Abstract
Severe primary hypothyroidism is a presumed rare cause of pseudoprecocious puberty (PsPP). Here, we report a 24% incidence of PsPP among 33 children with profound hypothyroidism. Those with PsPP were older and trended toward a higher thyroid stimulating hormone. Increased awareness of PsPP can hasten diagnosis and appropriate treatment.
Early secondary sexual development in the setting of profound primary hypothyroidism is a form of pseudoprecocious puberty (PsPP), also known as Van Wyk-Grumbach syndrome. In addition to classic features of hypothyroidism such as delayed linear growth and skeletal maturation, affected girls have breast development, galactorrhea, and/or vaginal bleeding, and boys have testicular enlargement without virilization.1 Pelvic ultrasounds in girls demonstrate large multicystic ovaries and an enlarged uterus.2 Biochemical evaluation reveals a markedly elevated thyroid stimulating hormone (TSH) and low thyroxine, elevated prolactin, normal to high estradiol and follicle stimulating hormone (FSH), and a suppressed luteinizing hormone level consistent with profound hypothyroidism and FSH-predominant gonadotropin-independent puberty.1-6 FSH-predominance is believed to be due to a prolactin-induced reduction in the frequency of gonadotropin-releasing hormone pulsatility, a condition that increases FSH expression while inhibiting luteinizing hormone expression.7
Although rare, the exact incidence of PsPP attributable to hypothyroidism is unknown. Whether there are different features of children with primary hypothyroidism who develop PsPP compared with those who do not is similarly unknown. The aim of this study was to determine the incidence of PsPP in a cohort of children with profound primary hypothyroidism. In addition, we sought to determine whether there were differences in the characteristics of children with PsPP compared with those who had hypothyroidism alone.
Methods
Following institutional review board approval, a retrospective review was performed for all children diagnosed with Hashimoto thyroiditis (International Classification of Diseases, 9th Revision code 245.2) over a 10-year period at our institution. Children with conditions known to affect growth and puberty were excluded. Children with an initial TSH ≥100 mIU/L were considered for further analysis. Of these, only children with a prepubertal chronologic age (CA) and/or bone age (BA) (defined as ≤9 years in boys and ≤8 years in girls) were included in the final analysis. The history of presenting illness, review of systems, physical examination, anthropometric information, BA, and initial TSH were extracted from the medical record. TSH values reported as “greater than X” were conservatively captured as “X” for statistical interpretation. PsPP was defined as a testicular volume ≥4 mL in boys and breast development and/or vaginal bleeding in girls.
Data was analyzed with SPSS v. 19 (SPSS Inc, Chicago, Illinois). A test of normality was performed. For data not normally distributed (TSH, BA-SDS, body mass index [BMI]-SDS), the Mann-Whitney U test was used. The remaining normally distributed outcomes were analyzed using 2-tail t tests. P values of <.05 were considered statistically significant.
Results
A total of 785 children with Hashimoto thyroiditis (International Classification of Diseases, 9th Revision, code 245.2) were identified; 62 of these children were found to have an initial TSH ≥100 mIU/L (78% female, CA of 10.2 ± 2.9 years, height-SDS −1.4 ± 1.9, weight-SDS 0.5 ± 1.9, and BMI-SDS 1.4 ± 1.1). A goiter was documented and in 34%. Twenty-three of these children had adult height data available, 56% of whom had final heights more than 2 SDS below their target height. Thirty-three of the 62 children were of prepubertal CA and/or BA and were, thus, included in final analysis (79% female, mean CA of 8.6 ± 2.7 [range 3.5-14.3] years, height-SDS −1.9 ± 1.9, weight-SDS of 0.4 ± 2.0, and BMI-SDS of 1.6 ± 0.9).
Eight children (24%) had evidence of PsPP (75% female) of whom 1 had a goiter (Table). Of the 6 girls, 4 had vaginal bleeding interpreted as menarche in 3, and 2 had isolated breast development without vaginal bleeding. Both boys had isolated testicular enlargement. All patients had stabilization or regression of pubertal development following initiation of thyroid hormone replacement.
Table.
Baseline characteristics of 8 children presenting with profound primary hypothyroidism and PsPP
| Pt | Sex | Reason for lab draw/referral |
Symptoms exposed by medical doctor |
CA(y) | BA (y) | BA-SDS | Height- SDS |
Weight- SDS |
BMI- SDS |
Goiter | Breast stage* |
PH stage |
Vaginal bleed |
Testes volume |
TSH (mIU/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | Vaginal bleeding | 6.8 | 4.2 | −3.8 | −0.9 | 1.6 | 2.3 | N | 2 | 1 | Y | 2632 | ||
| 2 | F | Fatigue, constipation | 10.1 | 6.8 | −3.7 | −1.2 | −0.7 | 0.1 | Y | 2 | 1 | N | >600 | ||
| 3 | F | LENG | Vaginal bleeding | 10.8 | 7.0 | −3.7 | −1.9 | 0.5 | 1.7 | N | 4 | 1 | Y × 1 | 648 | |
| 4 | F | LENG | 11.2 | 7.8 | −4.1 | −2.3 | 0.2 | 1.6 | N | 3 | 1 | N | 814 | ||
| 5 | F | LENG | Vaginal bleeding | 11.2 | 7.0 | −3.3 | −1.4 | −0.2 | 0.8 | N | 3 | 1 | Y × 2 | 11 553 | |
| 6 | F | Weight gain, fatigue | Vaginal bleeding, galactorrhea |
14.3 | 8.0 | −6.0 | −4.6 | 0.4 | 2.0 | N | 1 | 2 | Y × 3 | 430 | |
| 7 | M | LENG | 4.3 | 1.3 | −13.5 | −4.6 | −2.0 | 2.0 | N | 1 | 1 | 4mL | 967 | ||
| 8 | M | LENG | 13.3 | 7.0 | −6.8 | −3.8 | −2.2 | 0.4 | N | 1 | 2 | 8mL | >170 |
F, female; LENG, lack of expected normal growth; M, male; N, no (not present); PH, pubic hair Tanner stage; Pt, patient; Y, yes (present).
Breast stage = breast Tanner stage.
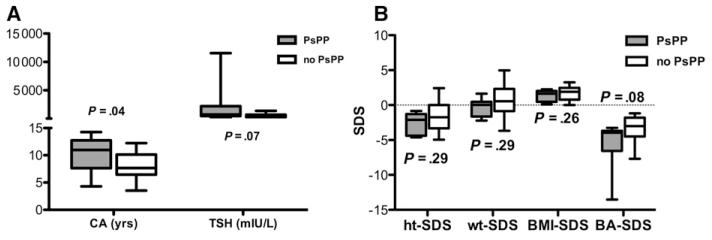
Compared with the 25 children without PsPP, children with PsPP were older (10.2 vs 8.1 years, P = .04) and trended toward a higher TSH (2226.8 vs 534.0, P = .07; Figure, A). A trend toward a more delayed BA-SDS was also seen (−5.6 vs −3.5, P = .08), although no differences in height-SDS, weight-SDS, or BMI-SDS were found (Figure, B).
Figure.
A, Comparison of CA and TSH at presentation in children with (gray) and without (white) PsPP. B, Comparison of height-SDS (ht-SDS), weight-SDS (wt-SDS), BMI-SDS, and BA-SDS at presentation in children with (gray) and without (white) PsPP. Data is shown as box-and-whiskers plot.
Discussion
Although the exact cause of PsPP is unknown, suggested mechanisms include cross-reactivity at the structurally similar FSH and TSH glycoprotein receptors, which share a common a subunit8 or direct stimulation of FSH release by elevated thyroid releasing hormone1 resulting in an FSH/estradiol-predominant phenotype. Despite knowledge of this entity since 1960, most reports of PsPP are sporadic cases, implying rarity. In our institution, however, PsPP was present in nearly one-quarter of prepubertal children with severe primary hypothyroidism, suggesting the incidence may be significantly higher than classically referenced. Further, the incidence may actually be higher than reported here as we did not include those children with CA and/or BA in the pubertal age range.
This study compared clinical features of children with and without PsPP in a cohort of patients with profound primary hypothyroidism. Although our study design precludes statements of causation, the older age and trend toward higher TSH and greater BA delay suggests that PsPP may result from prolonged exposure to markedly elevated circulating TSH levels in a dose-dependent manner. Our study likely underestimated differences in these measurements because we conservatively used a value of “X” when the TSH was above the limit of detection (reported as TSH >X), and sex steroids could have decreased the degree of BA delay in patients with PsPP at diagnosis. Alternatively, peripubertal aged children may have FSH receptors and/or a pubertal axis “primed” to respond to circulating TSH. PsPP appears to be underappreciated as pathologic by families and primary care providers as it can occur at a suitable CA for pubertal on-set but at a time that is inappropriate for the BA. For example, vaginal bleeding was interpreted as menarche in 4 of 6 girls despite classic features of hypothyroidism and severely delayed linear growth. This lack of recognition is likely to contribute to the delay in diagnosis.
Although our study was not specifically geared toward height outcomes, it is notable that more than one-half of our patients with profound hypothyroidism with adult height data were greater than −2 SDs from their target height. This is similar to what has been previously reported and does not appear to be ameliorated by the addition of growth promoting therapies.9 Unfortunately, a marked acceleration in the rate of skeletal maturation invariably occurs following initiation of thyroid hormone in the setting of profound hypothyroidism. Longer term studies capturing adult height within this population should better elucidate growth outcomes and spark discussions of the role of pubertal suppression and/or growth hormone therapy to augment final height.
It is expected that improved recognition of primary hypothyroidism as a cause of PsPP will attenuate unnecessary tests and expedite referral to pediatric endocrinology for initiation of hormone replacement. Prospective studies including careful investigation of the reproductive and thyroidal axes in children with PsPP would likely yield novel insights into the pathophysiology of this paradoxical phenomenon.
Glossary
- BA
Bone age
- BMI
Body mass index
- CA
Chronologic age
- FSH
Follicle stimulating hormone
- PsPP
Pseudoprecocious puberty
- TSH
Thyroid stimulating hormone
Footnotes
The authors declare no conflicts of interest.
This study was presented in part as a poster at the Pediatric Academic Societies’ Meeting, Boston, MA, April 28-May 1, 2012.
References
- 1.Van Wyk JJ, Grumbach MM. Syndrome of precocious menstruation and galactorrhea in juvenile hypothyroidism: an example of hormonal overlap in pituitary feedback. J Pediatr. 1960;57:416–35. [Google Scholar]
- 2.Browne LP, Boswell HB, Crotty EJ, O’Hara SM, Birkemeier KL, Guillerman RP. Van Wyk and Grumbach syndrome revisited: imaging and clinical findings in pre- and postpubertal girls. Pediatr Radiol. 2008;38:538–42. doi: 10.1007/s00247-008-0777-1. [DOI] [PubMed] [Google Scholar]
- 3.Baranowski E, Hogler W. An unusual presentation of acquired hypothyroidism: the Van Wyk-Grumbach syndrome. Eur J Endocrinol. 2012;166:537–42. doi: 10.1530/EJE-11-0494. [DOI] [PubMed] [Google Scholar]
- 4.Bassam T, Ajlouni K. A case of ovarian enlargement in severe primary hypothyroidism and review of the literature. Ann Saudi Med. 2006;26:66–8. doi: 10.5144/0256-4947.2006.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shu J, Xing L, Zhang L, Fang S, Huang H. Ignored adult primary hypothyroidism presenting chiefly with persistent ovarian cysts: a need for increased awareness. Reprod Biol Endocrinol. 2011;9:119. doi: 10.1186/1477-7827-9-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wormsbecker A, Clarson C. Acquired primary hypothyroidism: vaginal bleeding in a quiet child. CMAJ. 2010;182:588–90. doi: 10.1503/cmaj.090883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalkin AC, Haisenleder DJ, Ellis TR, Marshall JC. The frequency of gonadotropin-releasing-hormone stimulation differentially regulates gonadotropin subunit messenger ribonucleic acid expression. Endocrinology. 1989;125:917–24. doi: 10.1210/endo-125-2-917. [DOI] [PubMed] [Google Scholar]
- 8.Anasti JN, Flack MR, Froehlich J, Nelson LM, Nisula BC. A potential novel mechanism for precocious puberty in juvenile hypothyroidism. J Clin Endocrinol Metab. 1995;80:276–9. doi: 10.1210/jcem.80.1.7829625. [DOI] [PubMed] [Google Scholar]
- 9.Nebesio TD, Wise MD, Perkins SM, Eugster EA. Does clinical management impact height potential in children with severe acquired hypothyroidism? J Pediatr Endocrinol Metab. 2011;24:893–6. doi: 10.1515/jpem.2011.310. [DOI] [PubMed] [Google Scholar]