Abstract
The Na/K pump hydrolyzes ATP to export three intracellular Na (Nai) as it imports two extracellular K (Ko) across animal plasma membranes. Within the protein, two ion-binding sites (sites I and II) can reciprocally bind Na or K, but a third site (site III) exclusively binds Na in a voltage-dependent fashion. In the absence of Nao and Ko, the pump passively imports protons, generating an inward current (IH). To elucidate the mechanisms of IH, we used voltage-clamp techniques to investigate the [H]o, [Na]o, and voltage dependence of IH in Na/K pumps from ventricular myocytes and in ouabain-resistant pumps expressed in Xenopus oocytes. Lowering pHo revealed that Ho both activates IH (in a voltage-dependent manner) and inhibits it (in a voltage-independent manner) by binding to different sites. Nao effects depend on pHo; at pHo where no Ho inhibition is observed, Nao inhibits IH at all concentrations, but when applied at pHo that inhibits pump-mediated current, low [Na]o activates IH and high [Na]o inhibits it. Our results demonstrate that IH is a property inherent to Na/K pumps, not linked to the oocyte expression environment, explains differences in the characteristics of IH previously reported in the literature, and supports a model in which 1), protons leak through site III; 2), binding of two Na or two protons to sites I and II inhibits proton transport; and 3), pumps with mixed Na/proton occupancy of sites I and II remain permeable to protons.
Introduction
The Na, K-ATPase, or Na/K pump, is the P-type ATPase that builds the Na+ gradient that energizes most plasma membrane processes in animal cells, including excitability and secondary active transport. Under physiological and near-physiological conditions, this pump transports three Na+ ions out of the cell while importing two K+ ions for every ATP molecule hydrolyzed, with a fixed stoichiometry (1,2). The crystal structures of the Na/K pump with two K+ ions bound (3) and with three Na+ ions bound (4) show the location of the ion-binding sites and the residues that provide oxygen for ion coordination. There is one site, site III, that exclusively binds Na+, whereas sites I and II can bind either K+ or Na+ (although the conformation of the two sites slightly differs between the Na-bound and K-bound states).
This article is concerned with the characteristics of a noncanonical mode of passive inward H+ transport that occurs through Na/K pumps. Noncanonical, passive fluxes have also been described in other active transporters (5,6). Normal Na/K pumps (wild-type pumps or pumps with mutations that reduce their affinity for ouabain) present an inward H+ current (IH) in the absence of external Na+ (Na+o) and K+ (K+o). Several mutations that induce large changes in the ion-binding characteristics, including C-terminal deletion of the α-subunit, allow this leak to appear in the presence of physiological external Na+ concentration (7–9).
Originally found in endogenous Na/K pumps of Xenopus oocytes by Rakowski et al. (10), IH has been studied subsequently by several laboratories in heterologously expressed Na/K pumps in Xenopus oocytes. Wang and Horisberger (11) demonstrated that this leak current is carried by H+ by showing that IH-induced acidification of the oocyte’s intracellular milieu and its reversal potential are dependent on external pH (pHo). The characteristics of IH vary depending on the particular Na/K pump expressed; Li et al. (12) showed that mutations on a putative Na+ binding site, which altered the apparent affinity for Na+o, changed the amount of leak current observed in the absence of Na+o; the higher the affinity of the mutant for Na+o, the larger was the IH at pH 6 in the absence of Na+o. Later, our laboratory showed that deletion of the last five C-terminal residues in α1 modified the characteristics of this leak, making it evident in the presence of physiological Na+o levels, a result confirmed by two other laboratories (8,9). Finally, mutation of a conserved aspartic acid that may form part of site III to asparagine completely eliminates IH, consistent with observations that transient titration of this aspartic acid contribute to the H+ translocation pathway through the pump (14).
A possible pathophysiological relevance of IH is highlighted by the work of Poulsen et al. (15) and Azizan et al. (16), who showed that human α2 mutations that induce familial hemiplegic migraine (15) and human α1 mutations that induce hyperaldosteronism and hypertension (16) present IH at physiological [Na+]o. Thus, for instance, a large IH may be responsible for the depolarization observed in adrenal-cortical adenomas that express these mutant α1 pumps (17).
This study addresses major gaps in the current understanding of IH to elucidate the mechanisms underlying its appearance and inhibition. We demonstrate that the H+ current, to date described only in oocytes, is present as well in a classical mammalian preparation used to study Na/K pumps under voltage clamp, a finding that could elucidate the contribution of this mode of action to the pathophysiology of human illness caused by mutants that disrupt ion binding. We also study the complex interaction between the effects of Na+ and those of H+ in ventricular myocytes and oocytes, focusing on the voltage dependence of the apparent affinities and on the interactions between the different ions. Our results 1), demonstrate that leak currents are a property inherent to Na/K pumps, that is, they are not attributable to changes in environment caused by their heterologous expression in oocytes; 2), shed light on the mechanisms that might be involved in the appearance of IH in illness-related mutants under normal physiological conditions; 3), explain the differences between several laboratory studies of different Na/K pump mutants under different conditions; and 4), provide additional support for the suggestion that the H+ permeation site is the site responsible for the normal Na/K pump voltage dependence, believed to be site III (also known as the Na+-exclusive site).
Methods
Cell preparation
Guinea pig myocytes were isolated with slight modification of previously described methods (18). The heart was rapidly removed under pentobarbital anesthesia, mounted on a Langendorf system, and perfused with normal Tyrode’s solution consisting of (in mM) 1.8 CaCl2, 145 NaCl, 5 KCl, 1 MgCl2, and 10 HEPES (pH 7.4) at 35°C. After the coronary arteries were clean of blood, the solution was changed to Ca2+-free Tyrode’s for 7 min, followed by application of an enzymatic solution (1 mg/mL collagenase type 1A and 0.2 mg/mL protease type XIV (Sigma, St. Louis, MO) dissolved in Ca2+-free Tyrode’s) for ∼7 min, depending on the degree of digestion. The enzymatic solution was washed with a solution containing (in mM) 70 K-glutamate, 25 KCl, 20 taurine, 10 KH2PO4, 1 MgCl2, 0.5 EGTA, 20 glucose, and 10 HEPES (pH 7.3). In this high-K+ solution, the heart was cut, and myocytes were stored at 4°C before use within 36 h.
Oocyte preparation and molecular biology
Oocytes were isolated enzymatically, injected with a mixture of α1β3 complelmentary RNAs, and maintained for 2–6 days postinjection as described previously (2). For electrophysiological experiments, we used the double mutant Q120R/N131D (RD), which has a lower ouabain sensitivity (IC50 ∼ 100 μM), allowing reversible inhibition of exogenous pumps and irreversible block of endogenous pumps (IC50 < 100 nM). For comparison, some experiments were performed with ouabain-sensitive wild-type Xenopus α1β3.
Electrophysiology
All experiments were performed at room temperature, 20–23°C.
Myocytes
Whole-cell patch-clamp recording of ventricular myocytes was performed with an Axopatch 200A, a Digidata 1322 A/D board, and pClamp 10 software (all from Molecular Devices, Sunnyvale, CA), with acquisition at 10 kHz, filtered at 2 kHz. Pipettes had resistances between 1 and 3 MΩ when filled with intracellular solution containing (in mM) 130 L-glutamic acid, 10 HEPES, 10 EGTA, 10 tetraethylammonium-Cl, 5 MgATP, 1 MgCl2, and 50 NaOH, pH 7.4 with ∼100 mM CsOH. The external solution included (in mM) 130 methanesulfonic acid (MS), 10 HEPES, 10 MES (2-(N-Morpholino)ethanesulfonic acid), 5 BaCl2, 1 MgCl2, 1 CaCl2, 1 CdCl2, and 150 N-methyl D-glucamine (NMG) or NaOH (final pH 5.5–7.4 attained by adding MS or NMG). Intermediate Na+ concentrations were achieved by mixing the two solutions. Na/K-pump-mediated current was identified by inhibition with either ouabain (nearly irreversible) or the reversible steroids dihydroouabain (DHO) or strophanthidin (19).
Oocytes
An OC-725C amplifier (Warner Instruments, Hamden, CT), a Digidata 1440 A/D board, a Minidigi 1A, and pClamp 10 software (Molecular Devices) were used for two-electrode voltage-clamp recordings. Signals were filtered at 2 kHz and digitized at 10 kHz. Resistance of both microelectrodes (filled with 3M KCl) was 0.5–1 MΩ. Oocytes were Na+-loaded by 1-h incubation in a solution containing (in mM) 150 HEPES, 20 tetraethylammonium-Cl, and 0.2 EGTA (pH 7.2 with NaOH). They were then kept in Na+ recording solution until they were used for measurements. During loading, ouabain (10–20 μM) was applied to ooctyes injected with RD-α1 but not to those injected with wild-type α1. External solutions were composed of (in mM) 120 NaOH or 120 mM NMG, 10 mM HEPES, 10 mM MES, 5 mM Ba(OH)2, 1 mM Mg(OH)2, 0.5 mM Ca(OH)2 (pH 4.0–7.6, as indicated, with MS). Intermediate Na+ concentrations were achieved by mixing the two solutions at the appropriate pH. External K+ was added from a 3 M K+-MS stock.
Results
Characteristics of Na/K-pump-mediated IH leak in guinea pig ventricular myocytes
To date, an Na/K-pump-mediated IH has been observed only in Xenopus oocytes under voltage clamp. In the absence of both Na+o and K+o, different levels of IH have been described in endogenous pumps (10), overexpressed Xenopus α1β3 pumps (11), or other exogenous ouabain-insensitive pumps (7,8,12,20). To our surprise, this current has not been reported in any other cell type, including ventricular myocytes (a classical preparation for studying Na/K pump electrophysiology); this is even more puzzling considering that oocytes expressing ouabain-insensitive pumps present robust IH even at pHo as high as 7.6, with current amplitude increasing as pHo is lowered to pH 6.0 (11,20).
We looked for a cardiotonic steroid-sensitive IH in guinea pig ventricular myocytes in the absence of Na+o (Fig. 1). Fig. 1, A and B, shows results from a representative myocyte in Na+-free external solutions (with NMG+ as the main external cation). Fig. 1 A shows the family of curves generated by step voltage pulses applied from a holding potential (Vh) of −40 mV to the indicated voltages, under four conditions: with and without 2 mM DHO (a reversible Na/K pump inhibitor) at two pH values (7.4 and 6.0). Fig. 1 B displays the steady-state current measured with voltage pulses like those applied in Fig. 1 A at pH 7.4 in the absence (open black squares) and presence (open blue triangles) of DHO and at pH 6.0 without (open red circles) and with DHO (open green triangles). The DHO-sensitive current is plotted as solid black squares (pH 7.4) and solid red circles (pH 6.0). Fig. 1 C plots the average cardiotonic steroid (CS)-sensitive current density at pH 7.4 (black squares) and at pH 6.0 (red circles). These experiments demonstrate for the first time, to our knowledge, that IH is present in nonoocyte preparations.
Figure 1.
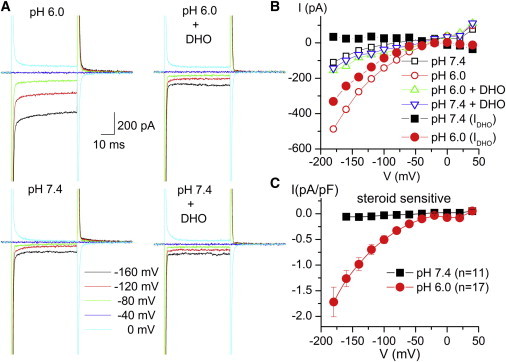
IH in whole-cell voltage-clamped ventricular myocytes at pH 6.0. (A) Currents induced by pulses from −40 mV to the indicated voltages in a ventricular myocyte bathed by 150 mM NMG external solution at pH 6.0 (upper) and pH 7.4 (lower) in the absence (left) and presence (right) of 2 mM DHO. (B) Average current (last 5 ms of voltage pulses) from the myocyte in A, in the absence (open black squares) and presence (open blue triangles) of DHO and at pH 6.0 without (open red circles) and with (open green triangles) DHO; the DHO-sensitive current is plotted as solid black squares (pH 7.4) and solid red circles (pH 6.0). (C) Average DHO-sensitive current density (current normalized by cell capacitance to correct for natural differences in myocyte size) at pH 7.4 (black squares) and at pH 6.0 (red circles). Error bars represent the mean ± SE.
To determine the H+o dependence of this current in myocytes, we also studied the pHo dependence of IH (Fig. 2). These experiments were performed in different myocytes where currents were normalized to cell capacitance to compare data from different cells. Fig. 2 A shows steady-state I/V curves and Fig. 2 B describes the [H+] effect obtained from these curves. It is clear that the inward currents increase with H+o concentration and appear to saturate at pHo 5.5, i.e., at 3 μM [H+]. The fits of Hill equations with shared Hill number (nH = 1.8) gave the parameters listed in the figure legend, which suggest that there is a reduction in the affinity for H+ as the voltage becomes more positive.
Figure 2.
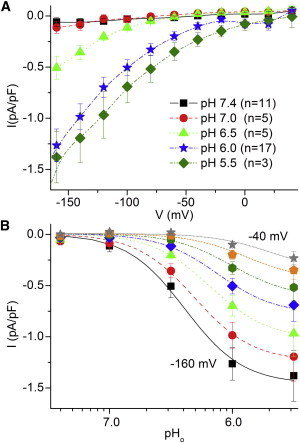
Leak currents depend on pHo in ventricular myocytes. Experiments similar to those in Fig. 1 were performed in several myocytes using either DHO (2 mM), strophanthidin (1 mM) or ouabain (2 mM) to inhibit Na/K pumps after exposure to the desired pHo. (A) Mean CS-sensitive current (normalized to cell capacitance) at indicated pH values is plotted as a function of voltage. Error bars represent the mean ± SE. (B) External pH dependence of the current at each voltage obtained from the average I/V curves in A. Lines represent the fit of a Hill equation to the average data with the following parameters and errors: shared nH = 1.8 ± 0.3, K0.5H (in μM) 0.41 ± 0.11 (−160 mV, squares), 0.49 ± 0.13 (−140 mV, circles), 0·65 ± 0.19 (−120 mV, triangles), 0.76 ± 0.24 (−100 mV, diamonds), 0.99 ± 0.37 (−80 mV, side triangles), 1.30 ± 0.54 (−60 mV, hexagons), and 2.05 ± 1.3 (−40 mV, stars). To see this figure in color, go online.
To obtain a clearer picture of the interaction between Na+ and H+, we evaluated the effects of Na+o on IH at pH 6.0 (Fig. 3). Fig. 3 A shows the voltage dependence of CS-sensitive current in the presence of different Na+ concentrations. At all voltages, 1 and 5 mM Na+ significantly increased IH, whereas higher Na+ concentrations inhibited IH. Fig. 3 B shows the Na+ concentration dependence at four voltages. IH is doubled by 5 mM Na+, whereas 14 mM Na+ brings the current back to the values observed at 0 Na+; higher concentrations reduce the current even further. This Na+ dependence is qualitatively similar to, but quantitatively different from, those reported in Xenopus oocytes with overexpressed α1β3 pumps at pHo 6.0 (11) and in Xenopus oocytes with endogenous pumps at pHo 5.6 (21). The experiments described below, performed in Xenopus oocytes, aim to explain these discrepancies.
Figure 3.
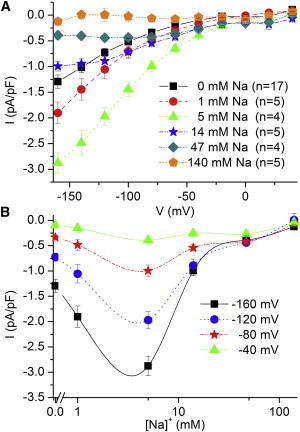
Effect of Na+ on IH at pH 6.0 in ventricular myocytes. (A) Average CS-sensitive steady-state current (normalized to cell capacitance) at different Na+ concentrations. (B) [Na+] dependence (from the data in A) at four voltages. To see this figure in color, go online.
Characteristics of Na/K pump-mediated inward H+ leak (IH+) in Xenopus oocytes heterologously expressing Xenopus α1β3 pumps
A detailed pHo dependence of the IH over a wide pHo range is an important aspect of this mode of operation that has not been studied with the different Na/K pumps expressed in Xenopus oocytes. Xenopus oocytes under two-electrode voltage clamp are more tolerant to extreme pH values than are ventricular myocytes, and expression of ouabain-resistant RD-α1β3 pumps makes it possible to perform full dose-response experiments in a single oocyte, thus avoiding the effects of cell-to-cell variation. Fig. 4 shows the pHo dependence of inward leak currents. Fig. 4 A displays the continuous recording from an oocyte held at −50 mV in which a full dose-response experiment was performed. Sharp vertical deflections of the current trace correspond to application of 100-ms pulses to measure voltage dependence. The experiment was initiated by adding 3 mM K+ to the NMG+ external solution, a maneuver that induces an outward-directed Na/K pump current (IP). The solution was then switched to an NMG+ external solution at pH 7.0, which induced a small inward current at −50 mV that was inhibited by 10 mM ouabain. This maneuver was repeated five times, switching to different pH values, with each iteration followed by ouabain washout for ∼10 min in a solution containing 120 mM Na+. The ouabain-sensitive inward current increased as pHo was reduced, but at pH values <5.6, an initial increase in current was followed by a slower reduction.
Figure 4.
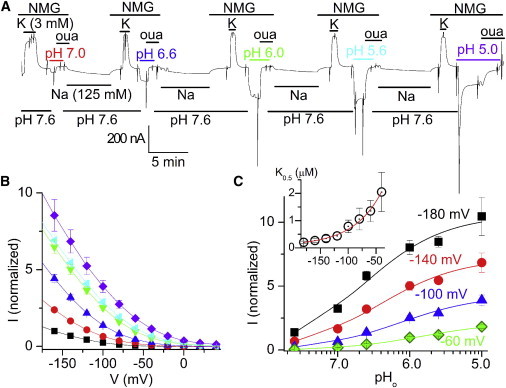
pHo dependence of IH without Na+o and K+o in Xenopus oocytes. (A) Continuous recording from an oocyte expressing RD-α1β3 held at −50 mV. Maximum IP was measured at 3 mM K+o without Na+o at pHo 7.6. pHo was then lowered, inducing an inward holding current that was inhibited by subsequent application of ouabain. After an 8-min ouabain washout with 120 mM Na+ (to maintain internal Na loading), the maneuver was repeated again, first applying K+, then lowering pHo, then adding ouabain. Note that IP is the same in each K+ application, indicating that all Na/K pumps recovered from inhibition before testing a different pHo. Ramp-shaped vertical deflections are 100-ms-long pulses to different voltages. The current at the end of those pulses was used to obtain I/V curves. (B) Ouabain-sensitive current (normalized to IH at −160 mV, pH 7.6, before lowering pHo) as a function of voltage. The data points are an average of eight oocytes where at least four different pHo values were tested. The currents at pH 5.6 and 5.0 were measured at the peak of the activation response, before significant inhibition had occurred. (C) pH dependence from the curves in B at the indicated voltages. The solid lines are global fits of Michaelis-Menten equations to the data from individual cells with K0.5 (in μM) ± fitting errors of 0.20 ± 0.04 (−180 mV, black), 0.34 ± 0.10 (−140 mV, red), 0.79 ± 0.23 (−100 mV, blue), and 1.36 ± 0.39 (−60 mV, green). The K0.5-versus-voltage curve is plotted in the inset; the red line is a fit of the equation K0.5= K00.5exp (λF/RT) + C to the data, with parameters K00.5 = 4.4 μM, λ = 0.51, and C = 0.07. R is the gas constant, F the Faraday constant, and T the temperature.
Fig. 4 B shows the normalized ouabain-sensitive I/V curves from eight experiments performed as in Fig. 4 A; for simplicity, we only plot the current at the peak of the response at very low pH values. From these I/V curves we built the external pHo-dependence plots at different voltages (Fig. 4 C). The fit of a Hill equation to these plots yielded a highly voltage-dependent K0.5H that increased > tenfold over the voltage range at which IH can be measured reliably (Fig. 4 C, inset). The high voltage sensitivity of activation by H+ is in contrast to the nearly voltage-independent inhibition by H+ (Fig. S1 in the Supporting Material). The different voltage dependencies indicate that activation and inhibition by H+ represent actions of this ion at at least two different sites.
To address whether the slow inhibition was due to intracellular H+ accumulation, and not to an effect of H+o on the binding sites, we modified the H+ gradient by changing the external voltage. That is, in terms of the H+ driving force, pH 5.0 at −50 mV is equivalent to pH 6.0 at −110 mV. Fig. 5 shows an example of such an experiment. External K+ reversibly activated IP. After the current returned to its baseline value (in NMG at pH 7.6), an NMG solution at pH 5.0 was applied at −50 mV, inducing a large inward current that slowly decreased, as described above. Two minutes after returning to pH 7.6, the voltage was switched to −110 mV and an NMG solution at pH 6.0 was applied. The total inward current induced was similar to the IH induced by pH 5.0 at −50 mV, consistent with the high voltage dependence for H+ activation of IH. However, the current did not show inhibition, consistent with the voltage dependence of inhibition being less than the voltage dependence for activation. To demonstrate that the currents induced by pHo were all mediated by Na/K pumps, we repeated the measurements after application of 10 mM ouabain at both −50 mV and −110 mV. The increase in H+ concentration did not induce changes in leak currents in the presence of ouabain. This experiment demonstrates that the inhibition by low pH is not due to intracellular accumulation of H+ leaking through the pump.
Figure 5.
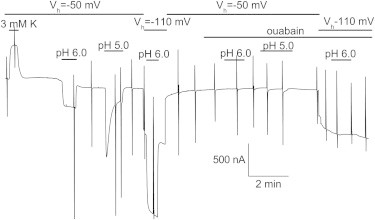
Current inhibition is not due to H+i accumulation. Shown is a continuous recording from an oocyte 3 days after injection with RNA for RD-α1β3 bathed with NMG external solutions. IP was activated by 3 mM K+ at pH 7.6 at −50 mV. After the current settled back to its baseline value in NMG, a pH 6.0 solution was applied, inducing a steady inward current that disappeared when pH was returned to 7.6. Subsequent perfusion of pH 5.0 solution induced a much larger current that decreased slowly. Two minutes after returning to pH 7.6, the holding potential was stepped to −110 mV and a pH 6.0 solution was applied, inducing a large inward steady current. After application of 10 mM ouabain, pH changes at −50 and −110 mV did not induce inward current. To see this figure in color, go online.
Na+o effects on leak currents were highly dependent on pHo (Figs. 6 and 7). Fig. 6 A shows the normalized I/V curves at pH 6.0 at different Na+o concentrations. Fig. 6 B shows the monotonic reduction of the current at all voltages as Na+o concentration is increased.
Figure 6.
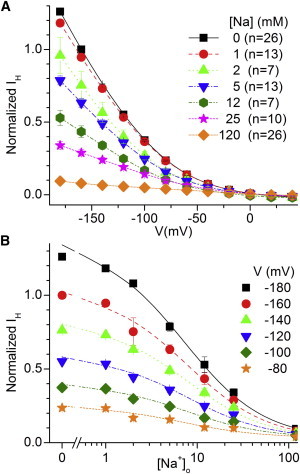
Na+o inhibits IH at pH 6.0. (A) Ouabain-sensitive I/V curves at the indicated [Na+]. Currents were normalized to IH measured at −160 mV in the absence of Na+. Data are expressed as the mean ± SE. (B) [Na+]o dependence of inward currents at different voltages. Line plots are fits of a Michaelis-Menten equation to the whole data set from individual cells at each voltage with K0.5 (in mM) 7.2 ± 0.8 (−180 mV), 7.9 ± 1.3 (−160 mV), 7.9 ± 0.9 (−140 mV), 7.9 ± 1.1 (−120 mV), 7.5 ± 1.2 (−100 mV), and 6.7 ± 1.4 (−80 mV). To see this figure in color, go online.
Figure 7.
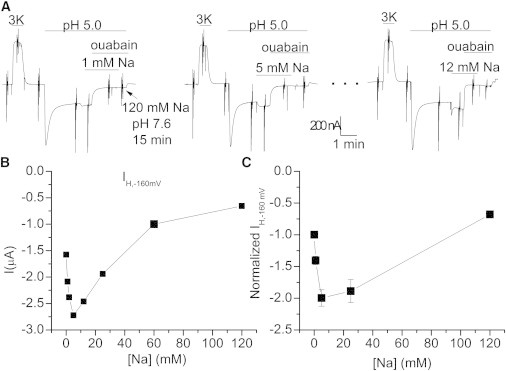
Effect of [Na+] on ouabain-sensitive currents at pH 5.0. (A) Excerpt traces from a continuous recording from an oocyte where 0, 1, 2, 5, 12, 25, 50, and 120 mM Na+ were tested. Each application of pH 5.0 solution was preceded by an application of 3 mM K+ to ensure that the total number of pumps did not change. Applications of 1, 5, and 12 mM are shown after stabilization of IH at pH 5.0. Ouabain was then added in the presence of Na+ and Na+ was then removed in the presence of steroid. Ouabain was washed out for 15 min in 120 mM Na+ (pH 7.6) before the next maneuver. (B) Full dose response for current activation at −160 mV in the oocyte shown in A. (C) Mean ± SE) at −160 mV, normalized to the steady-state current at 0 Na+, at concentrations where n > 8.
The whole data set fitted by a Hill equation (line plots) yielded a voltage-independent K0.5 (∼7 mM). A nearly identical inhibition by Na+o was observed at pH 7.6, with a similar voltage-independent apparent affinity (cf. Fig. 8 C). These results are qualitatively different from the effects observed in myocytes at pH 6.0, where a two-fold increase in IH was induced by 5 mM Na+o, after which higher Na+ concentrations inhibited IH (Fig. 3). Our results also differ from results reported for the wild-type α1β3 pump in Xenopus oocytes, where an ∼20% activation at 5 mM Na+o was followed by inhibition at higher [Na+o] (11). We did observe a small increase in current at 1 mM Na+o in oocytes overexpressing wild-type α1β3 pumps (Fig. S2).
Figure 8.
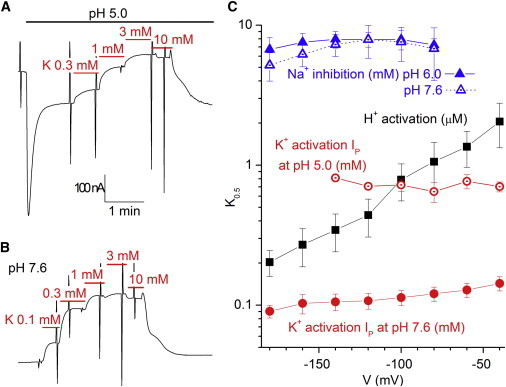
Reduction of K+ apparent affinity at high [H+]. (A) Continuous trace from an oocyte held at −50 mV, where step changes in [K+] were performed at pH 5.0. After the normal biphasic response to pH 5.0, step changes in [K+]o activated outward IP in a saturating, reversible fashion. (B) Continuous trace from the oocyte in A showing the increase in IP in response to step changes in [K+]o. Note the difference in percentage of current induced by subsaturating [K+]o compared to A. (C) Voltage dependence of K0.5 for the different effects on the RD-α1β3 pump described in this article. Note the logarithmic scale. The units for the different apparent dissociation constants are indicated in the plot label. K0.5 for K+ activation of IP at pH 5.0 represents the mean ± SE from five oocytes and those at pH 7.6 are the mean ± SE from 12 experiments. The other K0.5 and their error bars come from the fits described in Figs. 4 and 6 (except for Na inhibition at pH 7.6, from experiments similar to those in Fig. 6, but not shown).
Na+o had a drastically different effect on ouabain-sensitive currents at pH 5 (Fig. 7) and pH 4.0 (Fig. S3). Fig. 7 A shows part of an experiment in which 0, 1, 2, 5, 12, 25, 50, and 120 mM Na+o were tested at pH 5.0 in a single oocyte. Each application of the pH 5.0 solution was preceded by a brief application of 3 mM K+ to ensure that the total number of pumps did not change. Applications of 1, 5, and 12 mM Na+ (after the inhibitory phase had stabilized at pH 5.0) increased the inward current, whereas higher Na+ concentrations reduced IH. The current measured at −160 mV in this oocyte at all Na+ concentrations is shown in Fig. 7 B. The average normalized current data for concentrations where large numbers of oocytes were tested—1 (n = 13), 5 (n = 14), 25 (n = 8), and 120 mM Na+ (n = 18)—are shown in Fig. 7 C (2 and 12 mM were only tested in two oocytes). Note that 25 mM Na+ induced a twofold increase of the current compared to 0 mM Na+, similar to the observations on endogenous pumps at pHo 5.6 (cf. Fig. 5 in Vasilyev et al. (21)). Fig. S3 shows that the inhibitory effect of high external Na+ was not observed at pH 4, where 125 mM Na+ induced an even larger increase in current than did 5 mM Na+.
Figs. 6 and 7 demonstrate that the interactions between Na+ and H+ are complex. It appears that at high H+ concentration, the first Na+ to bind externally (thought to interact with site I or site II) kicks the protons off the inhibitory sites. If inhibitory protons occupied transport sites I and II, one would expect them to compete with K+ and thus reduce the apparent affinity for K+ activation of IP at pH 5.0. This is exactly the case, as shown in Fig. 8. Fig. 8 A shows the continuous recording from an experiment in which step changes in K+o concentration were performed at pH 5.0, inducing progressively larger outward current up to 3 mM K+. Fig. 8 B shows activation by K+ in the same oocyte at pH 7.6. There is a clear reduction in K+o affinity induced by 10 μM H+. The statistical significance of this difference is illustrated in Fig. 8 C, which plots the voltage dependence for the K0.5 for K+ in both conditions (i.e., nearly a 10-fold reduction in apparent affinity for K+ at pH 5.0 (open red circles) vs. pH 7.6 (solid red circles)). The same semilogarithmic plot also shows the effect of voltage on the apparent affinities described in this work. Note that activation of IH by H+ (black squares) is the only apparent affinity that presents strong voltage dependence, whereas inhibition of IH by Na+ at pH 6.0 (solid blue triangles) or 7.6 (open blue triangles) and activation of pump current by K+ at pH 7.6 (solid red circles) or 5.0 (open red circles) is nearly voltage-independent.
Discussion
Characterization of Na/K-pump-mediated leak currents in a mammalian preparation
The first goal of our study was to use a classical ventricular myocyte preparation to demonstrate that IH is a characteristic property intrinsic to Na/K pumps and not an artifact of the oocyte expression system. Such an artifact could arise, for instance, due to specific protein-lipid interactions in the oocyte membrane. Our results also demonstrate that the current has similar characteristics even in the presence of the normal regulatory subunits of the pump, in this case, phospholemman, FXYD1, which is prevalent in cardiac tissue (22–24).
Our results go beyond demonstrating that IH is not a curiosity of the oocyte membrane. Its characteristics in ventricular myocytes are important to understanding the mechanisms involved and the pathophysiological consequences that could arise when the features of IH are altered by Na/K-pump mutations that exhibit IH at physiological Na+o concentrations. In this respect, Beuschlein et al. (17) recently demonstrated that mutations of the Na/K pump α1 subunit in adenomas produce hyperaldosteronism-induced hypertension. Shortly thereafter, Azizan et al. (16) showed that most of the hyperaldosteronism-inducing mutants described the presence of leak currents at near-physiological salt concentration (∼100 mM Na+, 4 mM K+) in voltage-clamped oocytes. The authors suggested that this leak may be the cause of the depolarization reported by Beuschlein et al. in tumoral adrenal cells (17).
In addition, >50 mutations of the gene encoding the Na/K pump α2 subunit (ATP1A2) have been linked to familial hemiplegic migraine (FHM) type II (25,26). Reportedly, patients with α2 mutations present with varied symptoms that go from simple migraine to migraine with aura and epileptic episodes (see, e.g., Lebas et al. (27)). Although not all of the functional properties of these mutants have been reported, the mutants that have been characterized demonstrate important impairments in their enzymatic properties (25,28,29) or in their trafficking and expression at the plasma membrane (30). Indeed, leak currents at near-physiological Na+ and K+ concentrations have been reported in FHM-α2 mutants expressed in Xenopus oocytes (15,29). It is therefore conceivable, but has not yet been demonstrated, that the presence or absence of IH at mammalian physiological Na+ concentrations plays a role in the pathogenesis of FHM. As discussed below, the detailed description of H+- and Na+-concentration dependencies of leak currents in myocytes and in ouabain-resistant pumps in oocytes provides clues to the mechanisms underlying the leak at physiological Na+ concentrations upon mutation.
pHo dependence of Na/K-pump-mediated leak currents
The pHo dependence of leak currents has been studied previously in Xenopus oocytes using Na/K pumps from several sources and different Na-pump mutants. Most of the authors of those reports limited their evaluation to relatively high pH values, and the inhibitory effects of the very low pH values used here were never demonstrated. Our results show that H+ induces a more complex response than previously thought, and they strongly suggest that the two different phenomena featured in this response, activation and inhibition, result from interactions with different ion-binding sites.
Wang and Horisberger (11) demonstrated that protons are the permeating ion through this noncanonical mode of transport by the Na/K pump, whereas Efthymiadis et al. (31) studied the pH dependence of steady-state endogenous pump currents in Xenopus oocytes at pH values between 7.3 and 6.3. Those authors reported a mildly voltage-dependent K0.5 for H+ with a Hill coefficient of 1.79 and also observed activation by external Na+ at pH 6.5, indicating that in endogenous pumps, H+ partially inhibits the current at pH 6.5. Our results in ventricular myocytes were similar, with a relatively mildly voltage-dependent K0.5 for H+ and a Hill coefficient of 1.8. We believe that inhibition by H+ (discussed below) generates a higher apparent affinity for H+ activation (because the inhibition would appear initially as saturation of activation). It is important to note that even with this misleading increased affinity, the apparent affinity for H+ activation in myocytes is lower than that in oocytes.
Because we can separate inhibition from activation over a wide pHo range, the data in Fig. 4 C more accurately display the true pHo dependence of current activation. The inset convincingly shows a strong voltage dependence of H+ activation. The exponential voltage dependence of K0.5H (Fig. 4 C, inset) translates to an electrical distance, λ = 0.51, similar to the distance of λ = 0.49 reported by Sagar and Rakowski (32) for Na+o inhibition of endogenous Xenopus Na/K-pump currents, but lower than λ = 0.71 and 0.69, values reported for the slow component of Na+-dependent transient charge movement in squid giant axons (33) and endogenous Na/K pumps in oocytes (34), respectively. Nevertheless, Fig. 8 clearly shows the stronger voltage-dependent activation of IH compared to the activation of pump current by K+ or Na+ inhibition of leak currents at pH values of 7.6 and 6.0 (also shown in Fig. 6). Based on this strong voltage dependence, our data support previous proposals indicating that protons leak through the site responsible for Na+- and voltage-dependent inhibition of pump current (12,14).
Inhibition by H+
A finding of this work, that to our knowledge is new, is the dual effect of H+, i.e., that low pH not only activates a current but paradoxically also inhibits the current. Reasons why the biphasic activation/inhibition response described here may not have been reported before are that 1), it would not be evident if the inhibitory effect is nearly as fast as the activation, which could be the case in endogenous pumps, since they seem to have higher affinity for H+ inhibition; or 2), if the solution exchange is too slow, it may be impossible to distinguish the activation phase from the inhibition phase. Also, a small biphasic response could be easily overlooked as an effect unrelated to the pump, particularly if irreversible inhibitors are used, as is the case with ouabain in endogenous pumps in oocytes.
Initially, based on the slow kinetics of inhibition at pH 5.0 (τ = 11.1 ± 0.6 s (n = 13)), we favored the idea that intracellular proton accumulation was responsible for these effects. However, the experiment in Fig. 5 demonstrates that the protons leaking through the Na/K pump do not inhibit IH due to accumulation. Intracellular accumulation would also be unlikely, as we Na+-load the oocytes with a 150 mM HEPES solution (pH 7.2 with NaOH; see Methods) and HEPES (molecular mass 238 g/mol) is expected to enter the oocyte during the 1-h Na-loading procedure because it is clearly small enough to permeate connexins opened by the loading treatment. Molecules as big as gluconate− (molecular mass 196 g/mol) and tetrabutylammonium+ (molecular mass 259 g/mol) are just fivefold less permeable than K+ through endogenous connexins (35), whereas the larger sucrose, and even ATP, can permeate purified connexin 26 (36). A high HEPES concentration would thus provide ample intracellular buffering capacity. Consequently, the next logical mode of action to investigate was whether this effect may be due to H+ binding to ion-transport sites.
Transport of H+ as a substitute for Na+i or K+o in the Na/K pump has been reported previously (37,38). Apell et al. (38) demonstrated that the stoichiometry of the pump exchanging H+o for H+i is not electrogenic, but more relevant for our study, the authors addressed H+ binding to extracellular sites in E2P. It can be seen from Fig. 4 in their study using purified enzyme that only above 1 μM H+ (below pH 6.0) is there more than one site significantly occupied by H+, with the two sites being saturated at pH ∼5.0 (from extrapolation of the dotted line). These observations are in quantitative agreement with the idea that two protons occupy sites I and II at pH <5.0, where >80% of IH is inhibited in our RD-mutant pumps (Fig. S1). It seems that there is no significant 2H+o/3Na+i exchange, because maximal K+-induced currents at pH 5.0 have amplitudes similar to those at pH 7.6. However, we cannot rule out the possibility that the ATPase is working with a reduced Na+ stoichiometry, similar to that described for H+/H+ exchange (38). Also, it should be pointed out that when we talk about H+ at the ion-binding sites, we are suggesting not titration of acidic side chains, as recently reported to modulate K/Na selectivity (39), but rather binding of H+ or hydronium as substitutes for the transported ions.
The strong suggestion that protons occupy the K+ binding sites at 10 μM H+ is based on the large reduction in K+ apparent affinity at pH 5.0 (∼10-fold; Fig. 8), which is consistent with competition between the two cations. Taking this result together with the observation that neither inhibition by H+o nor that by Na+o is voltage-dependent at pH values 7.6 and 6.0 (Figs. S1 and S3, respectively, and Fig. 8 B), similar to the well established finding that external K+ binding is mildly voltage-dependent (32,40) (Fig. 8), directly supports a model in which inhibition of IH by external cations requires binding to the sites that are not responsible for the Na/K-pump voltage dependence. Although the contribution of sites I and II to voltage-dependent inhibition has not been established, reports indicate that disruption of site III almost completely abolishes the pump’s voltage dependence. Therefore, our results support the notion that binding of two protons, two K+, or two Na+ ions to sites I and II inhibits leak currents that normally occur through site III. Whether blocking of the leak reflects mere ion binding or the concomitant conformational change that follows binding of two ions from a single ion species to sites I and II remains to be elucidated.
Activation by Na+ at low pHo
A biphasic effect of Na+ on leak currents, activating at lower and inhibiting at higher Na+ concentrations, was previously reported for ouabain-sensitive pumps (11,21,31), but as those articles addressed Na+ effects at a single pHo, the link between H+ inhibition and Na+ activation was not identified. We believe that this complex interaction between Na+ and H+ underlies the difficulties and controversies regarding the mechanisms involved in leak-current generation, as many of the characteristic differences in IH reported to date in different systems can be explained by small shifts in apparent affinities for these two ions at the different sites.
Vedovato and Gadsby (8) reported that compared to wild-type α1β3 pumps, the ouabain-insensitive RD-α1β3 mutant (used here) has an approximately twofold lower affinity for both Na+o and K+o. Along these lines, the activation by Na+ observed at pH 6.0 in wild-type α1β3 pumps (Fig. S2) (11), endogenous Xenopus pumps (21,31), and guinea pig ventricular pumps (Fig. 3), compared to the RD mutant, may also reflect a higher affinity for H+ at sites I and II and thus have a higher occupancy of these sites by H+ at higher pHo values. This will make Na+-induced activation more evident, as it appears to reflect relief of H+ inhibition. In other words, the leak would be activated by Na+ when Na+ kicks off one H+ from the fraction of pumps with two bound H+ ions, giving rise to pumps with mixed occupancy of one H+ and one Na+, which permits leakage through site III.
It could be argued that our hypothesis is wrong because the nearly twofold increase induced by 5 mM Na+ in ventricular myocytes means that these pumps should have been at least ∼50% inhibited by H+ at pH 6, and that this should have been observed in the H+ dependence of IH shown in Fig. 2. However, we must note that the affinity for H+ activation at negative voltages is reduced in myocytes in steady state compared to that in oocytes at the peak of low pHo activation (cf. legends of Fig. 2 and Fig. 4). This is so even when the inhibitory effect of H+ gives a larger apparent affinity for activation by H+ in the myocyte. Thus, the lack of clear inhibition in the dose-response curve of Fig. 2 may reflect the reduced apparent affinity for H+ at site III in ventricular myocytes.
It is obvious that activation by Na+ cannot reflect binding to the H+ permeation site, as this will result in inhibition. It is also unlikely that permeation of Na+ occurs through the Na+-exclusive site, because if that were the case, we would expect to observe IH when applying low Na+o at higher pHo values, something that has not been reported in wild-type or ouabain-resistant pumps (except for mutations with large effects on ion binding, discussed below) in the ample body of literature dealing with various mammalian or oocyte systems. Likewise, we did not observe Na+ stimulation of IH at pH 7.6 or at pH 6.0 in RD pumps. Finally, the inhibitory effect of high Na+ at pH 5.0, where significant inward current is observed (Fig. 7), or the lack of inhibition by 125 mM Na+ at pH 4.0 (Fig. S3), where only current activation is observed, demonstrates a much lower apparent affinity for inhibition at pH 4.0 and 5.0 than at pH 6.0 or 7.6 (Figs. 6 and 8 B). Therefore, observations at pH 4.0 and 5.0 are consistent with a reduced affinity for binding of Na to site II due to H+ (or hydronium) occupancy of the sites, the interpretation we favor.
The biphasic effects of extracellular Na+ on inward current (activation and then inhibition) are similar but in opposite direction to its effects on Na-ATPase activity (41) or ATP-ADP exchange (42), two functional modes of the Na/K pump that are inhibited by low concentrations and activated by high concentrations of external Na+. These Na+ effects on IH may reflect interactions of the Na+ ions with the same sites they interact with in these other modes of operation. Alternatively, the activation by Na+ may reflect binding to a nontransport allosteric site similar to the one recently proposed in ventricular myocytes (43). However, this interpretation still requires the displacement of a previously bound H+ and cannot easily explain the large reduction of K+ apparent affinity at high H+ concentration.
Mechanism of the effects of mutations that modify leak currents
As discussed above, robust leak currents have been described at physiological external H+, K+, and Na+ concentrations in mutants that induce hypertension (16) or familial hemiplegic migraine (15,29). These leak currents are accompanied by large modifications of the Na/K-pump ion-binding reactions. Similar leaks associated with large modifications of ion-binding reactions have been observed at physiological pH and Na+ concentrations in Na/K pumps with C-terminal deleted α subunits (7,8,9). Although several mechanisms may be involved in induction of leak currents by the diverse mutations, our results lead us to speculate about one possible mechanism involved in induction of such leaks at physiological Na+ concentrations, and to explain the effects of Na+-activating leak currents in pumps with C-terminal deletions.
For instance, the monotonic increase in leak current induced by increasing Na+ concentrations at pHo 7.6 in C-terminally deleted pumps (RD-ΔKESYY (7)) may reflect an altered affinity between Na+ and H+ for sites I and II such that more double-H+ occupancy at pH 7.6 in the absence of Na+ leads to an increase in current. Then, as the Na+ concentration is raised at this very low H+ concentration, a mixed occupancy of sites I and II by Na+ and H+ at pH 7.6 is favored, which allows H+ leak through site III. Future studies of the combined effects of protons and Na+ on these mutants, over a broad range of ion concentrations, may provide a definitive resolution to this issue.
Acknowledgments
Note added in proof: At the time this article was under revision, an article addressing the effect of ion-binding site mutagenesis on leak currents mediated by Na/K pumps expressed in oocytes was published by Vedovato and Gadsby (44). Their proposal of protons transiting the Na-exclusive site III is consistent with our findings.
We thank Dr. Luis Reuss for comments on the manuscript, Beatriz A. Velez for excellent technical assistance in oocyte, myocyte, and solution preparation, and Sukanyalakshmi Chebrolu for help with the experiments at pH 4.0 (illustrated in Fig. S3).
This work was supported by grants from the National Science Foundation (MCB-1243842) to P.A. and From NIH (GM061583) to C.G.
Footnotes
Travis J. Mitchell and Camila Zugarramurdi contributed equally to this work.
Supporting Material
References
- 1.Sen A.K., Post R.L. Stoichiometry and localization of adenosine triphosphate-dependent sodium and potassium transport in the erythrocyte. J. Biol. Chem. 1964;239:345–352. [PubMed] [Google Scholar]
- 2.Rakowski R.F., Gadsby D.C., De Weer P. Stoichiometry and voltage dependence of the sodium pump in voltage-clamped, internally dialyzed squid giant axon. J. Gen. Physiol. 1989;93:903–941. doi: 10.1085/jgp.93.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shinoda T., Ogawa H., Toyoshima C. Crystal structure of the sodium-potassium pump at 2.4 Å resolution. Nature. 2009;459:446–450. doi: 10.1038/nature07939. [DOI] [PubMed] [Google Scholar]
- 4.Kanai R., Ogawa H., Toyoshima C. Crystal structure of a Na+-bound Na+,K+-ATPase preceding the E1P state. Nature. 2013;502:201–206. doi: 10.1038/nature12578. [DOI] [PubMed] [Google Scholar]
- 5.Binda F., Lute B.J., Galli A. The N-terminus of the norepinephrine transporter regulates the magnitude and selectivity of the transporter-associated leak current. Neuropharmacology. 2006;50:354–361. doi: 10.1016/j.neuropharm.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Ryan R.M., Mindell J.A. The uncoupled chloride conductance of a bacterial glutamate transporter homolog. Nat. Struct. Mol. Biol. 2007;14:365–371. doi: 10.1038/nsmb1230. [DOI] [PubMed] [Google Scholar]
- 7.Yaragatupalli S., Olivera J.F., Artigas P. Altered Na+ transport after an intracellular α-subunit deletion reveals strict external sequential release of Na+ from the Na/K pump. Proc. Natl. Acad. Sci. USA. 2009;106:15507–15512. doi: 10.1073/pnas.0903752106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vedovato N., Gadsby D.C. The two C-terminal tyrosines stabilize occluded Na/K pump conformations containing Na or K ions. J. Gen. Physiol. 2010;136:63–82. doi: 10.1085/jgp.201010407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meier S., Tavraz N.N., Friedrich T. Hyperpolarization-activated inward leakage currents caused by deletion or mutation of carboxy-terminal tyrosines of the Na+/K+-ATPase α subunit. J. Gen. Physiol. 2010;135:115–134. doi: 10.1085/jgp.200910301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rakowski R.F., Vasilets L.A., Schwarz W. A negative slope in the current-voltage relationship of the Na+/K+ pump in Xenopus oocytes produced by reduction of external [K+] J. Membr. Biol. 1991;121:177–187. doi: 10.1007/BF01870531. (K+) [DOI] [PubMed] [Google Scholar]
- 11.Wang X., Horisberger J.D. A conformation of Na+-K+ pump is permeable to proton. Am. J. Physiol. 1995;268:C590–C595. doi: 10.1152/ajpcell.1995.268.3.C590. [DOI] [PubMed] [Google Scholar]
- 12.Li C., Geering K., Horisberger J.D. The third sodium binding site of Na,K-ATPase is functionally linked to acidic pH-activated inward current. J. Membr. Biol. 2006;213:1–9. doi: 10.1007/s00232-006-0035-0. [DOI] [PubMed] [Google Scholar]
- 13.Reference deleted in proof.
- 14.Nyblom M., Poulsen H., Nissen P. Crystal structure of Na+, K+-ATPase in the Na+-bound state. Science. 2013;342:123–127. doi: 10.1126/science.1243352. [DOI] [PubMed] [Google Scholar]
- 15.Poulsen H., Khandelia H., Nissen P. Neurological disease mutations compromise a C-terminal ion pathway in the Na+/K+-ATPase. Nature. 2010;467:99–102. doi: 10.1038/nature09309. [DOI] [PubMed] [Google Scholar]
- 16.Azizan E.A., Poulsen H., Brown M.J. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat. Genet. 2013;45:1055–1060. doi: 10.1038/ng.2716. [DOI] [PubMed] [Google Scholar]
- 17.Beuschlein F., Boulkroun S., Reincke M. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat. Genet. 2013;45:440–444. doi: 10.1038/ng.2550. [DOI] [PubMed] [Google Scholar]
- 18.Artigas P., Ferreira G., Pizarro G. Effects of the enantiomers of BayK 8644 on the charge movement of L-type Ca channels in guinea-pig ventricular myocytes. J. Membr. Biol. 2003;193:215–227. doi: 10.1007/s00232-003-2020-1. [DOI] [PubMed] [Google Scholar]
- 19.Artigas P., Gadsby D.C. Large diameter of palytoxin-induced Na/K pump channels and modulation of palytoxin interaction by Na/K pump ligands. J. Gen. Physiol. 2004;123:357–376. doi: 10.1085/jgp.200308964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratheal I.M., Virgin G.K., Artigas P. Selectivity of externally facing ion-binding sites in the Na/K pump to alkali metals and organic cations. Proc. Natl. Acad. Sci. USA. 2010;107:18718–18723. doi: 10.1073/pnas.1004214107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vasilyev A., Khater K., Rakowski R.F. Effect of extracellular pH on presteady-state and steady-state current mediated by the Na+/K+ pump. J. Membr. Biol. 2004;198:65–76. doi: 10.1007/s00232-004-0660-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crambert G., Fuzesi M., Geering K. Phospholemman (FXYD1) associates with Na,K-ATPase and regulates its transport properties. Proc. Natl. Acad. Sci. USA. 2002;99:11476–11481. doi: 10.1073/pnas.182267299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Despa S., Bossuyt J., Bers D.M. Phospholemman-phosphorylation mediates the β-adrenergic effects on Na/K pump function in cardiac myocytes. Circ. Res. 2005;97:252–259. doi: 10.1161/01.RES.0000176532.97731.e5. [DOI] [PubMed] [Google Scholar]
- 24.Bossuyt J., Despa S., Bers D.M. Isoform specificity of the Na/K-ATPase association and regulation by phospholemman. J. Biol. Chem. 2009;284:26749–26757. doi: 10.1074/jbc.M109.047357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morth J.P., Poulsen H., Nissen P. The structure of the Na+,K+-ATPase and mapping of isoform differences and disease-related mutations. Philos. Trans. R. Soc. Lond. B, Biol. Sci. 2009;364:217–227. doi: 10.1098/rstb.2008.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pietrobon D., Moskowitz M.A. Pathophysiology of migraine. Annu. Rev. Physiol. 2013;75:365–391. doi: 10.1146/annurev-physiol-030212-183717. [DOI] [PubMed] [Google Scholar]
- 27.Lebas A., Guyant-Maréchal L., Parain D. Severe attacks of familial hemiplegic migraine, childhood epilepsy and ATP1A2 mutation. Cephalalgia. 2008;28:774–777. doi: 10.1111/j.1468-2982.2008.01603.x. [DOI] [PubMed] [Google Scholar]
- 28.Blostein R., Segall L., Gargus J.J. ATP1A2: a key player in familial hemiplegic migraine. Med. Sci. (Paris) 2006;22:341–343. doi: 10.1051/medsci/2006224341. [DOI] [PubMed] [Google Scholar]
- 29.Tavraz N.N., Friedrich T., Dichgans M. Diverse functional consequences of mutations in the Na+/K+-ATPase α2-subunit causing familial hemiplegic migraine type 2. J. Biol. Chem. 2008;283:31097–31106. doi: 10.1074/jbc.M802771200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tavraz N.N., Dürr K.L., Friedrich T. Impaired plasma membrane targeting or protein stability by certain ATP1A2 mutations identified in sporadic or familial hemiplegic migraine. Channels (Austin) 2009;3:82–87. doi: 10.4161/chan.3.2.8085. [DOI] [PubMed] [Google Scholar]
- 31.Efthymiadis A., Schwarz W. Conditions for a backward-running Na+/K+ pump in Xenopus oocytes. Biochim. Biophys. Acta. 1991;1068:73–76. doi: 10.1016/0005-2736(91)90062-d. [DOI] [PubMed] [Google Scholar]
- 32.Sagar A., Rakowski R.F. Access channel model for the voltage dependence of the forward-running Na+/K+ pump. J. Gen. Physiol. 1994;103:869–893. doi: 10.1085/jgp.103.5.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holmgren M., Wagg J., Gadsby D.C. Three distinct and sequential steps in the release of sodium ions by the Na+/K+-ATPase. Nature. 2000;403:898–901. doi: 10.1038/35002599. [DOI] [PubMed] [Google Scholar]
- 34.Holmgren M., Rakowski R.F. Pre-steady-state transient currents mediated by the Na/K pump in internally perfused Xenopus oocytes. Biophys. J. 1994;66:912–922. doi: 10.1016/s0006-3495(94)80867-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y., McBride D.W., Jr., Hamill O.P. The ion selectivity of a membrane conductance inactivated by extracellular calcium in Xenopus oocytes. J. Physiol. 1998;508:763–776. doi: 10.1111/j.1469-7793.1998.763bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fiori M.C., Figueroa V., Altenberg G.A. Permeation of calcium through purified connexin 26 hemichannels. J. Biol. Chem. 2012;287:40826–40834. doi: 10.1074/jbc.M112.383281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polvani C., Blostein R. Protons as substitutes for sodium and potassium in the sodium pump reaction. J. Biol. Chem. 1988;263:16757–16763. [PubMed] [Google Scholar]
- 38.Apell H.J., Benz G., Sauerbrunn D. Proton diet for the sodium pump. Biochemistry. 2011;50:409–418. doi: 10.1021/bi101576s. [DOI] [PubMed] [Google Scholar]
- 39.Yu H., Ratheal I.M., Roux B. Protonation of key acidic residues is critical for the K+-selectivity of the Na/K pump. Nat. Struct. Mol. Biol. 2011;18:1159–1163. doi: 10.1038/nsmb.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heyse S., Wuddel I., Stürmer W. Partial reactions of the Na,K-ATPase: determination of rate constants. J. Gen. Physiol. 1994;104:197–240. doi: 10.1085/jgp.104.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glynn I.M., Karlish S.J. ATP hydrolysis associated with an uncoupled sodium flux through the sodium pump: evidence for allosteric effects of intracellular ATP and extracellular sodium. J. Physiol. 1976;256:465–496. doi: 10.1113/jphysiol.1976.sp011333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaplan J.H., Hollis R.J. External Na dependence of ouabain-sensitive ATP:ADP exchange initiated by photolysis of intracellular caged-ATP in human red cell ghosts. Nature. 1980;288:587–589. doi: 10.1038/288587a0. [DOI] [PubMed] [Google Scholar]
- 43.Garcia A., Fry N.A., Clarke R.J. Extracellular allosteric Na+ binding to the Na+,K+-ATPase in cardiac myocytes. Biophys. J. 2013;105:2695–2705. doi: 10.1016/j.bpj.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vedovato N., Gadsby D.C. Route, mechanism, and implications of proton import during Na+/K+ exchange by native Na+/K+-ATPase pumps. J. Gen. Physiol. 2014;143:449–464. doi: 10.1085/jgp.201311148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


