Abstract
It has reported that human endometrial stromal cells (ESCs) express thymic stromal lymphopoietin (TSLP), and TSLP concentrations in the serum and peritoneal fluid were higher in women with endometriosis. Endometriosis is an estrogen-dependent disease. The present study aimed to elucidate whether and how estrogen regulates the growth of ESCs through TSLP. The ESCs behaviors in vitro were verified by SRB assay and Ki67 level detection, respectively. In addition, the effects of estrogen on TSLP and TSLP on the correspondent functional molecules were investigated by ELISA and flow cytometry. Here we found that estrogen stimulated the secretion of TSLP in a dosage-dependent manner. Recombinant human TSLP stimulates the secretion of MCP-1 and IL-8, and markedly promotes the viability and proliferation relative gene Ki-67 expression of ESCs. These effects could be abolished by the inhibitor for JNK or NF-κB signal, respectively. Moreover, not only anti-TSLP neutralizing antibody, but also blocking JNK or NF-κB signal by inhibitor abrogated the stimulatory role in the production of MCP-1 and IL-8, and the growth of ESCs induced by estrogen. Our current study has demonstrated that TSLP is involved in the regulation of estrogen on the secretion of MCP-1 and IL-8, and the growth of ESCs through JNK and NF-κB signal pathways, which suggests that the abnormal high expression of TSLP induced by estrogen may play an important role in ESCs growth and finally contribute to the origin and development of endometriosis.
Keywords: TSLP, estrogen, ESCs, MCP-1, IL-8, proliferation, endometriosis
Introduction
Endometriosis is a benign gynecological disorder characterized by the presence of tissue implants resembling endometrial glands and stroma in areas outside of the uterus [1]. The ectopic implants are found most commonly in the ovaries and on the visceral and peritoneal surfaces within the pelvis. As many as 10% of women aged 30-40 years can be affected, although many more can have asymptomatic disease [2]. Symptomatic cases have been associated with deregulated cytokine productions in both ectopic and eutopic endometrium [3,4].
Endometriosis is also an estrogen-dependent disease [5]. Our previous research has also demonstrated that 17β-estradiol stimulates CCL2 secretion and CCR2 expression, and promotes the invasion of ESCs via suppressing tumor metastasis suppressor CD82 expression. The enhanced interaction of CCL2–CCR2 recruited more macrophages into the ectopic milieu, and promoted the viability, proliferation and invasiveness [6,7]. Moreover, estrogen enhances the proliferation and invasiveness of ESCs, and promotes angiogenesis in the endometriotic milieu by down-regulating the expression of tumor metastasis suppressor NME1 [8,9].
Thymic stromal lymphopoietin (TSLP) is a novel cytokine that triggers Th2 cytokines, including thymus and activation regulated chemokine (TARC), and is involved in allergic responses, IgE production, and eosinophilia [10-12]. In addition, we have shown that human first-trimester trophoblasts secrete soluble TSLP, and that TSLP is in fact an effective inducer of proliferation and invasion of human trophoblast cells in an autocrine manner through down-regulating the expression of NME1 [13,14]. The TSLP-TSLPR interaction also induces the proliferation, activation and angiogenesis of HUVECs in a paracrine manner and plays an important role in the pathogenesis of cervical cancer [15].
Recently, Urata et al have reported that TSLP concentrations in the serum and peritoneal fluid (PF) are both higher in women with endometriosis than those without endometriosis. The stroma of endometrial tissue expresses TSLP, and IL-4 can enhance the IL-1β-induced TSLP secretion from ESCs [16]. However, there are still questions whether estrogen regulates the growth of ESCs through modulating the expression of TSLP. Therefore, the present study is undertaken to identify the role and mechanism of estrogen and TSLP in the viability and proliferation of ESCs in eutopic endometrium from women with endometriosis.
Materials and methods
Tissue collection, isolation and culture of ESC
All tissue samples were obtained with informed consent in accordance with the requirements of the Research Ethics Committee in Hospital of Obstetrics and Gynecology, Fudan University Shanghai Medical College. Eutopic endometrial tissue was obtained from fertile women (age 21-46 years) with ovarian (n=20) and pelvic (n=4) endometriosis, undergoing laparoscopy for pain or other benign indications. None of the women had received hormonal medication in the 3 months prior to the surgical procedure. All the samples were confirmed histologically according to established criteria. The samples were obtained only in the proliferative phase of the cycle.
The eutopic endometrial tissues were collected under sterile conditions and transported to the laboratory on ice in DMEM (Dulbecco’s modified Eagle’s medium)/F-12 (Gibco, USA) with 10% fetal calf serum (FCS; Hyclone, Logan, UT, USA). The ESCs were isolated according to the previous methods [6,17]. Immunocytochemistry showed >95% vimentin-positive and cytokeratin-negative ESCs.
Enzyme-linked immunosorbent assay (ELISA) for TSLP concentration
The ESCs were seeded at 1×105 cells in 24-well plates and treated with various concentrations of 17-β estradiol (E2) (10-11 M to 10-7 M) in phenol red-free DMEM (GIBCO) containing 10% dextran-coated charcoal-treated FBS (Hyclone, Logan, UT, USA). The controls were treated with 0.1% dimethyl sulfoxide (DMSO). In 48 h of culture, the culture supernatant was harvested, centrifuged to remove cellular debris, and stored at -80°C until being assayed by ELISA for TSLP determination (R&D Systems, Abingdon, UK).
Treatment with E2 and anti-human TSLP neutralizing antibody
ESCs (1×105 cells/well for ELISA assay) in 24-well plates or ESCs (1×104 cells/well for flow cytometry assay) in 96-well plates were treated with E2 and or anti-human TSLP neutralizing antibody (0.25 μg/ml, R&D Systems) for 48 h, and the supernatant was collected and analyzed the concentration of MCP-1, IL-8 and IL-6 by ELISA (R&D Systems), the viability and the expression of Ki67 of ESCs were detected by SRB assay and flow cytometry, respectively.
Treatment with signal inhibitors
Moreover, ESCs were incubated with or without WP1066 (STAT3 inhibitor, 10 μM, Santa Cruz Biotechnology, inc., USA), N’-((4-Oxo-4H-chromen-3-yl) methylene) nicotinohydrazide (STAT5 inhibitor, 10 μM, Santa Cruz Biotechnology), LY294002 (AKT signal pathway, 10 μM, Santa Cruz Biotechnology) SP600125 (inhibitor for JNK signal, 10 μM, Santa Cruz Biotechnology), SB203580 (inhibitor for p38/MAPK signal, 10 μM, Santa Cruz Biotechnology), U0126 (inhibitor for ERK1/2 signal, 10 μM, Santa Cruz Biotechnology) BAY11-7080 (NF-κB inhibitor, 10 μM, Santa Cruz Biotechnology), or pyrrolidine dithiocarbamate (PDTC, an antioxidant and an inhibitor of NF-κB, 10 μM, Santa Cruz Biotechnology) for 6 h, and then treated with or without rhTSLP (10 ng/ml) or E2 (10-8 M) for 48 h, as the vehicle with control. Finally, the supernatant was collected and the concentration of MCP-1, IL-8 and IL-6 of the supernatant was analyzed by ELISA (R&D Systems), and the viability of ESCs was detected by SRB assay.
Cell viability and proliferation assays
For SRB proliferation assay, 50 μl of 30% trichloroacetic acid was added for 60 min at 4°C. After washing and drying the plate, 100 μl of 0.4% SRB was added for 30 min. Next, the plate was rinsed with 0.1% acetic acid and air dried, and 100 μl of Tris base (10 mM) was added before shaking the plate for 10 min. The SRB value was measured at a wavelength of 570 nm. The experiment was performed in sextuplicate and repeated five times.
In addition, we analyzed the proliferation of ESCs by detecting the expression of Ki67 via flow cytometry. ESCs were trypsinized and collected. The cells were resuspended and washed with PBS, fixed and permilized the member and then incubated with mouse anti-human Ki67-PE monoclonal antibody (BD, NJ, USA) for another 30 min at room temperature, meanwhile, the isotypic control was used. After incubation, the cells were washed and analyzed immediately by a FACS Calibur flow cytometer (Becton Dickinson, NJ, USA) by using Cellquest software (Becton Dickinson). Statistical analysis was conducted by using isotype matched controls. The experiments were repeated three times.
Statistics
All values are shown as the mean ± SD. One-way ANOVA analysis of variance was used to detect the difference of TSLP, MCP-1, IL-8, IL-6, and Ki67 expression and the viability in ESCs. Differences were considered as statistically significant at P<0.05.
Results
Estrogen stimulates the secretion of TSLP in ESCs
In order to evaluate whether estrogen regulates the secretion of TSLP in ESCs, we treated eutopic ESCs from women with endometriosis with different concentration of 17-β estradiol (E2) for 48 h, and analyzed the secretion level of TSLP in the culture supernatant. As depicted in Figure 1, our results showed E2 significantly promoted the secretion level of TSLP in ESCs, and the optimal stimulus concentration was 10-8 M (P<0.05 or P<0.01) (Figure 1). These results suggest that estrogen promote the production of TSLP in ESCs in a dose-dependent manner, which may participate in regulating the biological behaviors of ESCs.
Figure 1.

Estrogen stimulates the secretion of TSLP in ESCs. ESCs (1×105 cells/well) of eutopic endometrium from women with endometriosis (n=6) were seeded in 24-well plates, and treated with 17-β estradiol (10-11 M-10-7 M) for 48 h, with the vehicle as control, and then ELISA was performed to analyze the secretion level of TSLP in the supernatant. E2: 17-β estradiol. Error bars depict the standard deviation of the mean. P<0.05, **P<0.01 and ***P<0.001 compared to control. ##P<0.01 compared to 10-11 M group.
TSLP enhances the viability and proliferation of ESCs
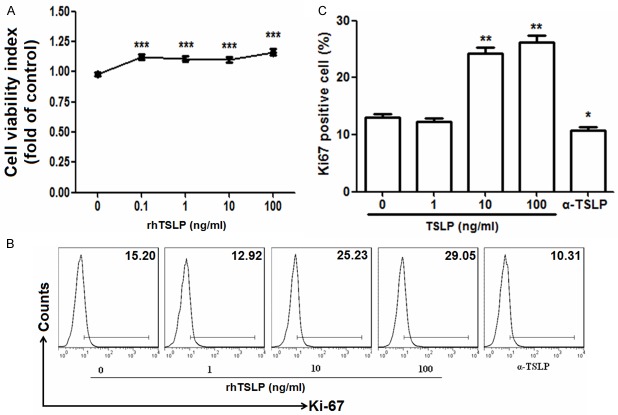
We next measured the effect of endogenous and exogenous TSLP on the viability and proliferation of ESCs. Date presented in Figure 2 showed that recombinant human TSLP (rhTSLP) from 0.1 to 100 ng/ml significantly increased the viability of ESCs (P<0.001) (Figure 2A). RhTSLP markedly up-regulated the expression of proliferation related gene Ki67 in ESCs, especially at the concentration of 10 ng/ml (P<0.01) (Figure 2B and 2C). In the contrast, blocking TSLP with anti-human TSLP neutralizing antibody (α-TSLP) decreased the expression of Ki67 in vitro (P<0.05) (Figure 2B and 2C). These results indicate that both the exogenous TSLP and TSLP derived from ESCs enhance the viability and proliferation of ESCs.
Figure 2.
TSLP enhances the viability and proliferation of ESCs. We incubated ESCs (n=6) (1×104 cells/well in 96-well plates for SRB assay; 1×105 cells/well in 96-well plates for flow cytometry) with recombinant human TSLP (rhTSLP) from 0.1 to 100 ng/ml or anti-human TSLP neutralizing antibody (0.25 μg/ml) (α-TSLP) for 48 h, and then the viability (A) and the expression of Ki67 (B, C) in ESCs were analyzed by SRB assay and flow cytometry. These pictures are representatives of three individual experiments. Data are expressed as the mean ± SD. P<0.05, **P<0.01 and ***P<0.001 as compared to control.
TSLP stimulates the secretion of MCP-1 and IL-8 and viability through JNK and NF-κB signal pathways
Taking into account the important role of cytokine in the regulation of ESC growth [4,18-21], we further investigated the influence of TSLP on MCP-1, IL-8 and IL-6 levels of ESCs. At first, we treated ESCs with different concentration of rhTSLP (0-100 ng/ml) for 48 h. Then we found that rhTSLP obviously increased the secretion of MCP-1 and IL-8 (P<0.05, P<0.01, P<0.001) (Figure 3A and 3B), but not changed IL-6 level in ESCs (P>0.05) (Figure 3C), suggesting that TSLP promote the viability and proliferation of ESCs possibly through stimulating the production of MCP-1 and IL-8.
Figure 3.

RhTSLP stimulates the secretion of MCP-1 and IL-8 of ESCs. ESCs (n=6) (1×105 cells/well) were seeded in 24-well plates, and treated with rhTSLP (0-100 ng/ml) for 48 h, and then ELISA was performed to analyze the secretion level of MCP-1 (A), IL-8 (B) and IL-6 (C) in the supernatant. Data are expressed as the mean ± SD. P<0.05, **P<0.01 and ***P<0.001 as compared to control.
In order to explore the down-stream signal of TSLP in regulating the biological behavior of ESCs, we analyzed the viability of ESCs after stimulation with rhTSLP and or different inhibitors for several signal pathways. As shown, combination of inhibitor for JNK (SB600125) or NF-κB (BAY-11-7080 and PDTC) signal significantly decreased the increase of ESCs viability induced by rhTSLP (P<0.001) (Figure 4A and 4B). However, the inhibitors for AKT, p38/MAPK, ERK1/2, STAT3 and STAT5 had no this effect (P>0.05) (Figure 4A). Our data indicated that the regulatory role of TSLP in the growth of ESCs required JNK and NF-κB signal pathways.
Figure 4.

TSLP up-regulates the production MCP-1 and IL-8 and viability of ESCs through JNK and NF-κB signal pathways. (A) The ESCs (n=6) were treated with or without inhibitor for STAT3 (WP1066, 10 μM), STAT5 [N’-((4-Oxo-4H-chromen-3-yl) methylene] nicotinohydrazide, 10 μM], AKT (LY294002, 10 μM), JNK (SP600125, 10 μM), p38/MAPK (SB203580, 10 μM), ERK1/2 (U0126, 10 μM) or NF-κB (BAY11-7080, 10 μM; PDTC, 10 μM) signal pathways for 6 h, and then incubated with rhTSLP (10 ng/ml) or α-TSLP (0.25 μg/ml) for 48 h, as the vehicle with control. The SRB assay was performed to detect the viability of these ESCs. After treatment with or without SP600125, BAY11-7080 or PDTC for 6 h, and stimulation with or without rhTSLP for another 48 h, SRB assay and ELISA were used to analyze the viability (B) and the secretion of MCP-1 (C) and IL-8 (D) of ESCs (n=6), respectively. Error bars depict the standard deviation of the mean. *P<0.05, **P<0.01, and ***P<0.001 as compared to the control. #P<0.05, ##P<0.01, ###P<0.001 as compared to rhTSLP treatment alone group. n.s.: no statistically difference.
Subsequently, in order to test whether TSLP regulates MCP-1 and IL-8 levels through JNK and NF-κB signal pathways, we performed ELISA to measure the secretion level of MCP-1 and IL-8 of ESCs treated with rhTSLP and or inhibitor for JNK or NF-κB signal. It was found that rhTSLP promoted the production of MCP-1 and IL-8 of ESCs, and this effect mediated by rhTSLP on MCP-1 could be significantly abrogated by JNK or NF-κB inhibitor (Figure 4C). However, only blocking NF-κB signals with BAY-11-7080 or PDTC could abolish the stimulatory effect on IL-8 production induced by rhTSLP (Figure 4C, left). Thus, it could be concluded that TSLP up-regulated the secretion of MCP-1 and IL-8, and therein stimulated the viability and proliferation by JNK and NF-κB signal pathways.
Estrogen promotes the viability and proliferation of ESCs by stimulating TSLP secretion
To further analyze whether estrogen regulate the growth of ESCs by TSLP, we used SRB assay and flow cytometry to detect the viability and proliferation of ESCs, after treatment with E2 (10-8 M), α-TSLP or E2 plus α-TSLP for 48 h. As shown in Figure 5, estrogen significantly promoted the viability (P<0.01 or P<0.001) (Figure 5A) and the expression of Ki67 (P<0.01) (Figure 5A) in ESCs, and these effects could be reversed by α-TSLP (Figure 5A-C). Collectively, our data indicated that estrogen was involved in increasing TSLP expression, and might further modulate the growth of ESCs.
Figure 5.

Estrogen promotes the viability and proliferation of ESCs by stimulating TSLP secretion. After incubation with E2 (10-8 M), α-TSLP (0.25 μg/ml), or E2 plus α-TSLP for 48 h, the viability (A) and the expression of Ki67 (B, C) in ESCs (n=6) were detected. Data are expressed as the mean ± SD. *P<0.05, **P<0.01, and ***P<0.001 as compared to the control. ###P<0.001 compared to E2 treatment alone group. ΔP<0.05 as compared to α-TSLP treatment alone group.
Estrogen promotes MCP-1 and IL-8 secretion and the viability of ESCs through TSLP and its downstream JNK and NF-κB signal pathways
To test whether estrogen modulates the biological function and cytokines production of ESCs by increasing TSLP expression and downstream JNK and NF-κB signals, we analyzed the secretion level of MCP-1 and IL-8, and the viability of ESCs after treatment with E2 (10-8 M), α-TSLP, E2 plus α-TSLP, inhibitor for JNK or NF-κB signal pathway, or E2 plus inhibitor for JNK or NF-κB signal pathway. As shown in Figure 6, estrogen could markedly up-regulate the expression of MCP-1 and IL-8, and the viability of ESCs (P<0.05 or P<0.01) (Figure 6A-C). In contrast, α-TSLP and inhibitor for JNK or NF-κB signal not only suppressed MCP-1 secretion and the viability of ESCs (P<0.05 or P<0.01) (Figure 6A and 6C), but also reversed the effect on MCP-1 secretion and ESCs viability induced by estrogen. However, α-TSLP and BAY-11-7080 did not inhibit NF-κB signal to reverse the increase of IL-8 production in ESCs triggered by estrogen (Figure 6A-C).
Figure 6.

Estrogen promotes MCP-1 and IL-8 secretion and the viability of DSCs through TSLP and its downstream JNK and NF-κB signal pathways. ESCs (n=6) were treated with or without SP600125, BAY11-7080 or PDTC for 6 h, and then stimulation with or without E2 and or α-TSLP for 48 h. Then ELISA and SRB assay were used to analyze the secretion of MCP-1 (A) and IL-8 (B) and the viability (C) of ESCs. Data are expressed as the mean ± SD. *P<0.05, **P<0.01, and ***P<0.001 compared to the control. #P<0.05, ##P<0.01, ###P<0.001 compared to E2 treatment alone group. ΔP<0.05 compared to α-TSLP treatment alone group. &P<0.05 compared to SP600125 or PDTC treatment alone group.
Our results demonstrate that estrogen may raise the secretion of MCP-1 and IL-8, and the viability and proliferation of ESCs by TSLP and the down-stream JNK and NF-κB signal pathways. Thus, the abnormal high level of TSLP mediated by estrogen may be involved in the pathogenesis of endometriosis through stimulating the growth of ESCs in the endometriotic milieu.
Discussion
The mechanisms contributing to the establishment of endometriotic lesions still remains controversial despite extensive research. However, an increasing body of evidence shows that the primary defect in endometriosis can be located in the eutopic endometrium. Different characteristics of the eutopic endometrium from women with endometriosis, such as aberrant production of cytokines [3,22,23], growth, adhesion and angiogenic factors as well as specific cancer-related molecules [6,8,9], are believed to contribute to the occurrence and continuation of this disease.
TSLP is an IL-7-like cytokine, originally isolated from a murine thymic stromal cell line [24] and characterized as a lymphocyte growth factor [25]. Both human and mouse TSLP exert their biological activities by binding to a high-affinity TSLP receptor (TSLPR) complex that is a heterodimer of TSLPR chain, which is closely related to the common receptor c chain (cc) and interleukin 7 receptor-a (IL-7Ra) [26,27]. TSLP is expressed mainly by epithelial cells, epidermal keratinocytes, and other types of cells such as mastocyte, smooth muscle, fibroblast, dendritic cell, trophoblast and cancer cells. This cytokine is a critical factor at interfaces between the body and environment (skin, airway, gut, placenta, and so on), which is involved in regulating Th2 cell differentiation, cell growth, invasion and angiogenesis, and participating in several physiological and pathological processes [13-15,28-32]. The report by Urata et al [16] was the first to show that the stroma cells of endometrial tissue express TSLP, and the TSLP concentrations in the serum and PF in women with endometriosis are higher than that in women without endometriosis. However, the cause and role for high level of TSLP of women with endometriosis were still unknown.
Taking into account endometriosis is an estrogen-dependent disease [5,33], as shown in Figure 7, the key finding of the present study is that up-regulation of TSLP secretion induced by estrogen can promote the viability and proliferation of ESCs. In addition, we have found that these effects are dependent on the elevated expression of downstream molecules, such as MCP-1, IL-8 and Ki67, in JNK and NF-κB signals-dependent manners. These findings suggest that estrogen may lead to the abnormal high level of TSLP from ESCs, and stimulate the proliferation and growth of ESCs in abdominal cavity, and further participate in the establishment and maintenance of endometriosis.
Figure 7.
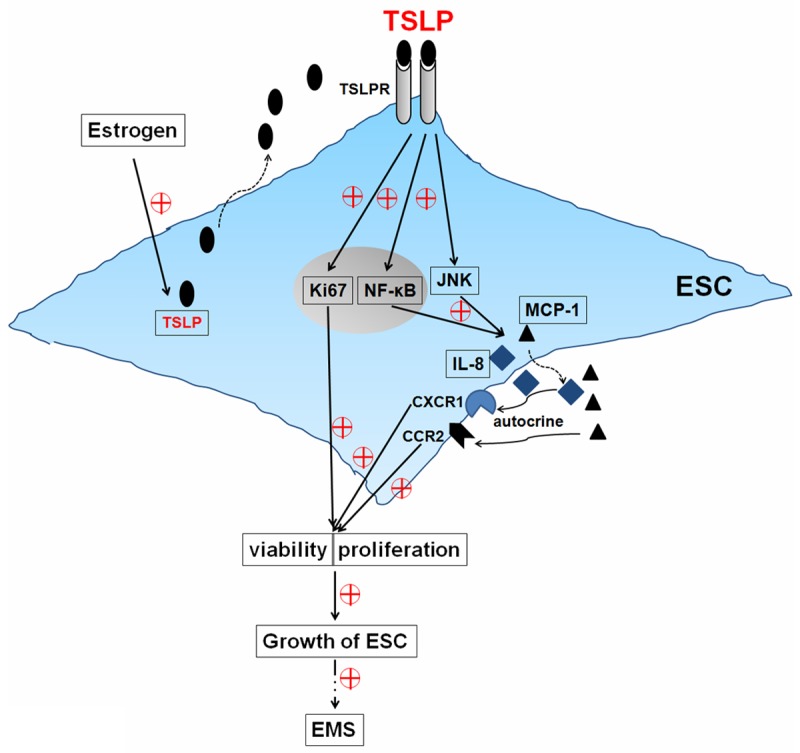
Schematic roles of estrogen and TSLP in regulating biological behaviorof ESCs. TSLP up-regulates the expression of Ki67, stimulates the secretionof MCP-1 and IL-8, and further promotes the viability and proliferation by JNK and NF-κB signal pathways in an autocrine manner. The abnormal high expression of TSLP induced by estrogen contributes to the growth of ESCs, finally participates in the origin and development of endometriosis.
Estradiol is the biologically active estrogen. It is produced in primarily three body sites (ovary, the peripheral tissues, the endometrial tissue itself) in a woman with endometriosis [34,35]. Estrogen action is mediated by two receptors, ERa and ERβ, the two transcription factors of a large family of nuclear receptors. ERβ suppresses ERα expression and results in strikingly high ERβ-to-ERα ratios in endometrial cells [35]. In our current study, 17β-estradiol stimulated the viability and Ki67 expression by stimulating TSLP secretion of eutopic ESCs from women with endometriosis in a dosage-dependent manner. However, whether these effects depend on ERβ and or ERα remains unclear.
A series of research has shown that cytokine produced in the endometriotic milieu may contribute to a feed-forward cascade of events, which changes the biological behavior of ESCs and accentuates the recruitment of leukocytes into the peritoneal cavity of patients with endometriosis [4,22]. Estrogen amplifies interleukin-1-induced monocyte chemotactic protein-1 expression by ectopic endometrial cells of women with endometriosis [22]. IL-1β has been suggested to induce TSLP secretion from ESCs. In addition, IL-1β also supports the development of endometriosis through the production of various inflammatory molecules including IL-6, IL-8 and MCP-1 [16]. Therefore, we next analyzed the expression of IL-6, IL-8 and MCP-1 of ESCs after stimulation with rhTSLP and estrogen, and found that estrogen stimulates the production of IL-8 and MCP-1 not IL-6 from ESCs through TSLP. Whether this process requires IL-1β signals also need further elucidation. Based on the role of IL-8 and MCP-1 in modulating the biological behavior of ESCs and development of endometriosis [7,17], it is not difficult to speculate the role of TSLP in endometriosis through IL-8 and MCP-1, including regulation of ESCs growth and invasion in peritoneal cavity, and the recruitment of leukocytes to local ectopic foci.
Estrogen also is involved in promoting the growth, adhesion, invasion of ESCs and angiogenesis through down-regulating the expression of NME1 [9]. And TSLP regulates the proliferation and invasion of trophoblasts by decreasing NME1 [14]. Thus, the up-regulation of TSLP induced by estrogen possible down-regulates the expression of NME1, and further modulates the biological behavior of ESCs.
Although a lot of research focused on STATs signals [36,37] in regulating cells biological function induced by TSLP, in view of the key role of several signals in cell growth, we evaluated whether the effects of estrogen and TSLP on the cytokine secretion and the growth of ESCs requires MAPK, AKT, STATs and NF-κB signal pathways. It was found that blocking JNK or NF-κB signal pathway significantly abolished these effects mediated by estrogen and TSLP, however, the inhibitor for p38/MAPK, AKT, STAT3 or STAT5 had no similar effect.
Therefore, our finding indicates that TSLP produced by ESCs increases the expression of Ki67, the secretion of MCP-1 and IL-8, and further promotes viability and proliferation of ESCs through JNK and NF-κB signal pathways. The abnormal high expression of TSLP from ESCs triggered by estrogen, on the one hand, promotes ESCs growth through Ki67, MCP-1 and IL-8; on the other hand, participates in the formation of immune microenvironment in ectopic foci through recruiting leukocytes via MCP-1 and directly educating the differentiation and development of immune cells. These integral effects will promote the survival and growth of ESCs and benefit to the origin and development of endometriosis. Further research is warranted to elucidate the functions and significance of estrogen and TSLP in the coordination between ESCs and other immune cells in peritoneal cavity, which will lead to a deeper understanding of the cross-talking mechanisms and biological functions between the immune cells and non-immune cells in the endometriotic milieu.
Acknowledgements
This study was supported by National Natural Science Foundation of China (NSFC) 31101064 to Ming-Qing Li, Ministry of Education Research Fund for Doctoral Program (20110071120092) to Ming-Qing Li; Training Program for young talents of Shanghai Health System (XYQ2013104) to Ming-Qing Li; Research Program of Shanghai Health Bureau (2011Y080) to Ming-Qing Li; Program for ZhuoXue of Fudan University to Ming-Qing Li; Project for enhancing the research ability of young teachers of Fudan University (20520133320) to Ming-Qing Li.
Disclosure of conflict of interest
None.
References
- 1.Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 2.Baldi A, Campioni M, Signorile PG. Endometriosis: pathogenesis, diagnosis, therapy and association with cancer (review) Oncol Rep. 2008;19:843–846. [PubMed] [Google Scholar]
- 3.Ulukus M, Cakmak H, Arici A. The role of endometrium in endometriosis. J Soc Gynecol Investing. 2006;13:467–476. doi: 10.1016/j.jsgi.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Barrier BF. Immunology of endometriosis. Clin Obstet Gynecol. 2010;53:397–402. doi: 10.1097/GRF.0b013e3181db7c33. [DOI] [PubMed] [Google Scholar]
- 5.Rizner TL. Estrogen metabolism and action in endometriosis. Mol Cell Endocrinol. 2009;307:8–18. doi: 10.1016/j.mce.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 6.Li MQ, Hou XF, Lv SJ, Meng YH, Wang XQ, Tang CL, Li DJ. CD82 gene suppression in endometrial stromal cells leads to increase of the cell invasiveness in the endometriotic milieu. J Mol Endocrinol. 2011;47:195–208. doi: 10.1530/JME-10-0165. [DOI] [PubMed] [Google Scholar]
- 7.Li MQ, Li HP, Meng YH, Wang XQ, Zhu XY, Mei J, Li DJ. Chemokine CCL2 enhances survival and invasiveness of endometrial stromal cells in an autocrine manner by activating Akt and MAPK/Erk1/2 signal pathway. Fertil Steril. 2012;97:919–929. doi: 10.1016/j.fertnstert.2011.12.049. [DOI] [PubMed] [Google Scholar]
- 8.Li MQ, Shao J, Meng YH, Mei J, Wang Y, Li H, Zhang L, Chang KK, Wang XQ, Zhu XY, Li DJ. NME1 suppression promotes growth, adhesion and implantation of endometrial stromal cells via Akt and MAPK/Erk1/2 signal pathways in the endometriotic milieu. Hum Reprod. 2013;28:2822–2831. doi: 10.1093/humrep/det248. [DOI] [PubMed] [Google Scholar]
- 9.Chang KK, Liu LB, Jin LP, Meng YH, Shao J, Wang Y, Mei J, Li MQ, Li DJ. NME1 suppression of endometrial stromal cells promotes angiogenesis in the endometriotic milieu via stimulating the secretion of IL-8 and VEGF. Int J Clin Exp Pathol. 2013;6:2030–2038. [PMC free article] [PubMed] [Google Scholar]
- 10.Liu YJ. Thymic stromal lymphopoietin and OX40 ligand pathway in the initiation of dendritic cell-mediated allergic inflammation. J Allergy Clin Immunol. 2007;120:238–244. doi: 10.1016/j.jaci.2007.06.004. quiz 245-246. [DOI] [PubMed] [Google Scholar]
- 11.Liu YJ, Soumelis V, Watanabe N, Ito T, Wang YH, Malefyt Rde W, Omori M, Zhou B, Ziegler SF. An epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 12.Guo PF, Du MR, Wu HX, Lin Y, Jin LP, Li DJ. Thymic stromal lymphopoietin from trophoblasts induces dendritic cell-mediated regulatory Th2 bias in the decidua during early gestation in humans. Blood. 2010;116:2061–2069. doi: 10.1182/blood-2009-11-252940. [DOI] [PubMed] [Google Scholar]
- 13.Wu HX, Guo PF, Jin LP, Liang SS, Li DJ. Functional regulation of thymic stromal lymphopoietin on proliferation and invasion of trophoblasts in human first-trimester pregnancy. Hum Reprod. 2010;25:1146–1152. doi: 10.1093/humrep/deq051. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Fan DX, Duan J, Li MQ, Zhu XY, Jin LP. Thymic stromal lymphopoietin downregulates NME1 expression and promotes invasion in human trophoblasts via the activation of STAT3 signaling pathway. Clin Immunol. 2012;143:88–95. doi: 10.1016/j.clim.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Xie F, Meng YH, Liu LB, Chang KK, Li H, Li MQ, Li DJ. Cervical carcinoma cells stimulate the angiogenesis through TSLP promoting growth and activation of vascular endothelial cells. Am J Reprod Immunol. 2013;70:69–79. doi: 10.1111/aji.12104. [DOI] [PubMed] [Google Scholar]
- 16.Urata Y, Osuga Y, Izumi G, Takamura M, Koga K, Nagai M, Harada M, Hirata T, Hirota Y, Yoshino O, Taketani Y. Interleukin-1β stimulates the secretion of thymic stromal lymphopoietin (TSLP) from endometrial stromal cells: possible involvement of TSLP in endometriosis. Hum Reprod. 2012;27:3028–3035. doi: 10.1093/humrep/des291. [DOI] [PubMed] [Google Scholar]
- 17.Li MQ, Luo XZ, Meng YH, Mei J, Zhu XY, Jin LP, Li DJ. CXCL8 enhances proliferation and growth and reduces apoptosis in endometrial stromal cells in an autocrine manner via a CXCR1-triggered PTEN/AKT signal pathway. Hum Reprod. 2012;27:2107–2116. doi: 10.1093/humrep/des132. [DOI] [PubMed] [Google Scholar]
- 18.Wu MY, Ho HN. The role of cytokines in endometriosis. Am J Reprod Immunol. 2003;49:285–296. doi: 10.1034/j.1600-0897.2003.01207.x. [DOI] [PubMed] [Google Scholar]
- 19.Kyama CM, Mihalyi A, Simsa P, Falconer H, Fulop V, Mwenda JM, Peeraer K, Tomassetti C, Meuleman C, D’Hooghe TM. Role of cytokines in the endometrial-peritoneal cross-talk and development of endometriosis. Front Biosci (Elite Ed) 2009;1:444–454. doi: 10.2741/e40. [DOI] [PubMed] [Google Scholar]
- 20.Guo Y, Chen Y, Liu LB, Chang KK, Li H, Li MQ, Shao J. IL-22 in the endometriotic milieu promotes the proliferation of endometrial stromal cells via stimulating the secretion of CCL2 and IL-8. Int J Clin Exp Pathol. 2013;6:2011–2020. [PMC free article] [PubMed] [Google Scholar]
- 21.Borrelli GM, Carvalho KI, Kallas EG, Mechsner S, Baracat EC, Abrão MS. Chemokines in the pathogenesis of endometriosis and infertility. J Reprod Immunol. 2013;98:1–9. doi: 10.1016/j.jri.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Akoum A, Jolicoeur C, Boucher A. Estradiol amplifies interleukin-1-induced monocyte chemotactic protein-1 expression by ectopic endometrial cells of women with endometriosis. J Clin Endocr Metab. 2000;85:896–904. doi: 10.1210/jcem.85.2.6348. [DOI] [PubMed] [Google Scholar]
- 23.Arici A. Local cytokines in endometrial tissue: the role of interleukin-8 in the pathogenesis of endometriosis. Ann N Y Acad Sci. 2002;955:101–109. doi: 10.1111/j.1749-6632.2002.tb02770.x. [DOI] [PubMed] [Google Scholar]
- 24.Friend SL, Hosier S, Nelson A, Foxworthe D, Williams DE, Farr A. A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp Hematol. 1994;22:321–328. [PubMed] [Google Scholar]
- 25.Sims JE, Williams DE, Morrissey PJ, Garka K, Foxworthe D, Price V, Friend SL, Farr A, Bedell MA, Jenkins NA, Copeland NG, Grabstein K, Paxton RJ. Molecular cloning and biological characterization of a novel murine lymphoid growth factor. J Exp Med. 2000;92:671–680. doi: 10.1084/jem.192.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park LS, Martin U, Garka K, Gliniak B, Di Santo JP, Muller W, Largaespada DA, Copeland NG, Jenkins NA, Farr AG, Ziegler SF, Morrissey PJ, Paxton R, Sims JE. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med. 2000;192:659–670. doi: 10.1084/jem.192.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pandey A, Ozaki K, Baumann H, Levin SD, Puel A, Farr AG, Ziegler SF, Leonard WJ, Lodish HF. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol. 2000;1:59–64. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- 28.Quentmeier H, Drexler HG, Fleckenstein D, Zaborski M, Armstrong A, Sims JE, Lyman SD. Cloning of human thymic stromal lymphopoietin (TSLP) and signaling mechanisms leading to proliferation. Leukemia. 2001;15:1286–1292. doi: 10.1038/sj.leu.2402175. [DOI] [PubMed] [Google Scholar]
- 29.Saenz SA, Taylor BC, Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev. 2008;226:172–190. doi: 10.1111/j.1600-065X.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol. 2010;11:289–293. doi: 10.1038/ni.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burgess DJ. Tumour immunology: Context is key for TSLP. Nat Rev Cancer. 2012;12:796. doi: 10.1038/nrc3405. [DOI] [PubMed] [Google Scholar]
- 32.Erdmann RB, Gartner JG, Leonard WJ, Ellison CA. Lack of functional TSLP receptors mitigates Th2 polarization and the establishment and growth of 4T1 primary breast tumours but has different effects on tumour quantities in the lung and brain. Scand J Immunol. 2013;78:408–418. doi: 10.1111/sji.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dizerega GS, Barber DL, Hodgen GD. Endometriosis: role of ovarian steroids in initiation, maintenance and suppression. Fertil Steril. 1980;33:649–653. doi: 10.1016/s0015-0282(16)44780-1. [DOI] [PubMed] [Google Scholar]
- 34.Bulun SE, Lin Z, Imir G, Amin S, Demura M, Yilmaz B, Martin R, Utsunomiya H, Thung S, Gurates B, Tamura M, Langoi D, Deb S. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev. 2005;57:359–383. doi: 10.1124/pr.57.3.6. [DOI] [PubMed] [Google Scholar]
- 35.Bulun SE, Monsavais D, Pavone ME, Dyson M, Xue Q, Attar E, Tokunaga H, Su EJ. Role of estrogen receptor-β in endometriosis. Semin Reprod Med. 2012;30:39–45. doi: 10.1055/s-0031-1299596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sebastian K, Borowski A, Kuepper M, Friedrich K. Signal transduction around thymic stromal lymphopoietin (TSLP) in atopic asthma. Cell Commun Signal. 2008;6:5. doi: 10.1186/1478-811X-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziegler SF, Roan F, Bell BD, Stoklasek TA, Kitajima M, Han H. The biology of thymic stromal lymphopoietin (TSLP) Adv Pharmacol. 2013;66:129–155. doi: 10.1016/B978-0-12-404717-4.00004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]