FSGS, that was similar to the levels found in the other NPGs but significantly lower than in the proliferative forms of glomerulonephritides.
In primary nephrotic syndrome, podocyte fusion causes the destabilization of cell-cell adhesion and induces the structural rearrangements of the slit diaphragm and cytoskeleton, redistributing and down-regulating the expression of podocyte proteins in the glomerulus [6,29]. Lahdenkari et al [30] showed that, in MCD, effaced podocytes did not detach from the basement membrane and did not show cell membrane rupture, but there was a redistribution of nephrin to the apical membrane. Our findings of low tissue levels of podocyte mRNAs and a lesser degree of podocyturia in the NPGs could reflect the ultrastructural GBM alterations reported by Lahdenkari’s group.
In contrast, inflammation and/or antibody-mediated insults in PGs may alter podocyte functions and morphology through different pathways, in accordance with recent studies. As examples, in experimental antibody-mediated nephropathy, there are disruptions of the cell cycle balance, the down-regulation of slit diaphragm proteins, and severe proteinuria [31]. In an animal model of lupus nephritis, the mRNA levels of glomerular nephrin and podocin were decreased in focal and diffuse proliferative nephritis in correlation with distorted slit diaphragms seen by electron microscopy [32]. The levels of urinary podocyte-associated mRNAs were higher in patients with active lupus nephritis compared with those with inactive disease; they also correlated with proteinuria and decreased renal function [33]. The increased number of urinary podocytes correlated with the severity of glomerular injury in patients with active IgA nephropathy [34]. In our study, we demonstrated a positive correlation between proteinuria and most of the urine podocyte mRNAs measured in the acute phase of proliferative forms of glomerulonephritis, which decreased over time, concomitantly with the use of immunosuppressive therapy.
In agreement with other authors [10,27], we found a high intra-renal correlation among nephrin, podocin, podocalyxin, and alpha actinin-4 mRNAs, as well as a lower but still significant correlation of these podocyte mRNAs in the urine. These correlations suggest that injury to podocytes may result in a stereotyped phenomena, in the sense that in active disease podocyte cells or its fragments rupture and detach from GBM, and are excreted in the urine. Thus, the relationship between tissue and urinary podocyte mRNAs should be inversely correlated, assuming that one follows the other. Our data confirm such findings. A threshold of podocyte injury resulting in podocytopenia has been suggested by Hara et al [35], after which point there is a continuum of podocyte excretion reflecting repeated hits of acute glomerular injury. A decrease in glomerular podocytes followed by their cumulative excretion in urine was well demonstrated in clinical studies, such as in lupus nephritis [25], IgA nephropathy [35], and diabetic nephropathy [36]. A hypothesis to explain the podocyte shedding was suggested by Yu et al [14], using the puromycin-induced nephrosis and anti-Thy nephritis rat models, which showed that the onset of proteinuria and podocyturia occurred in parallel after the acute insult. Following the transient glomerular lesion, the proteinuria persisted at late stages, but podocyturia subsided, suggesting that podocyturia, but not proteinuria, can distinguish persistent defects from ongoing glomerular injury. In the study of Wickman et al [22], the highest urinary podocin mRNAs were most prevalent in patients with acute inflammatory glomerulonephritides, that returned to levels not different from controls when the diseases went into remission.
The association between the rate of podocyturia and progression of renal disease has been demonstrated in experimental [14] and clinical [12,22,33,37,38] works in patients with different glomerulopathies. Recently, Wickman et al [22] studied a large number of patients with diverse nephropathies showing that, in those with biopsy-proven glomerular disease the urine podocyte mRNAs increased 79-fold in relation to normal controls, and these patients halved their kidney function or progressed to end-stage kidney disease, thus reinforcing the podocyte depletion hypothesis. After treatment, urine podocyte mRNAs returned to baseline values on disease remission, similar to what we found. Therefore, monitoring these events may impact the management and outcome of patients with glomerulopathies, as podocyturia increases the risk of progression to chronic kidney disease. In our study, urine podocyte mRNAs did not correlate with renal function at baseline, but at twelve months there was a trend toward an inverse correlation between eGFR and urine mRNA of podocin, podocalyxin, and alpha actinin-4. This lack of significance may be related to our small sample size, as only 43 patients completed one year of follow up.
Synaptopodin was expressed at similar levels in the tissue and urine of patients and healthy individuals and did not change after treatment. In addition, synaptopodin did not correlate with histological disease, proteinuria, or renal function. This molecule is regarded as a housekeeping gene because its expression is relatively constant in the podocyte cytoskeleton. Possibly, it does not affect glomerular permeability to protein in disease processes [39]. Other authors did not find either a correlation of synaptopodin with nephrin or podocin mRNA, or between synaptopodin and the rate of decline in eGFR [12].
Presently, targeting podocyte-specific proteins with drugs that could modify the course of glomerular diseases is a subject of investigation. Recent studies have provided insight into the potential of anti-proteinuric therapies to retain the expression of slit diaphragm and cytoskeleton proteins and to restore the integrity of the glomerular filtration barrier. Experimental and clinical studies tested the effects of corticosteroids and cytotoxic drugs [16,40], cyclosporine [41], ACEi and ARB-2 [18,42,43], or anti-diabetics [17], demonstrating their benefits in podocyte diseases. In our study, conventional immunosuppressive therapy, such as steroids, cytotoxics, and calcineurin inhibitors, reduced the urinary mRNA levels of podocin, podocalyxin, and alpha actinin-4 concomitantly with the decrease in proteinuria, which possibly indicates at least a partial recovery of the podocyte function in glomeruli.
The current investigation had some limitations. First, we did not evaluate whether the reduction of podocyturia after treatment was accompanied by an increase in glomerular mRNA of the podocyte markers; our patients did not have clinical indication for a second biopsy during the study period. Second, the number of patients in each histological group was small. In addition, as the majority of the patients were taking ACEi or ARB-2, we would have needed to control for their effects on the level of podocyturia.
In summary, this study showed that podocyte-associated mRNAs are inhibited in the kidney tissue and concomitantly are increased in the urine. The finding of ‘podocytopenia’ occurring concomitantly with ‘podocyturia’, although at different degrees in NPGs and PGs, reinforces the podocyte depletion hypothesis. Additionally, we found that the immunosuppressive therapy can improve the podocyte function, in parallel with the reduction of proteinuria and improvement of renal function. Further investigation is needed to evidence the pathways of podocyte injury in patients with glomerular diseases of different etiopathogenesis.
Acknowledgements
The authors thank the Program of Support for the Restructuring and Expansion of Brazilian Federal Universities (REUNI) for providing a Master’s grant to PGR, the Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS) and the Hospital de Clínicas de Porto Alegre Research Funding (FIPE/HCPA) for financial support, and the Laboratory of Molecular Biology Applied to Nephrology of HCPA for technical support.
Disclosure of conflict of interest
The authors declare no conflicts of interest.
References
- 1.Shankland SJ. The podocyte’s response to injury: Role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–2147. doi: 10.1038/sj.ki.5000410. [DOI] [PubMed] [Google Scholar]
- 2.Kriz W. Podocyte is the major culprit accounting for the progression of chronic renal disease. Microsc Res Tech. 2002;15:189–195. doi: 10.1002/jemt.10072. [DOI] [PubMed] [Google Scholar]
- 3.Ichikawa I, Ma J, Motojima M, Matsusaka T. Podocyte damage damages podocytes: autonomous vicious cycle that drives local spread of glomerular sclerosis. Curr Opin Nephrol Hypertens. 2005;14:205–210. doi: 10.1097/01.mnh.0000165884.85803.e1. [DOI] [PubMed] [Google Scholar]
- 4.Wiggins RC. The spectrum of podocytopathies: A unifying view of glomerular diseases. Kidney Int. 2007;71:1205–1214. doi: 10.1038/sj.ki.5002222. [DOI] [PubMed] [Google Scholar]
- 5.Barisoni L, Schnaper W, Kopp JB. Advances in the Biology and Genetics of the Podocytopathies. Implications for Diagnosis and Therapy. Arch Pathol Lab Med. 2009;133:201–216. doi: 10.1043/1543-2165-133.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doublier S, Ruotsalainen V, Salvidio G, Lupia E, Biancone L, Conaldi PG, Reponen P, Tryggvason K, Camussi G. Nephrin redistribution on podocytes is a potential mechanism for proteinuria in patients with primary acquired nephrotic syndrome. Am J Pathol. 2001;158:1723–1731. doi: 10.1016/S0002-9440(10)64128-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai KN, Leung JC, Chan LY, Saleem MA, Mathieson PW, Tam KY, Xiao J, Lai FM, Tang SC. Podocyte injury induced by mesangial-derived cytokines in IgA nephropathy. Nephrol Dial Transplant. 2009;24:62–72. doi: 10.1093/ndt/gfn441. [DOI] [PubMed] [Google Scholar]
- 8.Vogelmann SU, Nelson WJ, Myers BD, Lemley KV. Urinary excretion of viable podocytes in health and renal disease. Am J Physiol Renal Physiol. 2003;285:F40–F48. doi: 10.1152/ajprenal.00404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hara M, Yanagihara T, Kihara I, Higashi K, Fujimoto K, Kajita T. Apical cell membranes are shed into urine from injured podocytes: a novel phenomenon of podocyte injury. J Am Soc Nephrol. 2005;16:408–416. doi: 10.1681/ASN.2004070564. [DOI] [PubMed] [Google Scholar]
- 10.Koop K, Eikmans M, Baelde HJ, Kawachi H, De Heer E, Paul LC, Bruijn JA. Expression of podocyte-associated molecules in acquired human kidney diseases. J Am Soc Nephrol. 2003;14:2063–2071. doi: 10.1097/01.asn.0000078803.53165.c9. [DOI] [PubMed] [Google Scholar]
- 11.Schmid H, Henger A, Cohen CD, Frach K, Gröne HJ, Schlöndorff D, Kretzler M. Gene expression profiles of podocyte-associated molecules as diagnostic markers in acquired proteinuric diseases. J Am Soc Nephrol. 2003;14:2958–2966. doi: 10.1097/01.asn.0000090745.85482.06. [DOI] [PubMed] [Google Scholar]
- 12.Szeto CC, Lai KB, Chow KM, Szeto CY, Yip TW, Woo KS, Li PK, Lai FM. Messenger RNA expression of glomerular podocyte markers in the urinary sediment of acquired proteinuric diseases. Clin Chim Acta. 2005;36:182–190. doi: 10.1016/j.cccn.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura T, Ushiyama C, Suzuki S, Hara M, Shimada N, Sekizuka K, Ebihara I, Koide H. Urinary Podocytes for the Assessment of Disease Activity in Lupus Nephritis. Am J Med Sci. 2000;320:112–116. doi: 10.1097/00000441-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Yu D, Petermann A, Kunter A, Rong S, Shankland SJ, Floege J. Urinary Podocyte Loss Is a More Specific Marker of Ongoing Damage than Proteinuria. J Am Soc Nephrol. 2005;16:1733–1741. doi: 10.1681/ASN.2005020159. [DOI] [PubMed] [Google Scholar]
- 15.Skoberne A, Konieczny A, Schiffer M. Glomerular epithelial cells in the urine: what has to be done to make them worthwhile? Am J Physiol Renal Physiol. 2009;296:F230–F241. doi: 10.1152/ajprenal.90507.2008. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura T, Ushiyama C, Hara M, Osada S, Ugai K, Shimada N, Hayashi K, Ebihara I, Koide H. Comparative effects of plasmapheresis and intravenous cyclophosphamide on urinary podocyte excretion in patients with proliferative Lupus nephritis. Clin Nephrol. 2002;57:108–113. doi: 10.5414/cnp57108. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura T, Ushiyama C, Osada S, Hara M, Shimada N, Koide H. Pioglitazone reduces urinary podocyte excretion in type 2 diabetes patients with microalbuminuria. Metabolism. 2001;50:1193–1196. doi: 10.1053/meta.2001.26703. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura T, Ushiyama C, Suzuki S, Hara M, Shimada N, Sekizuka K, Ebihara I, Koide H. Effects of angiotensin-converting enzyme inhibitor, angiotensin II receptor antagonist and calcium antagonist on urinary podocytes in patients with IgA nephropathy. Am J Nephrol. 2000;20:373–379. doi: 10.1159/000013619. [DOI] [PubMed] [Google Scholar]
- 19.Investigation and Treatment of Glomerular Diseases in Adults: Guidelines of the Brazilian Society of Nephrology. J Bras Nefrol. 2005;26:1–40. [Google Scholar]
- 20.Cattran DC, Alexopoulos E, Heering P, Hoyer PF, Johnston A, Meyrier A, Ponticelli C, Saito T, Choukroun G, Nachman P, Praga M, Yoshikawa N. Cyclosporin in idiopathic gomerular disease associated with the nephrotic syndrome: Workshop recommendations. Kidney Int. 2007;72:1429–1447. doi: 10.1038/sj.ki.5002553. [DOI] [PubMed] [Google Scholar]
- 21.Abramson JH. WINPEPI (PEPI-for-Windows): computer programs for epidemiologists. Epidemiol Perspect Innov. 2004;1:6. doi: 10.1186/1742-5573-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wickman L, Afshinnia F, Wang SQ, Yang Y, Wang F, Chowdhury M, Graham D, Hawkins J, Nishizono R, Tanzer M, Wiggins J, Escobar GA, Rovin B, Song P, Gipson D, Kershaw D, Wiggins RC. Urine Podocyte mRNAs, Proteinuria, and Progression in Human Glomerular Diseases. J Am Soc Nephrol. 2013;24:2081–2095. doi: 10.1681/ASN.2013020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kriz W, Shirato I, Nagata M, LeHir M, Lemley KV. The podocyte’s response to stress: the enigma of foot process effacement. Am J Physiol Renal Physiol. 2013;304:F333–F347. doi: 10.1152/ajprenal.00478.2012. [DOI] [PubMed] [Google Scholar]
- 24.Hara M, Yanagihata T, Kihara I. Urinary podocytes in primary focal segmental glomerulosclerosis. Nephron. 2001;89:342–347. doi: 10.1159/000046097. [DOI] [PubMed] [Google Scholar]
- 25.Bollain-y-Goytia JJ, González-Castañeda M, Torres-del-Muro F, Daza-Benitez L, Zapata-Benavides P, Rodríguez-Padilla C, Avalos-Díaz E, Herrera-Esparza R. Increased excretion of urinary podocytes in lupus nephritis. Indian J Nephrol. 2011;21:166–171. doi: 10.4103/0971-4065.83029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang G, Lai FM, Lai KB, Chow KM, Kwan BC, Li KT, Szeto CC. Intra-renal and urinary mRNA expression of podocyte-associated molecules for estimation of glomerular filtration loss. Ren Fail. 2010;32:372–379. doi: 10.3109/08860221003611737. [DOI] [PubMed] [Google Scholar]
- 27.Navarro-Muñoz M, Ibernon M, Pérez V, Ara J, Espinal A, López D, Bonet J, Romero R. Messenger RNA expression of B7-1 and NPHS1 in urinary sediment could be useful to differentiate between minimal-change disease and focal segmental glomerulosclerosis in adult patients. Nephrol Dial Transplant. 2011;26:3914–3923. doi: 10.1093/ndt/gfr128. [DOI] [PubMed] [Google Scholar]
- 28.Petermann A, Floege J. Podocyte damage resulting in podocyturia: a potencial diagnostic marker to assess glomerular disease activity. Nephron Clin Pract. 2007;106:c61–c66. doi: 10.1159/000101799. [DOI] [PubMed] [Google Scholar]
- 29.Wernerson A, Dunér F, Pettersson E, Widholm SM, Berg U, Ruotsalainen V, Tryggvason K, Hultenby K, Söderberg M. Altered ultrastructural distribution of nephrin in minimal change nephrotic syndrome. Nephrol Dial Transplant. 2003;18:70–76. doi: 10.1093/ndt/18.1.70. [DOI] [PubMed] [Google Scholar]
- 30.Lahdenkari AT, Lounatmaa K, Patrakka J, Holmberg C, Wartiovaara J, Kestilä M, Koskimies O, Jalanko H. Podocytes are firmly attached to glomerular basement membrane in kidneys with heavy proteinuria. J Am Soc Nephrol. 2004;15:2611–8. doi: 10.1097/01.ASN.0000139478.03463.D9. [DOI] [PubMed] [Google Scholar]
- 31.Kawachi H, Han GD, Miyauchi N, Hashimoto T, Suzuki K, Shimizu F. Therapeutic targets in the podocyte: findings in anti-slit diaphragm antibody-induced nephropathy. J Nephrol. 2009;22:450–456. [PubMed] [Google Scholar]
- 32.Perysinaki GS, Moysiadis DK, Bertsias G, Giannopoulou I, Kyriacou K, Nakopoulou L, Boumpas DT, Daphnis E. Podocyte main slit diaphragm proteins, nephrin and podocin, are affected at early stages of lupus nephritis and correlate with disease histology. Lupus. 2011;20:781–91. doi: 10.1177/0961203310397412. [DOI] [PubMed] [Google Scholar]
- 33.Wang G, Lai FM, Tam LS, Li KM, Lai KB, Chow KM, Li KT, Szeto CC. Messenger RNA expression of podocyte-associated molecules in urinary sediment of patients with lupus nephritis. J Rheumatol. 2007;34:2358–2364. [PubMed] [Google Scholar]
- 34.Asao R, Asanuma K, Kodama F, Akiba-Takagi M, Nagai-Hosoe Y, Seki T, Takeda Y, Ohsawa I, Mano S, Matsuoka K, Kurosawa H, Ogasawara S, Hirayama Y, Sekine S, Horikoshi S, Hara M, Tomino Y. Relationships between Levels of Urinary Podocalyxin, Number of Urinary Podocytes, and Histologic Injury in Adult Patients with IgA Nephropathy. Clin J Am Soc Nephrol. 2012;7:1385–1393. doi: 10.2215/CJN.08110811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hara M, Yanagihara T, Kihara I. Cumulative excretion of urinary podocytes reflects disease progression in IgA nephropathy and Schönlein-Henoch purpura nephritis. Clin J Am Soc Nephrol. 2007;2:231–8. doi: 10.2215/CJN.01470506. [DOI] [PubMed] [Google Scholar]
- 36.Wang G, Lai FM, Lai KB, Chow KM, Li KT, Szeto CC. Messenger RNA expression of podocyte-associated molecules in the urinary sediment of patients with diabetic nephropathy. Nephron Clin Pract. 2007;106:c169–79. doi: 10.1159/000104428. [DOI] [PubMed] [Google Scholar]
- 37.Zheng M, Lv LL, Ni J, Ni HF, Li Q, Ma KL, Liu BC. Urinary Podocyte-Associated mRNA profile in Various Stages of Diabetic Nephropathy. PLoS One. 2011;6:e20431. doi: 10.1371/journal.pone.0020431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.do Nascimento JF, Canani LH, Gerchman F, Rodrigues PG, Joelsons G, dos Santos M, Pereira S, Veronese FV. Messenger RNA levels of podocyte-associated proteins in subjects with different degrees of glucose tolerance with or without nephropathy. BMC Nephrology. 2013;14:214. doi: 10.1186/1471-2369-14-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mundel P, Heid HW, Mundel TM, Kruger M, Reiser J, Kriz W. Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J Cell Biol. 1997;139:193–204. doi: 10.1083/jcb.139.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moysiadis DK, Perisynakis G, Bertsias G, Stratakis S, Kyriacou K, Nakopoulou L, Boumpas DT, Daphnis E. Early treatment with glucocorticoids or cyclophosphamide retains the slit diaphragm proteins nephrin and podocin in experimental lupus nephritis. Lupus. 2012;21:1196–1207. doi: 10.1177/0961203312451784. [DOI] [PubMed] [Google Scholar]
- 41.Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim K, Reiser J, Mundel P. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med. 2008;14:931–938. doi: 10.1038/nm.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang G, Lai FM, Lai KB, Chow KM, Kwan BC, Li PK, Szeto CC. Urinary messenger RNA expression of podocyte-associated molecules in patients with diabetic nephropathy treated by angiotensin-converting enzyme inhibitor and angiotensin receptor blocker. Eur J Endocrinol. 2008;158:317–322. doi: 10.1530/EJE-07-0708. [DOI] [PubMed] [Google Scholar]
- 43.Kelly DJ, Aaltonen P, Cox AJ, Rumble JR, Langham R, Panagiotopoulos S, Jerums G, Holthöfer H, Gilbert RE. Expression of the slit-diaphragm protein, nephrin, in experimental diabetic nephropathy: differing effects of anti-proteinuric therapies. Nephrol Dial Transplant. 2002;17:1327–1332. doi: 10.1093/ndt/17.7.1327. [DOI] [PubMed] [Google Scholar]


