Abstract
Pinopode, a progesterone-dependent endometrial projection which appears during uterine receptivity period, participates in blastocyst implantation. Blastocyst loosely attaches to pinopode via L-selectin ligand (MECA-79). We hypothesized that pinopode and MECA-79 expressions were affected by testosterone. Therefore, the effect of testosterone on pinopode and MECA-79 expressions during uterine receptivity period were investigated. Methods: Ovariectomized adult female rats received 8 days sex-steroid replacement intended to mimic hormonal changes in early pregnancy with day 6 to 8 represents uterine receptivity period. Testosterone (1 mg/kg/day) was injected together with flutamide or finasteride during the period of uterine receptivity. At the end of treatment, rats were sacrificed and uteri were removed. The existence of pinopodes in the endometrium was visualized by electron microscopy and uterine expression and distribution of MECA-79 protein were examined by Western blotting and immunohistochemistry (IHC) respectively. Results: Abundant pinopodes and MECA-79 expressions were observed in rats received normal steroid replacement regime. Administration of testosterone during uterine receptivity period reduced pinopodes and MECA-79 expressions, which were antagonized by flutamide and not finasteride. Conclusions: The decrease in uterine pinopodes and MECA-79 expressions during uterine receptivity period by testosterone may cause failure of blastocyst to implant in conditions associated with high level of this hormone.
Keywords: Pinopode, testosterone, MECA-79, uterine receptivity
Introduction
Pinopodes are dome-like projections arising from the apical membrane of uterine luminal epithelia [1]. In rats, this projection appears at day 4, peaking at day 5 and rapidly decline at day 6 of early pregnancy, coincide with uterine receptivity or implantation window period [2]. Due to its existence within a limited time period, pinopode has been used as a marker of uterine receptivity [3]. The appearance of pinopodes coincides with the expression of other uterine receptivity markers such as leukaemic inhibitory factor (LIF) [4], mucin, L-selectin, integrin αvβ3 and heparin-binding epidermal growth factor (HBEGF) [5,6]. In humans however, its role as uterine receptivity marker is debatable [7].
In the uterus, pinopode displays several functions. In rodents, this projection is involved in pinocytosis which assist the removal of fluid from the uterine lumen to initiate luminal closure preceding blastocyst attachment [8]. Additionally, this bulb-like projection has been reported to participate in embryo-endometrial interaction via L-selectin ligand [9] which contains a carbohydrate epitope, MECA-79 [10]. In humans, pinopodes were found to release secretory vesicles containing LIF into the uterine lumen [11]. Pinopode expression is progesterone dependent [12]. A strong correlation between pinopode with plasma P4 level and P4 receptor expression has been reported in the uterus of humans [12], rats [1] and mice [13]. Estrogen however causes loss of pinopode expression [1]. Ovariectomized mice were found to have high number of pinopodes, suggested that the removal of E2 most probably cause the increase in pinopode expression [14].
In primates [15] and humans [16], the level of testosterone increases at around the time of ovulation and in the late luteal phase of the menstrual cycle. In mice, serum testosterone level was the highest before estrogen and luteinizing hormone (LH) peak which occur 24 hours before implantation [17]. Evidence that testosterone is required for implantation was shown by Humprey [18] where the number of implantation sites were increased with an increase in plasma testosterone level. In female, testosterone is produced by the ovaries [19] and decidua and is required for decidualization [20]. In parallel to the increase in plasma testosterone level, high expression of uterine androgen receptor was also reported in the secretory phase of the cycle [21]. Although testosterone is required for normal implantation [22], high level of this hormone could interfere with the peri-implantation embryo and uterine developments resulting in early pregnancy loss [23]. The mechanism responsible for the adverse testosterone effect on the uterus during implantation is however unknown.
We hypothesize that testosterone could affect the pinopode and MECA-79 expressions in the uterus in early pregnancy which could interfere with the blastocyst-endometrial interaction, resulting in implantation failure. This study therefore investigated the effect of testosterone on pinopode and MECA-79 expression during uterine receptivity period in rats which could explain the adverse effect of testosterone on blastocyst implantation.
Materials and methods
Chemicals
Testosterone propionate, 17β-estradiol and progesterone were purchased from Sigma-Aldrich, St. Louis, USA. Other remaining chemicals were analytical grades.
Animals and hormones treatment
Adult female Wistar rats, purchased from Animal House, University of Malaya, weighted 200-250 g were maintained under 12:12 lighting conditions, with free access to food and water ad libitum. Ovariectomies were performed under isofluorane anesthesia at least 14 days prior to initiating any hormone treatment. All procedures performed were approved by the ethics committee with ethics number: 2013-07-15/FIS/R/NS. All experiments were carried out in accordance with international guideline outlined in the Guide for the Care and Use of Laboratory Animals [24]. Steroids were dissolved in arachis oil and injected subcutaneously in a volume of 0.1 ml. To mimic the hormonal profile in early pregnancy, 17β-estradiol (E2) and progesterone (P4) were administered according to the established protocol by Kennedy [25] however with slight modifications. In brief, the basic protocol involved injection of 1.0 μg/kg/day E2 for day 1 and day 2, 1.0 μg/kg/day E2 and 4 mg/kg/day P4 on day 3, no treatment on the 4th and 5th day, 16 mg/kg/day P4 and 0.5 μg/kg/day E2 on the next 3 days (days 6-8). Description of the study design was shown in Figure 1. Vehicle treated animals (control) received 8 daily injections of arachis oil. 1 mg/kg/day testosterone, regarded as supraphysiological dose in female [26] was administered for three (3) days from day 6-8 which was considered as uterine receptivity period with and without flutamide (5 mg/kg/day) or finasteride (1 mg/kg/day) where both inhibitors were administered 30 minutes prior to the testosterone injection.
Figure 1.
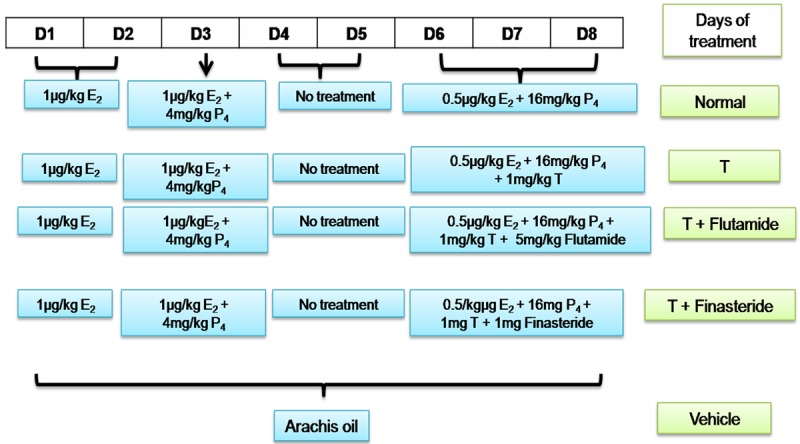
Protocol of sex-steroid replacement regime. Schematic representation of time-course of steroid treatment protocol administered to ovariectomized rats to mimic the fluctuating steroid hormones level in early pregnancy. The animals were kept at 12 hours light and 12 hours dark schedule. D: day, T: testosterone.
Field emission scanning electron microscopy (FESEM)
FESEM was performed to evaluate the presence of pinopodes on the endometrial surface. For FESEM preparation, uteri were cut longitudinally into 2 mm × 2 mm section and fixed at least 24 h in 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) at 4°C. The specimens were air-dried and then placed on carbon planchets and examined with FEG Quanta 450 EDX-OXFORD, Field Emission Scanning Electron Microscope, Netherlands at 5 kV, under low vacuum and at working distances of 11.0 mm and at magnifications of 3000 ×. Photographs were taken in slow scanning mode at 1280 × 1024 pixels.
Transmission electron microscopy (TEM)
Individual uteri were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4, and 2 mm-cross sections of each uterine horn were cut and incubated overnight at 4°C in 2.5% glutaraldehyde buffer. The buffer was removed, and the samples were rinsed three times, 15 minutes each in 0.1 M phosphate buffer. Samples were incubated in 1% osmium in 0.1 M phosphate buffer, rinsed and dehydrated in a series of ethanol (70%-100%) washes. Samples were incubated twice for 5 minutes in propylene oxide and then transferred to a rotor for 1 h at room temperature in a 1:1 mixture of propylene oxide and epon [47% Embed 812, 31% DDSA (dodenyl succinic anhydride), 19% NMA (nadic methyl anhydride), 3% BDMA (benzyldimethylamine)], followed by an overnight incubation in 1:2 propylene oxide-epon, and finally 100% epon for 2-3 hrs. Individual uterine samples were embedded in 100% epon in silicon flat embedding molds, and capsules were polymerized in a 60°C oven for over 48 hrs. Ultrathin transverse sections (70 nm) were prepared using a diamond knife (Diatome) on a MT 6000-XL ultramicrotome, captured on 300-mesh copper grids, and stained with 2% uranyl acetate. All reagents and materials were obtained from Electron Microscopy Sciences (Hatfield, PA, USA). The ultrathin sections were observed under a transmission electron microscope (Libra 120 Zeiss, Oberkochen, Germany).
Immunoperoxidase detection of MECA-79
Uterine tissues were fixed overnight in 4% paraformaldehyde (PFD) before processing and dehydrated through increasing concentrations of ethanol, cleared in chloroform and were then blocked in a paraffin wax. For IHC, tissues were cut into 5 μm sections, deparaffinized in xylene, rehydrated in reducing concentration of ethanol. Tri sodium citrate (pH 6.0) was used for antigen retrieval, while 1% H2O2 in phosphate buffered saline (PBS) was used to neutralize the endogenous peroxidase. Sections were then blocked in 5% BSA for the non-specific binding, prior to incubation with rat monoclonal primary antibody to MECA-79 (200 μg/0.1 ml, 1:500, Santa Cruz, SC 19602) at a dilution of 1:100 in 5% BSA at room temperature for an hour. After four times rinsing with PBS, the sections were then incubated with biotinylated secondary antibody for 30 min at room temperature, and were then exposed to avidin and biotinylated HRP complex (Santa Cruz, California, USA) in PBS for another half an hour. The sites of antibody binding were visualized using DAB (Diaminobenzidine HCl) (Santa Cruz, California, USA) which gave dark-brown stain. Sections were counterstained with hematoxylin for the nuclear staining. In this experiment, non-immune normal donkey serum was used as a negative control where no staining was observed.
Quantification of staining intensity by NIS-Element AR program
The slides were viewed using a Nikon Eclipse 80i microscope (Tokyo, Japan) with a Nikon DS Ri1 12 megapixel camera (Tokyo, Japan) attached. All images were captured under standard conditions of illumination. The voltage for illumination was chosen with the photographs taken at a fixed exposure time. Tiff images (1280 × 1024 pixels) were taken at objective lens magnification of 40 × and 100 ×. Using NIS-Element AR program (Nikon Instruments Inc, Melville, NY, USA), the exposure time and sensitivity were set prior to image capturing. At the outset of the session, part of the slide with no tissue (blank field) was viewed under the microscope and auto white balance was performed. Under hue-saturation-intensity (HSI) mode, the area of interest on the image (apical membrane of the uterine luminal epithelia) was selected and total counts (spots with dark-brown stained) was obtained. The mean intensity of the counts was determined which represents the average amount of protein expressed in the tissue.
Immunoblotting
Whole uterine tissues were snapped frozen in the liquid nitrogen and were then stored at -80°C prior to the protein extraction. Following total protein extraction with PRO-PREP solution (Intron, Seoul, Korea), an equal amount of protein from each tissue lysate were mixed with a loading dye, boiled for 5 min and separated using SDS-PAGE 12%. Protein was then transferred onto polyvinylidene difluoride (PVDF) membrane (BIORAD, Hercules, California, U.S) and blocked with 5% bovine serum albumin (BSA) for 90 min at a room temperature. The membrane was exposed overnight with rat monoclonal antibody to MECA-79 (200 μg/0.1 ml, 1:500, Santa Cruz, SC 19602L). Membrane incubation with non-immune normal donkey serum was used as a negative control. No bands were observed. The blots were then rinsed three times in PBS-tween with each rinse lasted for 5 minutes. The membranes were then incubated with rabbit anti rat IgG horseradish peroxidase conjugated secondary antibody (Santa Cruz, California, USA). The membrane was then rinsed and subjected to Opti-4CN™ Substrate Kit (Bio-Rad, Hercules, California, USA) to visualize the protein bands. Photos of the blots were captured by a gel documentation system and the density of each band was determined using Image J software (NIH Image J, version 1.46j, National Institute of Health, Bethesda, MD, USA). Ratio of each target band/β-actin was calculated and was considered as the expression level of the target proteins.
Statistical analyses
The density of protein bands in Western blotting was analyzed using Image-J software (version 1.46j; National Institutes of Health, Bethesda, MD, USA), and the results were presented as a ratio of the target protein to β-actin. Statistical differences were evaluated by analysis of variance (ANOVA) and Student’s t-test. A probability level of less than 0.05 (P < 0.05) was considered to be significant. Post-hoc statistical power analysis was performed for all the experiments conducted and all values obtained were > 0.8 which indicate adequate sample size. Meanwhile, the Shapiro-Wilk test was performed to test for data normality and all values obtained were > 0.05 indicated that these data were normally distributed.
Results
FESEM images of endometrial pinopodes
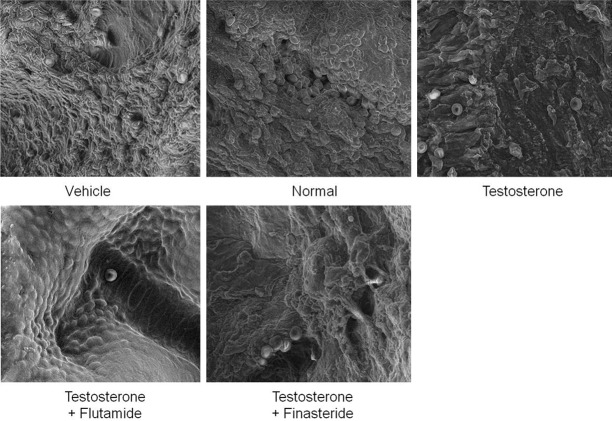
Figure 2 shows FESEM images of the endometrium in rats receiving arachis oil only (control), normal steroid replacement regime, normal steroid replacement regime with testosterone injection (days 6-8) and normal steroid replacement regime with testosterone injection (days 6-8) with flutamide or finasteride. Our findings showed abundant pinopodes expressed at the apical surface of the luminal epithelia in rats receiving normal steroid replacement. Concomitant administration of testosterone from days 6-8 resulted in a reduced pinopode expression as compared to rats receiving normal sex-steroid replacement. Meanwhile, concomitant administration of testosterone and flutamide from days 6-8 of the steroid replacement regime resulted in increased number of pinopodes at the endometrial surface as compared to testosterone only injected rats. Concomitant testosterone and finasteride treatment from days 6-8 however did not cause any significant changes in pinopodes expression as compared to testosterone only treatment.
Figure 2.
Field emission scanning electron microscopy (FESEM) of the endometrium in different experimental groups. Abundant bleb-like projections were seen in rats received normal steroid-replacement regime. Fewer pinopodes were seen following administration of testosterone during uterine receptivity period which were reverted by flutamide but not finasteride. scale bar: 40 μM.
TEM images of endometrial pinopodes
Figure 3 shows TEM images of endometrium of rats receiving arachis oil (control), normal steroid replacement, normal steroid replacement with testosterone injection (days 6-8) and normal steroid replacement with testosterone injection with flutamide or finasteride (days 6-8). Our findings showed multiple pinopodes were seen at the apical membrane of the endometrial epithelia in rats receiving normal steroid replacement regime. Testosterone administration from days 6-8 resulted in near disappearance of pinopodes with only microvilli was seen. Concomitant administration of flutamide with testosterone from days 6-8 of the normal steroid replacement regime caused the appearance of pinopodes at the apical surface. Meanwhile, few pinopodes appear in rats receiving finasteride and testosterone from days 6-8 which was almost similar to the effect of testosterone only treatment.
Figure 3.
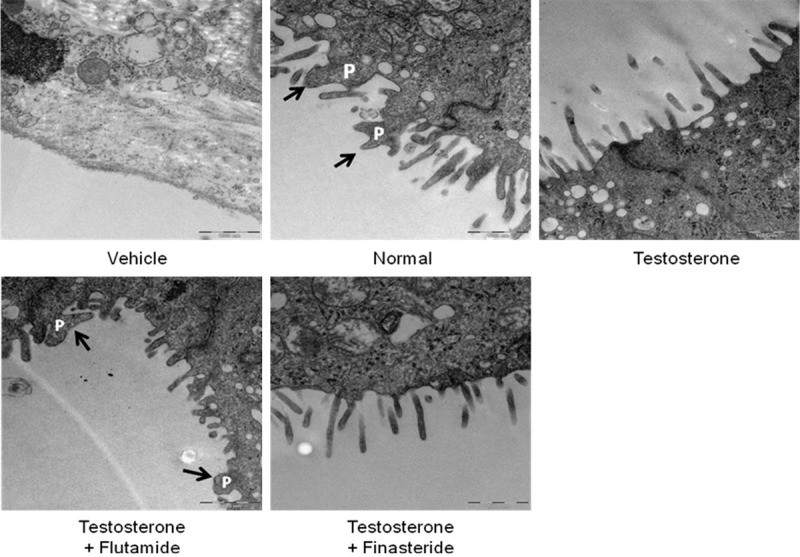
Transmission electron microscopy (TEM) of the endometrium in different experimental groups. Pinopode projections were seen in rats received normal steroid-replacement regime. Apical membrane with nearly absence pinopodes were seen following testosterone administration during uterine receptivity period which were reverted by flutamide but not finasteride. P = pinopode.
MECA-79 protein distribution
Figure 4 shows immunohistochemistry images of uterine sections while Figure 5 shows quantitative analyses of MECA-79 immunostaining intensity in different experimental groups. The highest staining intensity was observed in rats receiving normal steroid replacement regime. The staining intensity was markedly reduced following testosterone administration from days 6-8 of the normal steroid replacement regime. Concomitant administration of flutamide and testosterone from days 6-8 caused a significant increase in immunostaining intensity however no significant difference in intensity was noted following finasteride and testosterone treatment as compared to testosterone only treatment.
Figure 4.
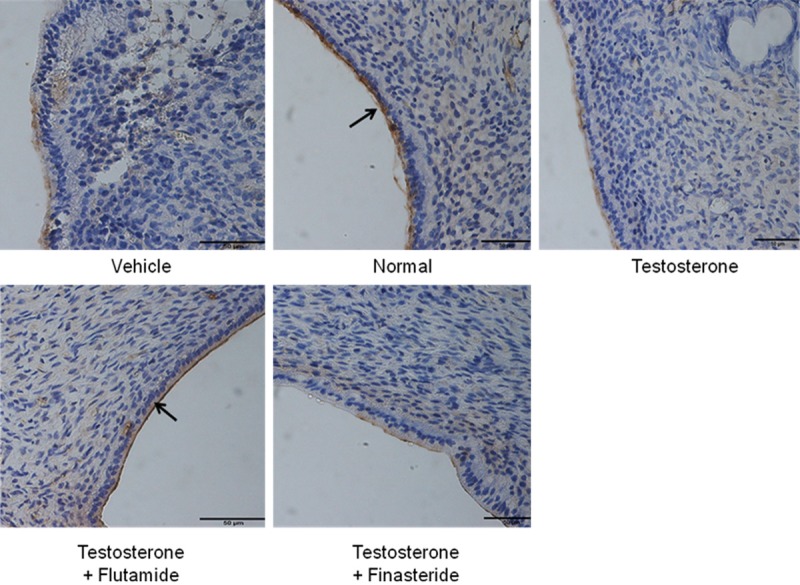
MECA-79 protein distribution at the apical membrane of the endometrial luminal epithelia in different experimental group. MECA-79 was seen to be distributed at the apical membrane of the luminal epithelia predominantly in rats received normal steroid replacement and concomitant testosterone and flutamide administration during uterine receptivity period. L: lumen, G: gland, scale bar: 50 μm. Arrow pointing towards the apical membrane with highest immunostaining intensity.
Figure 5.
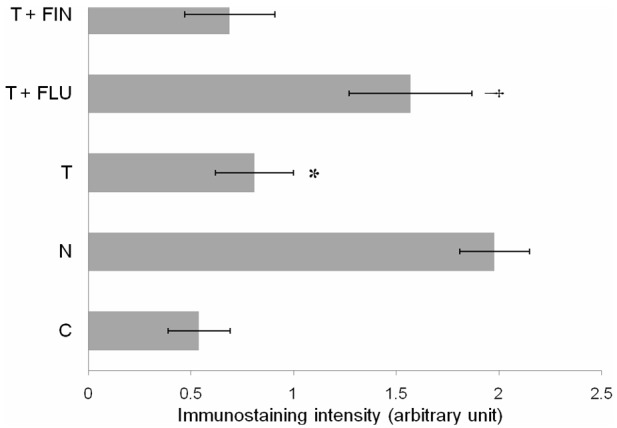
Quantitative analysis of MECA-79 immunoperoxidase staining intensity at the apical membrane of the luminal epithelia in different experimental groups. The highest intensity was observed in rats received normal steroid replacement regime and following co-administration of flutamide with testosterone during uterine receptivity period. Mean was obtained from intensity of 4 different sections per treatment group. *P < 0.05 as compared to normal steroid replacement, †P < 0.05 as compared with testosterone only treatment. C: vehicle control, N: normal steroid replacement regime, T: testosterone only injection with P4, T + FLU: testosterone and flutamide administration with P4 and T + FIN: testosterone and finasteride administration with P4.
MECA-79 protein expression
Figure 6A shows representative image of a Western blot band while Figure 6B shows analyses of protein band density of the uterine tissue homogenate following incubation with MECA-79 antibody in rats treated with arachis oil (vehicle control), normal steroid replacement regime, normal steroid replacement regime with testosterone (days 6-8) and normal steroid replacement regime with testosterone combined with flutamide or finasteride (days 6-8). Our findings indicate that density of MECA-79 protein band was the highest in rats receiving normal steroid replacement regime and the lowest following testosterone only treatment from days 6-8. Co-administration of flutamide and testosterone from days 6-8 resulted in a significant increase in the protein band density. Meanwhile, no significant difference was noted between concomitant testosterone and finasteride as compared to testosterone only treatments.
Figure 6.
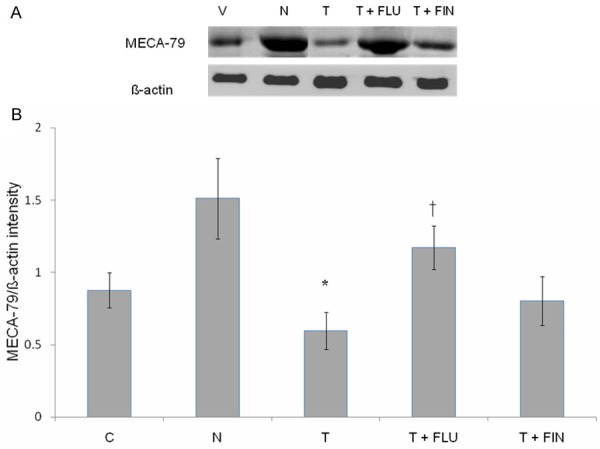
Immunoblotting analysis of MECA-79 protein in different experimental group. (A) Shows immunoblot image of protein band in different experimental group while (B) Shows analyses of the intensity of protein bands in different experimental groups. Our findings indicate that the highest MECA-79 protein intensity was observed in the group received normal sex-steroid replacement while the lowest intensity was observed following administration of testosterone during the uterine receptivity period. Mean was obtained from intensity of 4 different membranes per treatment group. *P < 0.05 as compared to normal steroid replacement, †P < 0.05 as compared with testosterone only treatment. C: vehicle control, N: normal steroid replacement regime, T: testosterone only injection with P4, T + FLU: testosterone and flutamide administration with P4 and T + FIN: testosterone and finasteride administration with P4.
Discussion
To the best of our knowledge, this study investigated for the first time the effect of testosterone on pinopode expression in rats during uterine receptivity period. In our study, the followings were observed: (i) testosterone administration from days 6-8 (uterine receptivity period) caused significant decrease in pinopode expression at the apical surface of the endometrium, (ii) testosterone administration (days 6-8) resulted in decreased expression of MECA-79 at the apical surface of the uterine luminal epithelia (iii) testosterone effect was antagonized by flutamide, which confirmed androgen receptor involvement and (iv) testosterone effects were not antagonized by finasteride, indicating that 5α-dihydrotestosterone (DHT) was not involved in causing these effects.
We have shown that in rats received normal steroid replacement regime, pinopodes appeared moderately wrinkled with oblong shaped structure protruding from the apical membrane of the luminal epithelium with a diameter of between 3.0 to 4.0 μm. These morphological appearances were consistent with the previous reports [27,28]. In addition, pinopodes were seen to project above the level of microvilli and contain multiple vacuoles. Several larger vacuoles were also seen at the base of these projections. These vacoules were thought to be involved in endocytosis which help to remove the uterine luminal fluid to initiate uterine closure [28,29]. In rats, uterine closure event is important as this could help to sandwich the implanting blastocyst between the two opposing endometrial surfaces, initiating the first physical contact between the blastocyst and the receptive endometrium. Parr and Parr, [28] reported that pinopode also contain actin-rich stalk which appears as bands on the pedicle. Meanwhile, it was estimated that in rats, between 5.5% to 20% of the endometrial epithelial cells were covered with pinopodes during the uterine receptivity period that could help to generate sufficient force to remove the fluid from uterine lumen [14].
In view that pinopode is progesterone-dependent [2], the appearance of this projection was expected following this hormone treatment. Concomitant administration of testosterone resulted in a near absence of pinopode from the endometrial surface. The involvement of androgen receptor (AR) indicated that testosterone effect was mediated via a genomic pathway. Our study has further shown that 5α-DHT was not involved in inhibiting uterine pinopode expression as evident from lack of finasteride inhibition on the testosterone-mediated decrease in pinopode expression. Uterine tissue has been reported to have higher affinity towards testosterone than 5α-DHT [30]. However, several uterine effect of testosterone has been shown to be mediated via 5α-DHT, including expression of estrogen receptor (ER) [31] as well as growth of this organ [32]. Additionally, 5α-DHT is also important during the secretory phase of the cycle in view that AR and 5α-reductase enzyme expressions in the uterus were reported the highest during this period [33].
L-selectin ligand (MECA-79) mediates blastocyst-endometrial contact prior to blastocyst attachment [34]. Our findings showed that MECA-79 expression was the highest in the endometrium of ovariectomized rats received normal steroid replacement regime. This protein was expressed mainly at the apical membrane of the uterine luminal epithelia, consistent with its role in embryo attachment to the receptive endometrium. In humans, L-selectin ligand was up-regulated from the day of ovulation to day 6-post ovulation [10] with peak expression in the mid-luteal phase of the cycle [35]. In addition, endometrial MECA-79 expression was reported to be higher in fertile than infertile humans [36]. In this study, a marked decrease in MECA-79 expression at the apical membrane of rat endometrial epithelia during uterine receptivity period following testosterone treatment indicated that the expression of this protein was inhibited by testosterone. In parallel with the effect on pinopode expression, testosterone effect on MECA-79 expression was also inhibited by flutamide, indicated the involvement of AR-mediated genomic mechanism. Meanwhile, 5α-DHT has no role in MECA-79 expression as evident from the lack of inhibition by 5α-reductase inhibitor on testosterone-mediated suppression of MECA-79 expression in the uterus during the receptive period.
This study has important contribution towards understanding the mechanism underlying testosterone effect on blastocyst implantation as blastocyst fails to implant in situation where testosterone level was high for example in polycystic ovarian disease (PCO) [37]. With high rate of implantation failure even after ovulation induction, the reduced fertility rate in PCO patient was not only due to anovulation however could also be due to endometrial dysfunction [38]. In PCO, other biomarkers of endometrial receptivity such as α(v)β3-integrin and glycodelin-were also decreased, while epithelial expression of estrogen receptor alpha (ERα) abnormally persists throughout the implantation window period. Additionally, stromal decidualization was also inhibited by the high insulin level [39] while expression of androgen receptor in the uterus was highly elevated in this condition [40]. In this study, we further reported that down-regulation of uterine pinopodes and L-selectin ligand expressions by testosterone could also contribute to failure of the blastocyst to implant, resulting in high infertility rate associated with this condition.
In conclusion, this study has provided mechanism underlying implantation failure as reported in female with high plasma testosterone level. The suppression of pinopode and L-selectin ligand expressions by testosterone could prevent initiation of physical contact between blastocyst and the receptive endometrium thus contributing to high infertility rate observed in this condition.
Acknowledgements
This study is supported by RG404/12 HTM and PG007-2013B, University of Malaya, Kuala Lumpur.
Disclosure of conflict of interest
None.
References
- 1.Martel D, Monier MN, Roche D, Psychoyos A. Hormonal dependence of pinopode formation at the uterine luminal surface. Hum Reprod. 1991;6:597–603. doi: 10.1093/oxfordjournals.humrep.a137386. [DOI] [PubMed] [Google Scholar]
- 2.Singh MM, Chauhan SC, Trivedi RN, Maitra SC, Kamboj VP. Correlation of pinopod development on uterine luminal epithelial surface with hormonal events and endometrial sensitivity in rat. Eur J Endocrinol. 1996;135:107–117. doi: 10.1530/eje.0.1350107. [DOI] [PubMed] [Google Scholar]
- 3.Nikas G, Drakakis P, Loutradis D, Mara-Skoufari C, Koumantakis E, Michalas S, Psychoyos A. Implantation: Uterine pinopodes as markers of the ‘nidation window’ in cycling women receiving exogenous oestradiol and progesterone. Hum Reprod. 1995;10:1208–1213. doi: 10.1093/oxfordjournals.humrep.a136120. [DOI] [PubMed] [Google Scholar]
- 4.Ding T, Song H, Wang X, Khatua A, Paria BC. Leukemia inhibitory factor ligand-receptor signaling is important for uterine receptivity and implantation in golden hamsters (Mesocricetus auratus) Reproduction. 2008;135:41–53. doi: 10.1530/REP-07-0013. [DOI] [PubMed] [Google Scholar]
- 5.Aghajanova L, Hamilton AE, Giudice LC. Uterine receptivity to human embryonic implantation: Histology, biomarkers, and transcriptomics. Sem Cell Dev Biol. 2008;19:204–211. doi: 10.1016/j.semcdb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Achache H, Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum Reprod Update. 2006;12:731–746. doi: 10.1093/humupd/dml004. [DOI] [PubMed] [Google Scholar]
- 7.Quinn CE, Casper RF. Pinopodes: a questionable role in endometrial receptivity. Hum Reprod Update. 2009;15:229–236. doi: 10.1093/humupd/dmn052. [DOI] [PubMed] [Google Scholar]
- 8.Parr MB, Parr PE. Relationship of apical domes in the rabbit uterine epithelium during the peri-implantation period to endocytosis apocrine secretion and fixation. J Reprod Fertil. 1982;66:739–744. doi: 10.1530/jrf.0.0660739. [DOI] [PubMed] [Google Scholar]
- 9.Nejatbakhsh R, Kabir-Salmani M, Dimitriadis E, Hosseini A, Taheripanah R, Sadeghi Y, Akimoto Y, Iwashita M. Subcellular localization of L-selectin ligand in the endometrium implies a novel function for pinopodes in endometrial receptivity. Reprod Biol Endocrinol. 2012;10:46. doi: 10.1186/1477-7827-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shamonki MI, Kligman I, Shamonki JM, Schattman GL, Hyjek E, Spandorfer SD, Zaninovic N, Rosenwaks Z. Immunohistochemical expression of endometrial L-selectin ligand is higher in donor egg recipients with embryonic implantation. Fertil Steril. 2006;86:1365–1375. doi: 10.1016/j.fertnstert.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 11.Kabir-Salmani M, Nikzad H, Shiokawa S, Akimoto Y, Iwashita M. Secretory role for human uterodomes (pinopods): secretion of LIF. Mol Hum Reprod. 2005;11:553–559. doi: 10.1093/molehr/gah218. [DOI] [PubMed] [Google Scholar]
- 12.Stavreus-Evers A, Nikas G, Sahlin L, Eriksson H, Landgren BM. Formation of pinopodes in human endometrium is associated with the concentrations of progesterone and progesterone receptors. Fertil Steril. 2001;76:782–791. doi: 10.1016/s0015-0282(01)01993-8. [DOI] [PubMed] [Google Scholar]
- 13.Rashidi B, Rad JS, Roshangar L, Miran RA. Progesterone and ovarian stimulation control endometrial pinopode expression before implantation in mice. Pathophysiology. 2012;19:131–135. doi: 10.1016/j.pathophys.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Quinn CE, Detmar J, Casper RF. Pinopodes are present in Lif null and Hoxa10 null mice. Fertil Steril. 2007;88:1021–1028. doi: 10.1016/j.fertnstert.2006.11.157. [DOI] [PubMed] [Google Scholar]
- 15.Rao AJ, Kotagi SG. Serum Testosterone Levels during the Menstrual Cycle and Early Pregnancy in the Bonnet Monkey (Macaca Radiata) Endocrinol Jap. 1982;29:271–275. doi: 10.1507/endocrj1954.29.271. [DOI] [PubMed] [Google Scholar]
- 16.Persky H, Lief HI, Strauss D, Miller WR, O’Brien CP. Plasma testosterone level and sexual behavior of couples. Arch Sex Behav. 1978;7:157–173. doi: 10.1007/BF01542376. [DOI] [PubMed] [Google Scholar]
- 17.Barkley MS, Michael SD, Geschwind II, Bradford GE. Plasma testosterone during pregnancy in the mouse. Endocrinology. 1977;100:1472–1475. doi: 10.1210/endo-100-5-1472. [DOI] [PubMed] [Google Scholar]
- 18.Humphreys EM, Ghione R, Gosden RG, Hobson BM, Wide L. Relationship between corpora lutea or fetal number and plasma concentrations of progesterone and testosterone in mice. J Reprod Fertil. 1985;75:7–15. doi: 10.1530/jrf.0.0750007. [DOI] [PubMed] [Google Scholar]
- 19.Fogle RH, Stanczyk FZ, Zhang X, Paulson RJ. Ovarian Androgen Production in Postmenopausal Women. J Clin Endocrinol Metab. 2007;92:3040–3043. doi: 10.1210/jc.2007-0581. [DOI] [PubMed] [Google Scholar]
- 20.Kramen MA, Johnson DC. Uterine decidualization in rats given testosterone propionate neonatally. J Reprod Fertil. 1975;42:559–562. doi: 10.1530/jrf.0.0420559. [DOI] [PubMed] [Google Scholar]
- 21.Mertens HJ, Heineman MJ, Theunissen PH, de Jong FH, Evers JL. Androgen, estrogen and progesterone receptor expression in the human uterus during the menstrual cycle. Eur J Obstet Gynecol Reprod Biol. 2001;98:58–65. doi: 10.1016/s0301-2115(00)00554-6. [DOI] [PubMed] [Google Scholar]
- 22.Lu Q, Shen H, Li Y, Zhang C, Wang C, Chen X, Liang R, Wei L. Low testosterone levels in women with diminished ovarian reserve impair embryo implantation rate: a retrospective case-control study. J Assist Reprod Genet. 2014;31:485–91. doi: 10.1007/s10815-014-0186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diao HL, Su RW, Tan HN, Li SJ, Lei W, Deng WB, Yang ZM. Effects of androgen on embryo implantation in the mouse delayed-implantation model. Fertil Steril. 2008;90:1376–1383. doi: 10.1016/j.fertnstert.2007.07.1341. [DOI] [PubMed] [Google Scholar]
- 24.Health. TNACRfbNIo. Guide for the Care and Use of Laboratory Animals. Washington (DC): National Academies Press (US); 2011. [PubMed] [Google Scholar]
- 25.Kennedy TG. Intrauterine infusion of prostaglandins and decidualization in rats with uteri differentially sensitized for the decidual cell reaction. Biol Reprod. 1986;34:327–335. doi: 10.1095/biolreprod34.2.327. [DOI] [PubMed] [Google Scholar]
- 26.Dehghan F, Yusof A, Salleh N. Sex-Steroid Regulation of Relaxin Receptor Isoforms (RXFP1 & RXFP2) Expression in the Patellar Tendon and Lateral Collateral Ligament of Female WKY Rats. Int J Med Sci. 2014;11:180–191. doi: 10.7150/ijms.6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enders A, Nelson DM. Pinocytotic activity of the uterus of the rat. Am J Anat. 1973;138:277–300. doi: 10.1002/aja.1001380302. [DOI] [PubMed] [Google Scholar]
- 28.Parr MP, Parr EL. Uterine luminal epithelium: protrusions mediate endocytosis not apocrine secretion in the rat. Biol Reprod. 1974;11:220–233. doi: 10.1095/biolreprod11.2.220. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson O. Ultrastructure of the process of secretion in the rat uterine epithelium at preimplantation. J Ultrastruct Res. 1972;40:572–580. doi: 10.1016/s0022-5320(72)80044-3. [DOI] [PubMed] [Google Scholar]
- 30.Giannopoulos G. Binding of Testosterone to Uterine Components of the Immature Rat. J Biol Chem. 1973;248:1004–1010. [PubMed] [Google Scholar]
- 31.Cárdenas H, Pope WF. Estrogen receptors in the uterus and ovarian follicles of gilts treated with dihydrotestosterone. Domest Anim Endocrinol. 2005;29:523–533. doi: 10.1016/j.domaniend.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Sun Y, Liu Y, Sun Y, Liao DJ. Synergistic effects of androgen and estrogen on the mouse uterus and mammary gland. Oncol Rep. 2004;12:709–716. [PubMed] [Google Scholar]
- 33.Ito K, Suzuki T, Akahira JI, Moriya T, Kaneko C, Utsunomiya H, Yaegashi N, Okamura K, Sasano H. Expression of androgen receptor and 5α-reductases in the human normal endometrium and its disorders. Int J Cancer. 2002;99:652–657. doi: 10.1002/ijc.10394. [DOI] [PubMed] [Google Scholar]
- 34.Genbacev OD, Prakobphol A, Foulk RA, Krtolica AR, Ilic D, Singer MS, Yang ZQ, Kiessling LL, Rosen SD, Fisher SJ. Trophoblast L-selectin-mediated adhesion at the maternal-fetal interface. Science. 2003;299:405–408. doi: 10.1126/science.1079546. [DOI] [PubMed] [Google Scholar]
- 35.Lai TH, Shih IM, Vlahos N, Ho CL, Wallach E, Zhao Y. Differential expression of L-selectin ligand in the endometrium during the menstrual cycle. Fertil Steril. 2005;83:1297–1302. doi: 10.1016/j.fertnstert.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 36.Margarit L, Gonzalez D, Lewis PD, Hopkins L, Davies C, Conlan RS, Joels L, White JO. L-Selectin ligands in human endometrium: comparison of fertile and infertile subjects. Hum Reprod. 2009;24:2767–2777. doi: 10.1093/humrep/dep247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi MH, Lee SH, Kim HO, Cha SH, Kim JY, Yang KM, Song IO, Koong MK, Kang IS, Park CW. Comparison of assisted reproductive technology outcomes in infertile women with polycystic ovary syndrome: In vitro maturation, GnRH agonist, and GnRH antagonist cycles. Clin Exp Reprod Med. 2012;39:166–171. doi: 10.5653/cerm.2012.39.4.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shang K, Jia X, Qiao J, Kang J, Guan Y. Endometrial Abnormality in Women With Polycystic Ovary Syndrome. Reprod Sci. 2012;19:674–683. doi: 10.1177/1933719111430993. [DOI] [PubMed] [Google Scholar]
- 39.Giudice LC. Endometrium in PCOS: Implantation and predisposition to endocrine CA. Best practice & research. Best Pract Res Clin Endocrinol Metab. 2006;20:235–244. doi: 10.1016/j.beem.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Apparao KBC, Lovely LP, Gui Y, Lininger RA, Lessey BA. Elevated Endometrial Androgen Receptor Expression in Women with Polycystic Ovarian Syndrome. Biol Reprod. 2002;66:297–304. doi: 10.1095/biolreprod66.2.297. [DOI] [PubMed] [Google Scholar]