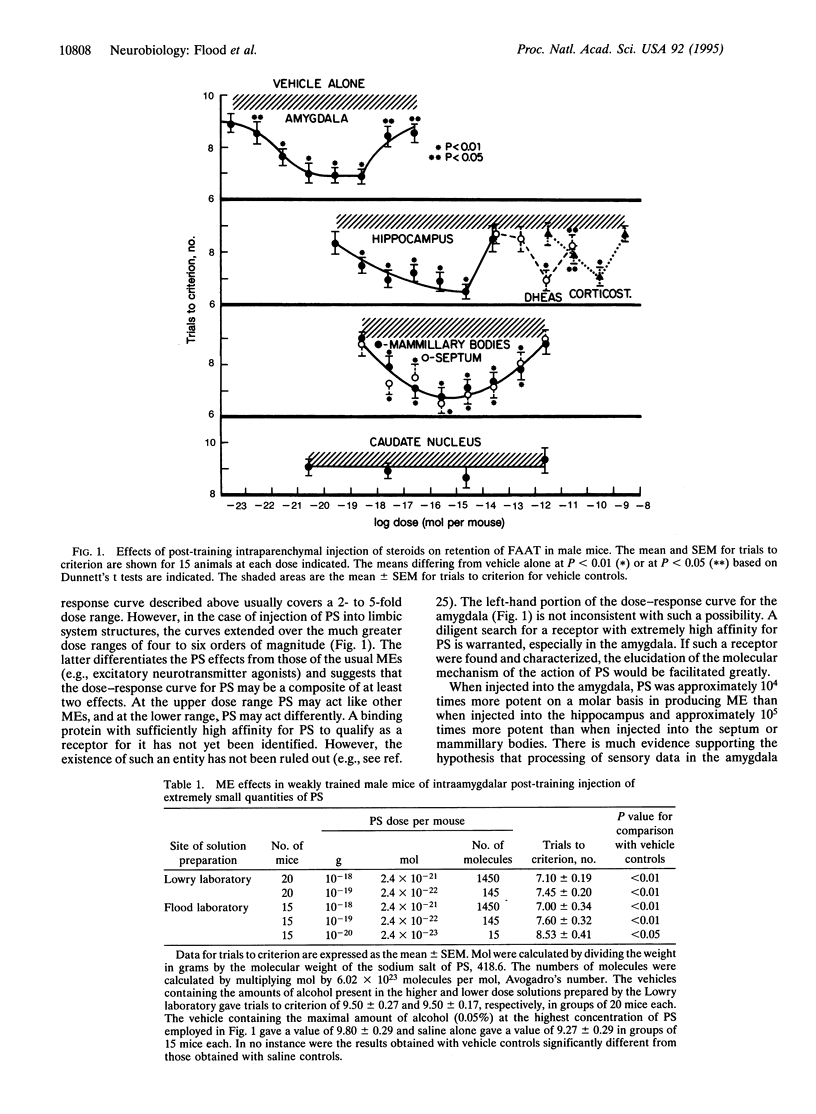
Abstract
Immediate post-training, stereotactically guided, intraparenchymal administration of pregnenolone sulfate (PS) into the amygdala, septum, mammillary bodies, or caudate nucleus and of PS, dehydroepiandrosterone sulfate, and corticosterone into the hippocampus was performed in mice that had been weakly trained in a foot-shock active avoidance paradigm. Intrahippocampal injection of PS resulted in memory enhancement (ME) at a lower dose than was found with dehydroepiandrosterone sulfate and corticosterone. Intraamygdally administered PS was approximately 10(4) times more potent on a molar basis in producing ME than when PS was injected into the hippocampus and approximately 10(5) times more potent than when injected into the septum or mammillary bodies. ME did not occur on injection of PS into the caudate nucleus over the range of doses tested in the other brain structures. The finding that fewer than 150 molecules of PS significantly enhanced post-training memory processes when injected into the amygdala establishes PS as the most potent memory enhancer yet reported and the amygdala as the most sensitive brain region for ME by any substance yet tested.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akwa Y., Young J., Kabbadj K., Sancho M. J., Zucman D., Vourc'h C., Jung-Testas I., Hu Z. Y., Le Goascogne C., Jo D. H. Neurosteroids: biosynthesis, metabolism and function of pregnenolone and dehydroepiandrosterone in the brain. J Steroid Biochem Mol Biol. 1991;40(1-3):71–81. doi: 10.1016/0960-0760(91)90169-6. [DOI] [PubMed] [Google Scholar]
- Arancio O., Kandel E. R., Hawkins R. D. Activity-dependent long-term enhancement of transmitter release by presynaptic 3',5'-cyclic GMP in cultured hippocampal neurons. Nature. 1995 Jul 6;376(6535):74–80. doi: 10.1038/376074a0. [DOI] [PubMed] [Google Scholar]
- Bologa L., Sharma J., Roberts E. Dehydroepiandrosterone and its sulfated derivative reduce neuronal death and enhance astrocytic differentiation in brain cell cultures. J Neurosci Res. 1987;17(3):225–234. doi: 10.1002/jnr.490170305. [DOI] [PubMed] [Google Scholar]
- Bowlby M. R. Pregnenolone sulfate potentiation of N-methyl-D-aspartate receptor channels in hippocampal neurons. Mol Pharmacol. 1993 May;43(5):813–819. [PubMed] [Google Scholar]
- Cohen P. Protein phosphorylation and hormone action. Proc R Soc Lond B Biol Sci. 1988 Jul 22;234(1275):115–144. doi: 10.1098/rspb.1988.0040. [DOI] [PubMed] [Google Scholar]
- Flood J. F., Baker M. L., Hernandez E. N., Morley J. E. Modulation of memory processing by neuropeptide Y varies with brain injection site. Brain Res. 1989 Nov 27;503(1):73–82. doi: 10.1016/0006-8993(89)91706-x. [DOI] [PubMed] [Google Scholar]
- Flood J. F., Morley J. E., Roberts E. An amyloid beta-protein fragment, A beta[12-28], equipotently impairs post-training memory processing when injected into different limbic system structures. Brain Res. 1994 Nov 14;663(2):271–276. doi: 10.1016/0006-8993(94)91273-4. [DOI] [PubMed] [Google Scholar]
- Flood J. F., Morley J. E., Roberts E. Memory-enhancing effects in male mice of pregnenolone and steroids metabolically derived from it. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1567–1571. doi: 10.1073/pnas.89.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood J. F., Roberts E. Dehydroepiandrosterone sulfate improves memory in aging mice. Brain Res. 1988 May 10;448(1):178–181. doi: 10.1016/0006-8993(88)91116-x. [DOI] [PubMed] [Google Scholar]
- Flood J. F., Smith G. E., Jarvik M. E. A comparison of the effects of localized brain administration of catecholamine and protein synthesis inhibitors on memory processing. Brain Res. 1980 Sep 15;197(1):153–165. doi: 10.1016/0006-8993(80)90441-2. [DOI] [PubMed] [Google Scholar]
- Flood J. F., Smith G. E., Roberts E. Dehydroepiandrosterone and its sulfate enhance memory retention in mice. Brain Res. 1988 May 3;447(2):269–278. doi: 10.1016/0006-8993(88)91129-8. [DOI] [PubMed] [Google Scholar]
- Gallagher M., Holland P. C. The amygdala complex: multiple roles in associative learning and attention. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):11771–11776. doi: 10.1073/pnas.91.25.11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnegy M. E. Calmodulin in neurotransmitter and hormone action. Annu Rev Pharmacol Toxicol. 1993;33:45–70. doi: 10.1146/annurev.pa.33.040193.000401. [DOI] [PubMed] [Google Scholar]
- Gu X., Spitzer N. C. Distinct aspects of neuronal differentiation encoded by frequency of spontaneous Ca2+ transients. Nature. 1995 Jun 29;375(6534):784–787. doi: 10.1038/375784a0. [DOI] [PubMed] [Google Scholar]
- Guth L., Zhang Z., Roberts E. Key role for pregnenolone in combination therapy that promotes recovery after spinal cord injury. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):12308–12312. doi: 10.1073/pnas.91.25.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin R. P., Maragakis N. J., Rogawski M. A., Purdy R. H., Farb D. H., Paul S. M. Pregnenolone sulfate augments NMDA receptor mediated increases in intracellular Ca2+ in cultured rat hippocampal neurons. Neurosci Lett. 1992 Jul 6;141(1):30–34. doi: 10.1016/0304-3940(92)90327-4. [DOI] [PubMed] [Google Scholar]
- Lavond D. G., Kim J. J., Thompson R. F. Mammalian brain substrates of aversive classical conditioning. Annu Rev Psychol. 1993;44:317–342. doi: 10.1146/annurev.ps.44.020193.001533. [DOI] [PubMed] [Google Scholar]
- Majewska M. D. Neurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog Neurobiol. 1992;38(4):379–395. doi: 10.1016/0301-0082(92)90025-a. [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Guthrie P. B., Kater S. B. A role for Na+-dependent Ca2+ extrusion in protection against neuronal excitotoxicity. FASEB J. 1989 Nov;3(13):2519–2526. doi: 10.1096/fasebj.3.13.2572500. [DOI] [PubMed] [Google Scholar]
- Monnet F. P., Mahé V., Robel P., Baulieu E. E. Neurosteroids, via sigma receptors, modulate the [3H]norepinephrine release evoked by N-methyl-D-aspartate in the rat hippocampus. Proc Natl Acad Sci U S A. 1995 Apr 25;92(9):3774–3778. doi: 10.1073/pnas.92.9.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orentreich N., Brind J. L., Rizer R. L., Vogelman J. H. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984 Sep;59(3):551–555. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]
- Prado-Alcalá R. A., Fernández-Samblancat M., Solodkin-Herrera M. Injections of atropine into the caudate nucleus impair the acquisition and the maintenance of passive avoidance. Pharmacol Biochem Behav. 1985 Feb;22(2):243–247. doi: 10.1016/0091-3057(85)90385-5. [DOI] [PubMed] [Google Scholar]
- Regelson W., Loria R., Kalimi M. Hormonal intervention: "buffer hormones" or "state dependency". The role of dehydroepiandrosterone (DHEA), thyroid hormone, estrogen and hypophysectomy in aging. Ann N Y Acad Sci. 1988;521:260–273. doi: 10.1111/j.1749-6632.1988.tb35284.x. [DOI] [PubMed] [Google Scholar]
- Roberts E. A systems approach to aging, Alzheimer's disease, and spinal cord regeneration. Prog Brain Res. 1990;86:339–355. doi: 10.1016/s0079-6123(08)63190-8. [DOI] [PubMed] [Google Scholar]
- Roberts E., Bologa L., Flood J. F., Smith G. E. Effects of dehydroepiandrosterone and its sulfate on brain tissue in culture and on memory in mice. Brain Res. 1987 Mar 17;406(1-2):357–362. doi: 10.1016/0006-8993(87)90807-9. [DOI] [PubMed] [Google Scholar]
- Roberts E. Pregneolone--from Selye to Alzheimer and a model of the pregnenolone sulfate binding site on the GABAA receptor. Biochem Pharmacol. 1995 Jan 6;49(1):1–16. doi: 10.1016/0006-2952(94)00258-n. [DOI] [PubMed] [Google Scholar]
- Slemmon J. R., Martzen M. R. Neuromodulin (GAP-43) can regulate a calmodulin-dependent target in vitro. Biochemistry. 1994 May 10;33(18):5653–5660. doi: 10.1021/bi00184a039. [DOI] [PubMed] [Google Scholar]
- Sonka J. Dehydroepiandrosterone. Metabolic effects. Acta Univ Carol Med Monogr. 1976;71:1-137, 146-71. [PubMed] [Google Scholar]
- Vesely D. L. Testosterone and its precursors and metabolites enhance guanylate cyclase activity. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3491–3494. doi: 10.1073/pnas.76.7.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein H., Mehler E. L. Ca(2+)-binding and structural dynamics in the functions of calmodulin. Annu Rev Physiol. 1994;56:213–236. doi: 10.1146/annurev.ph.56.030194.001241. [DOI] [PubMed] [Google Scholar]
- Witt M. R., Dekermendjian K., Frandsen A., Schousboe A., Nielsen M. Complex correlation between excitatory amino acid-induced increase in the intracellular Ca2+ concentration and subsequent loss of neuronal function in individual neocortical neurons in culture. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):12303–12307. doi: 10.1073/pnas.91.25.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F. S., Gibbs T. T., Farb D. H. Pregnenolone sulfate: a positive allosteric modulator at the N-methyl-D-aspartate receptor. Mol Pharmacol. 1991 Sep;40(3):333–336. [PubMed] [Google Scholar]



