Abstract
Neurodegenerative disorders are often associated with excessive neuronal apoptosis. It is well known that apoptosis is regulated by some intracellular proteases, such as, Caspases (cysteine-dependent, aspartate-specific proteases). In fact, Caspase-8 which is an initiator caspase, has been identified as a key mediator of neuronal apoptosis. In addition, Caspase-8 is found to be coupled with the regulation of various neurodegenerative disorders including Alzheimer׳s disease (AD), Parkinson׳s disease (PD), Huntington׳s Diseases (HD) and Dentatorubral Pallidoluysian Atrophy (DRPLA). Caspase-8 inhibition may provide an effective means of treatment for multiple neurodegenerative disorders. Therefore, the present study describes the molecular interaction of some selected natural compounds with known anti neurodegenerative properties with Caspase-8. Docking between Caspase-8 and each of these compounds (separately) was performed using ‘Autodock4.2’. Out of all the selected compounds, rosmarinic acid and curcumin proved to be the most potent inhibitors of Caspase-8 with binding energy (ΔG) of -7.10 Kcal/mol and -7.08 Kcal/mol, respectively. However, further in vitro and in vivo studies are needed to validate the anti-neurodegenerative potential of these compounds.
Keywords: Neurodegenerative disorders, Caspase 8, Natural compounds, Molecular Docking
Background
Neurodegenerative disorders are characterized by progressive loss of structure or function of neurons leading to neuronal death, and are frequently hereditary [1]. Neurodegenerative diseases include a variety of progressive disorders that result in cognitive and/or motor deterioration. It is thought that a combination of progressive neuronal loss and neuronal dysfunction underlies neurodegeneration and some of these common neurodegenerative disorders include Alzheimer׳s disease (AD), Parkinson׳s disease (PD), Huntington׳s Diseases (HD) and few others. It is thought that a combination of progressive neuronal loss and neuronal dysfunction underlies neurodegeneration [2]. Apoptosis has recently been implicated as a possible mechanism for neuronal death in neurodegenerative diseases [3]. Inhibition of apoptosis may be effective in the treatment of neurodegenerative diseases. However, the inhibitors of apoptosis must be selective enough to avoid blocking normal apoptosis. Much still remains to be elucidated about the specific pathways that intervene in neurodegeneration and neuroprotection [4].
Caspases form a unique class of cysteine aspartate-specific proteases according to their substrate specificities and biological functions [5, 6]. Caspases are proteolytic in nature and key executioners of apoptosis [7]. Excessive neuronal apoptosis leads to a variety of diseases such as stroke, Alzheimer׳s disease, Huntington׳s disease and Parkinson׳s disease [8, 9]. The caspase family consists of cysteine proteases that cleave the peptide bond after an aspartic acid in their substrates. They are classified as inflammatory and apoptotic caspases based on their function and prodomain structure. Caspases can be classified into two broad categories firstly, initiator caspases (caspase-2, caspase-8, caspase-9, and caspase- 10) and secondly effector caspases (caspase-3, caspase-6, and caspase-7). Initiator caspases generally act in early stages of a proteolytic cascade whereas effector caspases act downstream and are involved in the cleavage of specific cellular proteins [10]. The search for effective caspase inhibitors as possible therapeutic agents is an important area for the treatment of neurodegenerative disorders [11, 12].
AD is the most common form of dementia among the elderly population. It represents the fourth cause of death in industrialized countries and it is estimated that, by 2050, more than 100 million people worldwide will suffer from AD [13]. The caspases have been detected in brains of the patients suffering with AD. In fact, caspases-1, -2, -3, - 5, -6, -7, -8 and -9 have all been detected to be transcriptional elevated in AD [4]. PD and AD are known to be associated with neuroinflammation and the presence of activated microglia [14, 15]. Inflammation and activated microglia are implicated in the neuronal death and loss occurring in infectious, ischemic, traumatic, and neurodegenerative diseases [16]. The activation of caspase-8 in inflamed microglia prevents their death by necroptosis, and thus, caspase-8 inhibitors may protect neurons in the inflamed brain by selectively killing activated microglia [17]. Huntington's disease (HD) and dentatorubral pallidoluysian atrophy (DRPLA), have been shown to be caused by the expansion of CAG repeats [18, 19]. The CAG repeats are located in the coding regions of the respective responsible genes and are translated into polyglutamine. Previously it has been reported that activated caspase-8 was present within insoluble fractions of HD brains, further supporting the idea that caspase-8 is recruited to aggregates and is subsequently activated [20].
The medicinal use of natural products―compounds that are derived from natural sources such as plants, animals or micro-organisms―precedes recorded human history probably by thousands of years. The past few years, however, have seen a renewed interest in the use of natural compounds and, more importantly, their role as a basis for drug development [21].
Methodology
Protein preparation:
The crystal structure of caspase-8 was extracted from protein data bank (PDB ID: 1QTN). The structure was refined by removing the heteroatoms and water molecules. The minimization was performed by using a CHARMm force field [22] with Dependent Dielectric implicit solvent model along and conjugates gradient method.
Ligand preparation:
The 3D structure of natural compounds with known anti-neuro degenerative properties were retrieved from pubchem compounds database. These compounds include Bilobalide (pubchem id: 12308750, Quercetin (pubchem id: 5280343), EGCG (pubchem id: 65064), Resveratrol (pubchem id:445154), Curcumin (pubchem id: 969516), Huperzine A (pubchem id: 5912039), Rosmarinic acid (pubchem id: 5281792), Luteolin (pubchem id: 5280445), Apigenin (pubchem id: 5280443) and Berberine (pubchem id: 2353). Further the compounds were refined for correct protonation.
Validation:
Before conducting molecular docking, validation was performed, in which the peptide inhibitor present within the active site of the crystal structure of caspase-8 was extracted. This inhibitor was subjected to re-dock within the active site of caspase-8 using Autodock 4.2 [23]. The orientation of the crystal and redocked confirmation of inhibitor was compared with that obtained from Protein Data Bank, PDB ID: 1QTN.
Molecular docking:
All the selected compounds were docked into each of the energy minimized modeled enzyme-structures using Autodock4.2 [23]. For the compounds, charges of the Gasteiger type were assigned. The MMFF94 force field was used for energy minimization of ligand molecules. Non-polar hydrogen atoms were merged, and rotatable bonds were defined. Docking calculations were carried out on the protein models. Essential hydrogen atoms, Kollman charges, and solvation parameters were added with the aid of AutoDock tools. Affinity (grid) maps of 60×60×60 Å grid points and 0.375 Å spacing were generated using the Autogrid program. AutoDock parameter set and distance-dependent dielectric functions were used in the calculation of the Van der Waals and the electrostatic terms, respectively. Docking simulations were performed using the Lamarckian genetic algorithm (LGA). The docking parameters set to perform each docking experiment was derived from 10 different runs that were set to terminate after a maximum of 2500000 energy evaluations, elitism of 1, mutation rate 0.02, cross-over rate of 0.8, local search rate of 0.06. The population size was set to 150. The best run coordinates of the compounds with enzyme were visualized and analyzed through Pymol for analysis of their mode of interaction with binding site residues.
Result & Discussions
Caspases (cysteine-dependent aspartate-specific proteases) belong to the group of cysteine proteases. Caspases are synthesized as inactive pro-enzymes that are converted during apoptosis into active proteases by autoactivation or by amplification cascade through a highly regulated process [24]. In such a caspase cascade, initiator caspases (such as caspase 8) activate so-called effector caspases (such as caspase 3 or 6), which are responsible for the cleavage of various cellular proteins, leading to apoptotic death [24]. The prime objective of the present study was to identify the binding potential of some natural compounds with previously reported anti-neurodegenerative properties against caspase-8, a common therapeutic target involved in multi-neurodegenerative disorders using molecular docking approach. Here in this study we have performed in silico approach to identify natural compounds having the potential of being a best possible drug candidate against caspase-8. No docking program could reproduce the experimentally determined binding modes in a satisfactory manner if is used in the default parameter settings. Validation of the size and center of the coordinates of the grid box is the first step in molecular docking. Validation of the protocol and the size and center of the coordinates of the grid was carried out in order to ensure that ligands bind to the binding pocket in the correct conformation. For this purpose the peptide inhibitor présent within the active site of crystallized structure of CASPASE-8 was redocked into its respective binding site. Re-docked inhibitor was found to interact with the same amino acids of the active site as was in the crystal structure. The RMSD of all atoms between these two conformations was found to be 1.6610Å; indicating that the protocol set for molecular docking is accurate and faithfully reproduces the crystallographic complex with a high degree of similarity. The orientation of the crystallized and re-docked tetra peptide inhibitor (ACE-IETD-ALDEHYDE) is shown in Figure 1.
Figure 1.
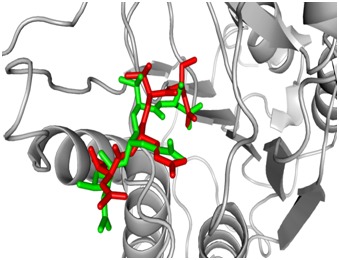
Binding mode of crystallized (red) and redocked (green) tetra peptide inhibitor within the active site of caspase- 8. The RMSD of all atoms between these two conformations is 1.6610Å.
Molecular docking simulations were used to investigate possible binding modes of selected natural compounds within the active site of caspase-8. Several plausible binding modes were detected and were ranked on the basis of their binding free energy. It was revealed that Rosmarinic acid and curcumin were capable of binding within the binding site of caspase-8 with higher affinity as compare to their other compounds. Rosmarinic acid is a polyphenol antioxidant carboxylic acid existing in many Lamiaceae herbs possesses several biological activities like antioxidant, antibacterial, anticancer, anti-inflammatory, antiviral, and neuroprotective effects [25, 26]. Previous studies on curcumin suggest that it may be useful for the treatment of a number of diseases, such as cancer, cystic fibrosis, inflammatory diseases as well as neural disorders [27]. For instance, Rosmarinic acid and curcumin were found to bind within the active site of caspase-8 with binding free energy of -7.10 Kcal/mol and -7.08 Kcal/mol respectively that was higher than other compounds which binds within the range of 5.67-6.18 Kcal/mol. Further the binding efficacy of Rosmarinic acid and curcumin were compared with know tetrapeptide inhibitor (ACE-IETD-ALDEHYDE) of caspase-8. It was found that this peptide inhibitor of caspase-8 interacts with binding free energy of -8.07 kcal/mol within the activesite of caspase-8. The binding efficacy of our finally selected natural compounds (Rosmarinic acid and curcumin) was comparable to their peptide counterpart. The binding mode of Rosmarinic acid and curcumin within the active site of caspase-8 is shown in (Figure 2 & Figure 3). Table 1 (see supplementary material) illustrates the binding score of all the compounds used in this study against caspase-8. In the present study, all the compounds were found to interact mainly through ten amino acid residues R258, R260, N261, G262, T263, H317, A359 and C360 (Table 1). The role of Arg 258 is conspicuous in the structure of caspase 8 as the large protrusion that sticks out in the top-middle of the active site cleft [28]. This residue was also very much involved in the positioning of compounds within the active site of caspase-8. Interaction of all the compounds against caspase-8 was observed to be dominated by both hydrogen bonds as well as hydrophobic interactions. Hence, the present study reveals that Rosmarinic acid and curcumin are efficient inhibitor of caspase- 8 in terms of amino acid interaction and Autodock binding free energy
Figure 2.
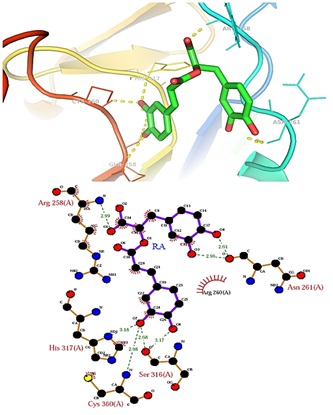
Binding mode of Rosmarinic acid within the active site of caspase-8.
Figure 3.
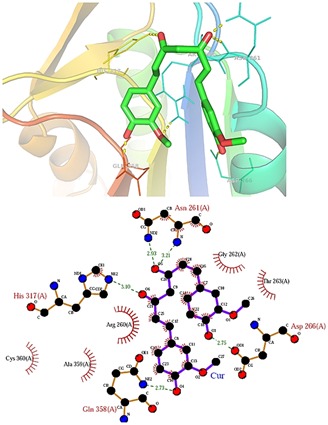
Binding mode of curcumin within the active site of caspase-8.
Conclusion
This study explores the molecular interactions of some selected natural compounds with caspase-8. However, these compounds were selected on the basis of their anti-neuro degenerative properties reported earlier. Both hydrogen bonds as well as hydrophobic interactions were found to play an important role during interaction of all these natural compounds to caspase-8. Out of all the selected compounds, Rosmarinic acid and curcumin were found to be the best potent inhibitors of Caspase-8 in terms of binding energy, which were calculated as -7.10 Kcal/mol and -7.08 Kcal/mol, respectively. Such information may open new prospects in the use of natural compounds and their derivatives as a potential drug candidate against caspase-8 for treatment of neurodegenerative disorders. Further experimental studies need to be performed to validate these data. Though, it can be safely stated that Rosmarinic acid and curcumin are efficient inhibitor of caspase-8.
Supplementary material
Acknowledgments
Authors are thankful to Integral University, Lucknow, India for providing necessary facilities to conduct the study and one of the authors (K. Ahmad) is thankful to UGC, New Delhi for granting Maulana Azad National Fellowship.
Footnotes
Citation:Ahmad et al, Bioinformation 10(4): 191-195 (2014)
References
- 1.Hebert LE, et al. Alzheimer Dis Assoc Disord. 2001;15:169. doi: 10.1097/00002093-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Kruttgen A, et al. J Neuropathol Exp Neurol. 2003;62:340. doi: 10.1093/jnen/62.4.340. [DOI] [PubMed] [Google Scholar]
- 3.Kanazawa I. Trends Mol Med. 2001;7:339. doi: 10.1016/s1471-4914(01)02017-2. [DOI] [PubMed] [Google Scholar]
- 4.Castro RE, et al. Curr Pharm Des. 2010;16:2851. doi: 10.2174/138161210793176563. [DOI] [PubMed] [Google Scholar]
- 5.Grutter MG. Curr Opin Struct Biol. 2000;10:649. doi: 10.1016/s0959-440x(00)00146-9. [DOI] [PubMed] [Google Scholar]
- 6.Fuentes-Prior P, Salvesen GS. Biochem J. 2004;384:201. doi: 10.1042/BJ20041142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohr A, Zwacka RM. Cell Biol Int. 2007;31:526. doi: 10.1016/j.cellbi.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Fleischer A, et al. Mol Immunol. 2006;43:1065. doi: 10.1016/j.molimm.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Taylor RC, et al. Nat Rev Mol Cell Biol. 2008;9:231. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 10.Marques CA, et al. J Biol Chem. 2003;278:28294. doi: 10.1074/jbc.M212265200. [DOI] [PubMed] [Google Scholar]
- 11.Friedlander RM, et al. N Engl J Med. 2003;348:1365. doi: 10.1056/NEJMra022366. [DOI] [PubMed] [Google Scholar]
- 12.Howley B, Fearnhead HO. J Cell Mol Med. 2008;12:1502. doi: 10.1111/j.1582-4934.2008.00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turner RS. Semin Neurol. 2006;26:499. doi: 10.1055/s-2006-951622. [DOI] [PubMed] [Google Scholar]
- 14.Block ML, et al. Nat Rev Neurosci. 2007;8:57. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 15.Aarli JA. Curr Med Chem. 2003;10:1931. doi: 10.2174/0929867033456918. [DOI] [PubMed] [Google Scholar]
- 16.Neher JJ, Brown GC. Biochem Soc Trans. 2007;35:1166. doi: 10.1042/BST0351166. [DOI] [PubMed] [Google Scholar]
- 17.Fricker M, et al. J Biol Chem. 2013;288:9145. doi: 10.1074/jbc.M112.427880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Huntington's Disease Collaborative Research Group. Cell. 1993;72:971. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 19.Nagafuchi S, et al. Nat Genet. 1994;6:14. doi: 10.1038/ng0194-14. [DOI] [PubMed] [Google Scholar]
- 20.Sánchez I, et al. Neuron. 1999;22:623. doi: 10.1016/s0896-6273(00)80716-3. [DOI] [PubMed] [Google Scholar]
- 21.Ji HF, et al. EMBO Rep. 2009;10:194. doi: 10.1038/embor.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brooks BR, et al. J Comp Chem. 2009;30:1545. [Google Scholar]
- 23.Morris GM, et al. Curr Protoc Bioinformatics. 2008;8:14. doi: 10.1002/0471250953.bi0814s24. [DOI] [PubMed] [Google Scholar]
- 24.Nicholson DW, et al. Cell Death Differ. 1999;6:1028. doi: 10.1038/sj.cdd.4400598. [DOI] [PubMed] [Google Scholar]
- 25.Petersen M, Simmonds MS. Phytochemistry. 2003;62:121. doi: 10.1016/s0031-9422(02)00513-7. [DOI] [PubMed] [Google Scholar]
- 26.Shaerzadeh F, et al. J Nat Med. 2011;65:455. doi: 10.1007/s11418-011-0519-9. [DOI] [PubMed] [Google Scholar]
- 27.Srivastava RM Int, et al. Immunopharmacol. 2011;11:331. doi: 10.1016/j.intimp.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 28.Reszka P, et al. ChemMedChem. 2010;5:103. doi: 10.1002/cmdc.200900356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


