Abstract
Background/Objectives
Caregivers of patients with serious illness endure significant burden, yet it is not clear at what stage of advanced illness patient and caregiver needs are greatest. This study compared prevalence and predictors of caregiver esteem and burden during two different stages of patients’ illnesses – advanced chronic illness and the last year of life.
Design
Longitudinal, observational cohort study.
Setting
Community sample recruited from outpatient clinics at Duke University and Durham VA Medical Centers.
Participants
Patients living with advanced cancer, congestive heart failure, or chronic obstructive pulmonary disease and their primary caregiver, retrospectively coded as chronic-illness (n=62) or end-of-life (n=62) patient-caregiver dyads.
Measurements
We measured caregiver experience monthly with the Caregiver Reaction Assessment (CRA), which includes caregiver esteem and 4 domains of burden: schedule, health, family, and finances.
Results
During both chronic-illness and end-of-life, high caregiver esteem was almost universal (95%); health, family, and financial burden were endorsed by <25% of the sample. Schedule burden was the most prevalent form of burden and was experienced more frequently by end-of-life caregivers (58%) than the chronic-illness caregivers (32%). Caregiver esteem and all dimensions of burden were relatively stable over one year. Few factors were associated with burden.
Conclusion
Caregiver experience is relatively stable over one year and similar among caregivers of patients in the last year of life and those further upstream in advanced illness. Schedule burden stands out as most prevalent and variable among dimensions of experience. Because prevalence of burden is not specific to stage of illness and is relatively stable over time, multidisciplinary healthcare teams should assess caregiver burden and refer burdened caregivers to supportive resources early in the course of chronic illness.
Keywords: caregiving, caregiver burden, chronic illness, end of life
INTRODUCTION
Informal caregivers provide extensive care for spouses, parents, and loved ones with serious chronic illness. Approximately 43.5 million U.S. adults provide an average of 19 hours of unpaid informal care per week for someone aged > 50 years.1 The demand for informal caregivers is expected to rise by more than 85% over the next few decades due to the growing population of older adults, many of whom will experience significant functional impairment related to chronic illness.2 Understanding and addressing the needs of informal caregivers will become increasingly important to physicians and other providers who care for older adults.
Caregivers’ tasks include both direct assistance (e.g., helping with activities of daily living, medication and lifestyle management) and less visible tasks such as monitoring symptoms and navigating health care systems.3, 4 The demands of caregiving often spawn additional stressors in caregivers’ lives, commonly referred to as caregiver burden.5–7 Caregiver burden is associated with anxiety, depression, and insomnia; decreased use of preventive services; poor self-rated health; and increased mortality for caregivers.8, 9 Caregiver burden is also associated with patient outcomes, including placement in long term care facilities.10
Although caregiver burden is associated with the care recipient’s functional impairment and illness severity,11, 12 available data suggest that caregiver characteristics may be more important than patient characteristics.13–17 Social support,7, 18 including desire for more help from family and friends,15 need for more help with daily tasks,13 and coping resources,8 may be especially important for burden. These studies offer important insight into factors which may increase risk for burden and poor caregiver outcomes, but there are a number of limitations to existing research. First, the majority of studies have been cross-sectional7, 16, 18 or retrospective,12 and the small amount of longitudinal research13, 19 has suffered from high rates of drop-out among caregivers of patients near the end of life. Longitudinal research with frequent data collection is needed to promote our understanding of caregiver burden as illness progresses.8, 14, 20, 21 Second, previous studies have focused on one patient population, often those with dementia or cancer. Levels of burden are similar between cancer caregivers and those caring for persons with organ failure like congestive heart failure (CHF) and chronic obstructive pulmonary disease (COPD),13, 15 but examination of burden across stages of illness is needed due to the unique disease trajectory associated with organ failure.8 Third, previous studies have used statistical methods that aggregate trends of change over time over a heterogeneous sample; person-centered analyses are needed to analyze individual and group-based trends over time.22, 23 Fourth, many studies have examined palliative care samples at the end of life, but few have compared different stages of advanced illness.8
The aims of these analyses were to: (a) describe the experience of caregivers for a diverse group of patients living with advanced cancer, COPD, and CHF (Aim 1); (b) compare experience (caregiver esteem, health burden, family burden, financial burden, and schedule burden) during one year of advanced illness (chronic-illness group) and the last year of a patient’s life (end-of-life group) (Aim 2); and (c) examine associations among patient factors, caregiver resources, and caregiver experience among the total sample and between groups (Aim 3).
METHODS
Data Source
Data are from the Pathways study, a longitudinal cohort study of patients living with serious illness, including advanced cancer, CHF, or COPD with hypercapnea; some patients identified a caregiver to participate in the study. Community-dwelling patients were recruited through hospital databases at Duke University Hospital and the Durham VA Medical Center. To observe illness trajectories from serious illness to death, patients were recruited based on clinical criteria associated with an estimated 50% two-year survival.24 The study was approved by the Duke University Medical Center and Durham VAMC Institutional Review Boards and both patients and caregivers provided informed consent. The current study analyzes patient-caregiver dyads within the Pathways dataset to address one specific aim of the parent study – to describe caregivers’ experiences of esteem and burden over time – and follow up on previous cross-sectional research concerning caregiver well-being in the Pathways Study.15
The Pathways study included 210 patients who participated in at least one interview; 146 identified a primary caregiver, the person who “spends the most time with you providing support, help, companionship, or care.” Patients and caregivers each participated in interviews every four weeks for 2–6 years. Although the recruitment criteria were designed to prospectively identify patients with an estimated 50% two year survival, there was significant variation in survival during the 2–6 years following study enrollment. Approximately half of the patients died during the study period, an average of 23 months after enrollment (range 3 to 66 months); the other half of the sample lived through the duration of the study. Therefore, at the time of enrollment in this prospective study, some patients were near the end of life while others were earlier in the trajectory of serious illness. This variation allowed us to retrospectively identify dyads that were observed during the patient’s last year of life and dyads that were observed during advanced chronic illness.
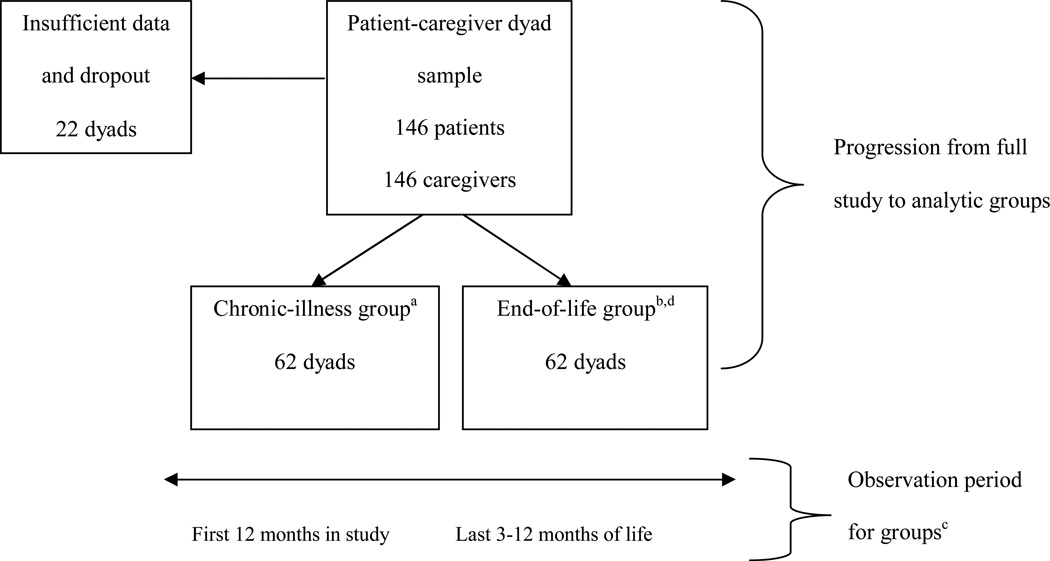
Within the patient-caregiver dyad sample (n=146), dyads with incomplete data or too few data points (<3 for end-of-life, <12 for chronic-illness) were excluded from the study (n=22; Figure 1). Our analytic sample included 124 patient-caregiver dyads (85% of the full sample); there was no significant difference in baseline caregiver burden between the final analytic sample and the 22 dyads with missing data, mostly due to geographic relocation. The analytic sample contains mutually exclusive groups representing two stages of advanced illness: advanced chronic illness prior to the last year of life (chronic-illness) and the last year of life (end-of-life). The chronic-illness group includes dyads that participated for 30 or more months after they enrolled and were alive at the end of the study (n=62); in this paper we analyze observations from the first 12 months of their study participation. The end-of-life group includes dyads in which the patient died at any time during the study period (n=62); dyads in which the patient lived for at least 30 months after enrollment and died during the study (n=18) were assigned to the end-of-life group to create balanced, mutually exclusive groups. They participated up to an average of 66 days before death, with a range of 5 to 364 days from last interview to date of death. In this paper we analyze observations from the last year of the patient’s life (3–12 months of data). A separate chronic-EOL exploratory group includes these 18 dyads with unique data during both stages. The presence of 18 dyads with sufficient chronic-illness and end-of-life data enabled exploratory examination of change from chronic-illness through end-of-life within dyads.
Figure 1. Analysis Groups and Observation Periods.
a The chronic-illness group includes 62 dyads that were retrospectively identified based on two criteria: (1) 12 months of complete data starting at baseline, and (2) at least 6 months of follow-up observation that does not include the last year of life or dropout from the study.
b The end-of-life group includes 62 dyads who were retrospectively identified based on two related criteria: (1) 3–12 months of complete data (2) during the last year of life. Eighteen of these dyads participated for several years and fit the criteria for both chronic-illness and end-of-life; they were assigned to the end-of-life group to create balanced, mutually exclusive groups and only their last year of data prior to death were used for these analyses.
c The observation period is different for each group: The chronic-illness group was observed for the first 0–12 months of the study. The end-of-life group was observed for the last year of the patient’s life, identified retrospectively based on date of death.
d The chronic-eol exploratory group includes dyads with complete data from the beginning of the study (0 months) through the end of life (0 days until death).
Measures
Caregiver Experience
The Caregiver Reaction Assessment (CRA)25 was administered every four weeks. This instrument is widely used to measure caregiver experience11, 18, 26, 27 and was chosen for its applicability to non-dementia care and its discrimination between different types of burden (in contrast to common burden scales such as the Zarit Caregiver Burden Interview). Subscales assess four dimensions of subjective burden: 1 - family burden (lack of family support; 5 items), 2 - financial burden (struggle with bills; 3 items), 3 - health burden (caregivers’ health decline; 4 items), and 4 - schedule burden (disruption of daily tasks; 5 items). The fifth dimension, caregiving esteem (7 items) is a positive subscale that measures enjoyment and importance of caregiving. Each subscale is internally consistent and reliable.15 We divided the total score for each subscale by the number of items in that subscale to produce an average score that ranged from one (strongly disagree) to five (strongly agree) for each of the five CRA dimensions. We then dichotomized each monthly assessment of each dimension of caregiver experience as high (4–5, indicating agreement with statements) or low (1–3, indicating disagreement with statements). We investigated change over time, defined as a clear upward or downward trend that crossed from low to high or high to low over the 1-year observation period. One in ten caregivers reported change in schedule burden over one year and 7% reported change in financial burden; change in other areas of burden and esteem was negligible. Because caregivers did not exhibit much change over time, we classified CRA subscales based on the modal average score across all available data points in the observation period (3–12) and dichotomized as high (4–5) or low (1–3) for both chronic-illness and end-of-life groups.
Caregiver Factors
All caregiver factors other than the CRA were measured only at baseline. Demographic factors included age (in years), gender, and race (nonwhite vs. white); socioeconomic factors included work status (working vs. unemployed), education (some college education vs. none), and financial security (difficulty paying bills vs. none). Relationship to patient was measured as spouse/partner versus other relative or friend. Caregiver social resources included social network size (number of relatives and close friends with whom the caregiver feels at ease, can talk to about private matters, and can call on for help); perceived adequacy of social support (the degree to which caregivers can count on and discuss problems with family and friends);28 and coping resources. We measured caregiver coping resources with the Mini-Mental Adjustment to Cancer Scale,29 which measures five coping styles: helplessness-hopelessness (8 items), fighting spirit (4 items), anxious preoccupation (8 items), cognitive avoidance (4 items), and fatalism (5 items). Mean scores for each coping style ranged from strongly disagree (1) to strongly agree (4).30
Patient Factors
Patient factors measured at baseline included diagnosis (cancer, COPD, or CHF) and age. Functional impairment was assessed every three months and included Katz basic activities of daily living (ADLs; e.g., bathing, dressing),31 Lawton and Brody instrumental ADLs (e.g., managing medicine, meal preparation),32 and Rosow Breslau functional health (e.g., walking up and down stairs, heavy housework).33 Disease severity was assessed every three months and combined self-reported health and days in bed during the last month.34 See Table 1 for more information on variable coding. These variables are used as study covariates to explain the likelihood of low caregiver esteem and high burden based on the needs and characteristics of the patient portion of the patient-caregiver dyad.
Table 1.
Sample Description
| Covariate | Full sample (n=124) |
Chronic-illness group (n=62) |
End-of-life group (n=62) |
P-Valuea |
|---|---|---|---|---|
| Baseline patient factors | ||||
| Age in years, median ± IQRb | 66.5 ± 19 | 66.0 ± 15 | 69.5 ± 22 | .50 |
| Diagnosis, % (n) | .04 | |||
| Cancer | 36.3 (45) | 25.0 (15) | 46.9 (30) | |
| COPDc | 31.5 (39) | 36.7 (22) | 26.6 (17) | |
| CHFd | 32.2 (40) | 38.3 (23) | 26.6 (17) | |
| Functional impairment levele, median ± IQR | 11.0 ± 7 | 11.0 ± 5 | 11.0 ± 7 | .60 |
| Disease severityf, % (n) | .56 | |||
| Low | 30.6 (38) | 30.0 (18) | 31.3 (20) | |
| Medium | 43.6 (54) | 40.0 (24) | 46.9 (30) | |
| High | 25.8 (32) | 30.0 (18) | 21.9 (14) | |
| Baseline caregiver factors | ||||
| Spouse/partner of patient, % (n) | 58.9 (73) | 55.0 (33) | 62.5 (40) | .40 |
| Age in years, median ± IQR | 58.0 ± 19.5 | 58.0 ± 16.5 | 57.5 ± 24 | .74 |
| Male, % (n) | 18.6 (23) | 16.7 (10) | 20.3 (13) | .60 |
| Nonwhite, % (n) | 29.0 (36) | 36.7 (22) | 21.9 (14) | .07 |
| Working, % (n) | 49.2 (61) | 51.7 (31) | 46.9 (30) | .59 |
| Some college education, % (n) | 62.1 (77) | 53.3 (32) | 70.3 (45) | .05 |
| Difficulty paying bills, % (n) | 22.6 (28) | 25.0 (15) | 20.3 (13) | .53 |
| Social network size, median ± IQR | 13.5 ± 8 | 13.0 ± 5 | 15.5 + 9.5 | .22 |
| Perceived supportg, median ± IQR | 6.0 ± 1 | 6.0 ± 1 | 6.0 ± 1 | .80 |
| Coping styleh, median ± IQR | ||||
| Helpless-hopeless | 1.8 ± 0.6 | 1.8 ± 0.5 | 1.9 ± 0.6 | .70 |
| Fighting spirit | 2.8 ± 0.5 | 2.8 ± 0.5 | 2.8 ± 0.8 | .35 |
| Anxious preoccupation | 2.5 ± 0.6 | 2.5 ± 0.6 | 2.6 ± 0.7 | .14 |
| Cognitive avoidance | 2.3 ± 0.8 | 2.3 ± 0.8 | 2.3 ± 0.5 | .74 |
| Fatalism | 3.4 ± 0.4 | 3.4 ± 0.6 | 3.4 ± 0.4 | .26 |
| Longitudinal patient factors | ||||
| Functional impairment leveli, % (n) | .03 | |||
| Stable high | 39.3 (48) | 30.5 (18) | 47.6 (30) | |
| Stable low/medium | 45.1 (55) | 57.6 (34) | 33.3 (21) | |
| Increasing | 15.6 (19) | 11.9 (7) | 19.1 (12) | |
| Disease severityj, % (n) | .06 | |||
| Stable low | 17.1 (21) | 25.0 (15) | 9.5 (6) | |
| Stable medium | 27.7 (34) | 26.7 (16) | 28.6 (18) | |
| Stable high | 21.9 (27) | 18.3 (11) | 25.4 (16) | |
| Increasing | 18.7 (23) | 11.7 (7) | 25.4 (16) | |
| Decreasing | 14.6 (18) | 18.3 (11) | 11.1 (7) |
Test for significant differences in variable distribution between the chronic-illness and end-of-life groups: Chi-square tests for dichotomous and categorical variables and Wilcoxon-Mann-Whitney tests for non-normally distributed continuous variables.
IQR = interquartile range.
COPD = chronic obstructive pulmonary disease.
CHF = congestive heart failure.
Responses to 17 items were dichotomized (need some help or unable to do the activity vs. need no help) and further coded as a hierarchic disability scale indicating the highest-order level of patient impairment from 0 to 17.44 12+ indicates ADL impairment.
High = poor/fair self-rated health (SRH) and at least half days in bed; Medium = poor/fair SRH or at least half days in bed but not both; Low = good/excellent SRH and fewer than half days in bed.
Scores range from 1 (hardly ever) to 6 (most of the time).
Score represents average response for coping style index and ranges from 1 (strongly disagree) to 4 (strongly agree).
Stable high = ADL impaired at all times; Stable low/medium = no I/ADL impairment or IADL impaired at all times; Increasing = change from low/medium to high impairment over one year.
Stable low = good/excellent SRH and < half days in bed per month for one year; stable medium = poor/fair SRH or at least half days in bed per month for one year; stable high = poor/fair SRH and at least half days in bed per month for one year; increase = change from low to medium, low to high, or medium to high over one year; decrease = change from high to medium, high to low, or medium to low over one year.
Statistical analysis
We described the sample with proportions for categorical variables and medians and interquartile ranges for non-normally distributed continuous variables. We compared characteristics by group with Chi-square tests for categorical variables and Wilcoxon-Mann-Whitney tests for continuous variables. Because the large number of statistical tests increased the potential for Type I errors, we used 99% confidence intervals to assess statistical significance.
We estimated percentages of caregivers reporting low caregiving esteem or high family, health, financial, or schedule burden to describe five separate dimensions of caregiver experience for a diverse group of patients with advanced illness (Aim 1; Figure 2). We used bivariate chi-square analyses to compare prevalence of these outcomes between chronic-illness and end-of-life groups (Aim 2; Figure 2). We used logistic regression analyses to examine patient and caregiver factors associated with high vs. low caregiver experience (esteem and 4 dimensions of burden) and whether associations varied between groups (Aim 3; Table 2 and Table 3). Because the small sample limited our ability to include substantive covariates in multivariable logistic regression models,35 we conducted bivariate, binary logistic regression analyses to investigate independent associations between covariates and caregiver outcomes.
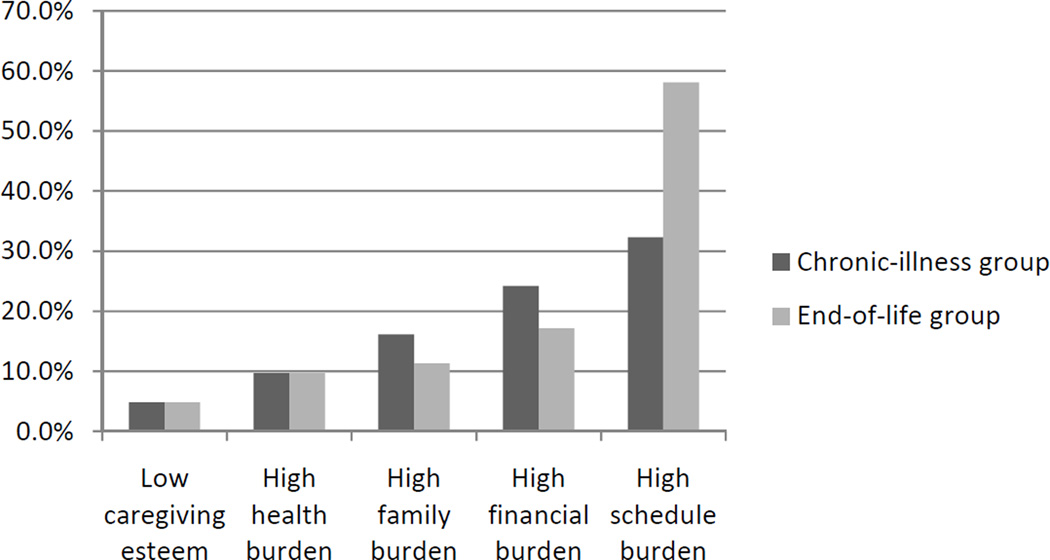
Figure 2. Prevalence of Caregiver Outcomes in the Chronic-Illness (n=62) and End-of-life (n=62) Groups.
Notes: Low caregiving esteem indicates an average score of three or lower (disagree, or neither agree nor disagree, with esteem statements). High burden (health, family, financial, and schedule) indicates an average score of four or higher (agree or strongly agree with burden statements). Chi-square tests reported a significant difference in prevalence of high schedule burden between chronic-illness and end-of-life groups (p=.01).
Table 2.
Selected Associations between Constant Covariates and Caregiver Outcomes in the Full Sample: Bivariate Odds Ratios and 99% Confidence Intervals
| Covariate | Low caregiving esteem |
High family burden | High health burden | High financial burden | High schedule burden |
|---|---|---|---|---|---|
| Baseline dyad factors | |||||
| Patient age (years) | 1.02 (0.93–1.12) | 1.01 (0.95–1.07) | 1.01 (0.95–1.08) | 0.98 (0.93–1.02) | 1.01 (0.97–1.05)c |
| Spouse (vs. other relationship) | 0.69 (0.08–5.93) | 0.76 (0.20–2.92) | 0.98 (0.20–4.77) | 0.63 (0.20–1.99) | 2.29 (0.87–6.08)b |
| Nonwhite (vs. white) | 2.58 (0.30–22.53) | 2.51 (0.64–9.91) | 1.87 (0.38–9.28) | 2.64 (0.81–8.60)b | 0.96 (0.34–2.68) |
| Difficulty w/ bills (vs. none) | 0.67 (0.04–11.98) | 0.70 (0.12–4.01) | 1.83 (0.34–9.89) | 4.39 (1.28–15.09)a | 1.87 (0.61–5.72) |
| Baseline caregiver social resources | |||||
| Social network size (#) | 0.99 (0.82–1.18) | 0.95 (0.84–1.07) | 0.93 (0.80–1.07) | 0.95 (0.86–1.05) | 1.00 (0.9–31.08)c |
| Perceived support (1–6) | 0.69 (0.30–1.55) | 1.02 (0.53–1.96) | 0.75 (0.40–1.42) | 0.92 (0.54–1.57) | 1.34 (0.82–2.19)c |
| Coping style (1–4) | |||||
| Helpless-hopeless | 0.41 (0.03–5.46) | 2.48 (0.46–13.21) | 5.78 (0.71–46.93)b | 0.58 (0.15–2.29) | 1.07 (0.35–3.27)d |
| Anxious preoccupation | 0.70 (0.08–6.12) | 1.85 (0.47–7.33) | 1.15 (0.63–3.28) | 1.24 (0.40–3.92) | 3.01 (1.05–8.60)a,d |
| Fighting spirit | 1.45 (0.13–16.85) | 0.98 (0.63–1.51) | 0.98 (0.76–1.27) | 0.98 (0.86–1.12) | 2.20 (0.77–6.28)c |
| Cognitive avoidance | 0.89 (0.11–7.12) | 2.08 (0.54–7.96) | 1.95 (0.41–9.20) | 0.75 (0.25–2.25) | 1.65 (0.66–4.13)c |
| Fatalism | 1.07 (0.71–1.61) | 0.98 (0.81–1.18) | 0.98 (0.83–1.17) | 0.98 (0.86–1.12) | 4.29 (1.02–17.96)a,d |
| Longitudinal patient impairment | |||||
| Stable high (vs. low/med.) | 1.15 (0.08–15.95) | 1.89 (0.44–8.16) | 0.62 (0.11–3.42) | 1.19 (0.36–3.97) | 2.45 (0.86–6.19)b |
| Increasing (vs. low/med.) | 3.12 (0.22–45.20) | 0.96 (0.10–8.89) | 0.38 (0.02–6.55) | 0.42 (0.05–3.45) | 0.81 (0.19–3.49) |
Notes: n=124 patient/caregiver dyads. Low caregiving esteem indicates a modal average score of three or lower (disagree, or neither agree nor disagree, with esteem statements). High burden (health, family, financial, and schedule) indicates a modal average score of four or higher (agree or strongly agree with burden statements). Modal score was based on all available data points (3–12).
Indicates significant association at p<.01.
Indicates significant association at p<.05.
Indicates non-homogeneity of odds between chronic-illness and end-of-life groups at p<.01.
Indicates non-homogeneity of odds between chronic-illness and end-of-life groups at p<.05.
Table 3.
Associations that Vary by Group for High Schedule Burden: Odds Ratios and 99% Confidence Intervals for Chronic-Illness and End-of-life Groups
| Covariate | Chronic-illness group (n=62) | End-of-life group (n=62) |
|---|---|---|
| Patient age (years) | 0.99 (0.93–1.05) | 1.02 (0.96–1.07) |
| Social network size (#) | 0.99 (0.87–1.12) | 0.99 (0.90–1.11) |
| Perceived support (1–6) | 0.97 (0.52–1.80) | 2.09 (0.87–5.02) |
| Coping style (1–4) | ||
| Helpless-hopeless | 4.21 (0.61–29.20) | 0.39 (0.08–1.98) |
| Anxious preoccupation | 9.56 (1.15–79.6)a | 1.49 (0.40–5.59) |
| Fighting spirit | 1.86 (0.38–9.26) | 2.30 (0.54–9.90) |
| Cognitive avoidance | 3.51 (0.78–15.91) | 1.00 (0.27–3.78) |
| Fatalism | 8.67 (0.92–82.15) | 1.72 (0.20–14.80) |
p<.01
We used Box-Tidwell transformations to test the assumption that continuous independent variables were linearly related to the log odds of dichotomous outcomes. No squared or logged transformations were significantly associated with the outcomes at p<.01, assuring linearity of the logit. We also tested whether the bivariate odds ratios were homogeneous between the chronic illness and end of life sub-samples, using the Breslow-Day test36 for dichotomous variables and testing an interaction term between the covariate and chronic/end-of-life indicator for categorical and continuous covariates. We conducted all analyses using SAS 9.3.
RESULTS
Sample description
Patient and caregiver characteristics are detailed in Table 1. The median age of patients and caregivers was 67 and 58 years, respectively. Most caregivers were female and white and more than half of the caregivers were the spouse or partner of the patient. Almost half of caregivers were working at baseline. The majority of caregivers had some college education, and a minority had difficulty paying bills. Caregivers reported a median of 13 people in their social network and high satisfaction with their network. There were no significant differences between the chronic-illness and end-of-life groups at p<.01 for baseline factors including age, diagnosis, functional impairment, and disease severity of the patient. Also, no significant differences were identified between the two groups for caregiver relationship, gender, race, employment, and education at baseline.
Caregiver esteem and burden between illness stages
Exploratory analyses (not shown) modeled random intercept growth curves and estimated burden scores for each CRA subscale within the chronic-illness and end-of-life groups. Slopes estimating change per month were negligible and non-significant.
Because caregiver esteem and burden were relatively stable over time, we examined the prevalence of high and low burden/esteem and differences between the chronic-illness and end-of-life groups (Aims 1 & 2; Figure 2). Schedule burden was the most prevalent form of burden, with more than one in three caregivers reporting that their daily schedules are disrupted by caregiving. Financial burden was reported by approximately one in five caregivers, while family burden (14%) and health burden (10%) were reported less frequently. Less than 5% of caregivers reported low caregiving esteem. Schedule burden was the only type of caregiver experience that varied between chronic-illness and end-of-life; 58% of caregivers reported schedule burden during end-of-life, compared to 32% of chronic-illness caregivers (p=.01).
A paired analysis of the 18 dyads observed for both one year of chronic illness and the last year of life revealed stability across the two periods for ten dyads. High schedule burden was most prevalent and most likely to increase between chronic-illness and end-of-life stages; increase in burden was more likely than decrease for all dimensions; and at least half of the caregivers who reported any dimension of burden during end-of-life also experienced that dimension of burden during chronic-illness.
Factors associated with caregiver experience
In bivariate logistic regressions, most covariates were not associated with low esteem or high burden during either chronic-illness or end-of-life (Aim 3; Table 2). No covariates were associated with odds of low caregiving esteem or high family burden. Caregivers who reported difficulty paying bills at baseline experienced increased odds of financial burden over one year (OR=4.39, 99% CI=1.28–15.09). Caregivers who reported higher levels of anxious preoccupation and fatalistic coping styles had higher odds of schedule burden (OR=3.01, 99% CI=1.05–8.60; OR=4.29, 99% CI=1.02–17.96, respectively).
Tests for non-homogeneity of odds ratios indicated that associations between caregiver social resources and high schedule burden differed between the chronic-illness and end-of-life groups (Table 2). Additional analyses (Table 3) show that greater endorsement of anxious preoccupation coping style was more strongly associated with high schedule burden in the chronic-illness group (OR=9.56, 99% CI=1.15–79.6), than in the end-of-life group.
DISCUSSION
Two aims of these analyses were to examine caregiver experience and whether it varies between stages of chronic-illness and end-of-life. High caregiving esteem was almost universal, emphasizing positive experiences with this role. Schedule burden was the most common type of burden for both subsamples but was even more common among caregivers of patients in the last year of life. Regarding aim 3, few patient or caregiver characteristics were associated with odds of low esteem or high burden. Patient functional impairment, even when measured longitudinally, was only marginally related to schedule burden. Caregiver social support, an important component of stress process models, was not significantly associated with caregiver outcomes; however, fatalistic and anxious preoccupation coping styles were associated with higher schedule burden. This study extends prior research on caregiver experience in advanced serious illness by including a diverse sample and examining change over time while differentiating between chronic-illness and end-of-life caregiving.
These results suggest three conclusions. First, many caregivers experience substantial schedule burden, especially during the last months of patients’ lives. Second, esteem and other types of burden are relatively stable within 1-year periods and similar between chronic-illness and end-of-life stages. Third, few demographic, health-related, or social factors are associated with caregiver outcomes. Although these results are exploratory due to small sample sizes, they raise new implications for the assessment of family palliative care needs and the design of interventions to support the growing number of informal caregivers.
Caregivers experience burden throughout advanced illness
This study examined separate components of caregiver burden to inform interventions for specific caregiver needs at all stages of illness. Prevalence of burden observed in the current study were consistent with previous studies that used the CRA to measure caregiver experience in cancer, heart failure, and terminal illness; schedule burden is most problematic for caregivers and often increases over time, and, on average, at least half of caregivers report some level of burden.7, 12, 16, 19 Family, health, and financial burden can strain social network support and limit caregivers’ abilities to continue to provide care in the community. The prevalence of these three types of burden was similar in the chronic-illness and end-of-life groups, indicating that need for intervention is not specific to stage of illness. This is important given that prognostication is difficult and we often do not know when people are in their last months of life.37
One exception is schedule burden, the most prevalent form of burden in our sample. This finding indicates that there is a significant negative impact of caregiving on the caregiver’s daily activities, work, social life, and personal time. Many caregivers need help managing their personal lives and routines while managing patients’ needs, especially during end-of-life caregiving. While most caregivers need support throughout advanced illness, the last year of patients’ lives is a period of heightened need for caregivers juggling multiple social roles. Exploratory analyses suggested that coping resources may be less effective against schedule burden during the last year of life. Future research with larger samples is needed to examine the dynamic nature of this complex form of caregiver burden.
Caregiver experience is relatively stable
The stability in caregiving experience observed in this study confirmed previous longitudinal studies of caregiver burden immediately following cancer diagnosis19 and during advanced chronic-illness.13 Observed stability over time is sometimes a consequence of statistical methods that aggregate and obscure divergent individual trends.22, 23 This study reduced this possibility by examining individuals’ subscale scores over 3–12 months and demonstrated that caregiver experience is relatively stable within and between stages of advanced illness.
The similar prevalence of caregiver burden between chronic-illness and end-of-life stages supports recent findings that caregiver mental health and well-being do not vary by stage of illness.38 Our finding that coping styles are associated with burden supports the idea that caregiver experience is likely trait-based, not state-based. Esteem and burden did not vary with patient need, demonstrated stability over 1-year periods, and were similar during chronic-illness and end-of-life caregiving. Caregivers who struggle with caring for a patient at the end of life will likely show signs of burden during the earlier chronic illness stage. This continuity in need is in line with integrated models of palliative care that emphasize support for multiple dimensions of family need as early in the disease course as necessary rather than waiting until the patient is near the end-of-life.39
Analyses that compare average levels across two groups (chronic-illness and end-of-life caregivers) have the potential to obscure relatively infrequent or divergent trends. 40 Within our small sub-sample of dyads observed over both chronic-illness and end-of-life stages, seven of eighteen caregivers experienced an increase in one type of burden, from un-burdened during chronic-illness to burdened during end-of-life. These trends are not highly represented in our sample, but invite further investigation with larger samples to identify caregivers who are likely to experience periods of heightened need later in the illness trajectory.
Few “predictors” of burden
Explanatory models of caregiver burden such as stress process theory have been widely extended to cancer caregiving,11, 18, 19, 27, 41 yet the issue of caregiver burden only recently has been examined in families living with advanced COPD17 and CHF.16 We examined several socio-demographic and social resource traits and found no consistent associations with odds of low esteem or high burden. In this sample, social support and coping resources were only associated with schedule burden, and the association may be stronger during chronic-illness compared to end-of-life.
The lack of associations with social support and coping may be due to properties of the sample. First, the patients were relatively high functioning, with more than three quarters of patients requiring no ADL assistance at baseline; social resources may be more important for caregivers with more intensive caregiving demands. Second, the health systems from which our sample was drawn provide fairly comprehensive and accessible family support services, which may mitigate the association between social resources and caregiver burden. Third, social resources may predict increases in burden over time, but not differentiate between fairly stable levels of burden. Future studies should utilize within-person analyses with larger longitudinal datasets to investigate these possibilities.
Limitations
This study is limited by its small sample size, which affected the depth of analyses and our ability to control for substantive covariates. It was not feasible to model additional covariates, including hospitalizations, patient symptoms, caregiver depression and anxiety, and use of formal services. Other factors, such as caregiver health status, co-residence with patient, duration of caregiving, and feelings of responsibility to provide care were not available in the data. Some factors that are likely to change over time (e.g., financial security, work status, and social resources) were only measured at baseline. Although our measure of burden (CRA) enabled a nuanced investigation of types of burden, it limits comparisons with more common measures of overall caregiver burden and there is speculation about its responsiveness to change over time.25, 42 Considering these limitations, conclusions should be interpreted as exploratory.
Implications for care
This study found that burden is fairly common and stable between stages for cancer, CHF, and COPD caregivers. We recommend that multidisciplinary teams caring for patients with advanced illness be available and attuned to signs of caregiver burden. Simple screening questions, such as “do you need help,” can identify need for supportive services during all stages in the disease trajectory.15 Because caregivers who experience burden show signs early in the disease course, earlier screening and supportive services could prevent unnecessary suffering and negative impact on care provision. However, clinical support for these recommendations is often lacking until patients qualify for hospice benefit, sometimes years after caregiving burden has begun to take its toll. Integrated models of early palliative care are more likely to provide needed interdisciplinary support services at any stage of serious illness that requires home caregiving. Our exploratory results identified a small proportion of caregivers who experience an increase in burden over time; support should be specially targeted to these families to support their ability to provide informal care in the community. Educational and supportive services to reduce schedule burden, such as caregiver respite and care coordination, may be valuable to caregivers for all illnesses and should be further implemented and evaluated.43
ACKNOWLEDGMENTS
Funding source: This research was supported by a grant from the National Institute of Nursing Research #1R01NR/AG08249, “Trajectories of Serious Illness: Patients and Caregivers.” The first author was supported by AHRQ post-doctoral training grant T32HS000079.
This work was also supported with resources from the Durham VA Center for Health Services Research.
Sponsor’s Role: The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions
Study concept and design: Jessica M. Sautter, Kimberly S. Johnson, Maren K. Olsen, James A. Tulsky, Karen E. Steinhauser
Acquisition and management of subjects and data: James A. Tulsky, Karen E. Steinhauser, Maren K. Olsen, Jennifer Hoff Lindquist
Analysis and interpretation of data: Jessica M. Sautter, Maren K. Olsen, Jennifer Hoff Lindquist, Karen E. Steinhauser, James A. Tulsky
Preparation of manuscript: Jessica M. Sautter, KJ, Karen E. Steinhauser, James A. Tulsky
Revision of manuscript: Jessica M. Sautter, KJ, Maren K. Olsen, Allison M. Burton-Chase, James A. Tulsky, Jennifer Hoff Lindquist, Sheryl Zimmerman, Karen E. Steinhauser
The corresponding author affirms that all who contributed significantly to this study were listed as authors.
REFERENCES
- 1.National Alliance for Caregiving, AARP. Caregiving in the U.S.: A focused look at those caring for someone age 50 or older. 2009 http://www.caregiving.org/data/FINALRegularExSum50plus.pdf.
- 2.Department of Health and Human Services and Assistant Secretary for Planning and Evaluation. The future supply of long-term care workers in relation to the aging baby boom generation. Report to Congress. Washington, DC: Department of Health and Human Services; 2003. http://aspe.hhs.gov/daltcp/reports/ltcwork.htm#note7. [Google Scholar]
- 3.Clark AM, Reid ME, Morrison CE, et al. The complex nature of informal care in home-based heart failure management. J Adv Nurs. 2008;61:373–383. doi: 10.1111/j.1365-2648.2007.04527.x. [DOI] [PubMed] [Google Scholar]
- 4.Molloy GJ, Johnston DW, Whitham MD. Family caregiving and congestive heart failure: Review and analysis. Eur J Heart Failure. 2005;7:592–603. doi: 10.1016/j.ejheart.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Pearlin LI, Mullan JT, Semple SJ, et al. Caregiving and the stress process: An overview of concepts and their measures. Gerontologist. 1990;30:583–594. doi: 10.1093/geront/30.5.583. [DOI] [PubMed] [Google Scholar]
- 6.Aneshensel CS, Pearlin LI, Mullan JT, et al. Profiles in Caregiving: The Unexpected Career. San Diego, CA: Academic Press, Inc; 1995. [Google Scholar]
- 7.Gaugler JE, Linder J, Given CW, et al. The proliferation of primary cancer caregiving stress to secondary stress. Cancer Nurs. 2008;31:116–123. doi: 10.1097/01.NCC.0000305700.05250.9d. [DOI] [PubMed] [Google Scholar]
- 8.Stajduhar K, Funk L, Toye C, et al. Part 1: Home-based family caregiving at the end of life: a comprehensive review of published quantitative research (1998–2008) Palliat Med. 2010;24:573–593. doi: 10.1177/0269216310371412. [DOI] [PubMed] [Google Scholar]
- 9.Schulz R, Beach SR. Caregiving as a risk factor for mortality: The caregiver health effects study. JAMA. 1999;282:2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 10.Yaffe K, Fox P, Newcomer R, et al. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA. 2002;287:2090–2097. doi: 10.1001/jama.287.16.2090. [DOI] [PubMed] [Google Scholar]
- 11.Andrews SC. Caregiver burden and symptom distress in people with cancer receiving hospice care. Oncol Nurs Forum. 2001;28:1469–1474. [PubMed] [Google Scholar]
- 12.Brazil K, Bedard M, Willison K, et al. Caregiving and its impact on families of the terminally ill. Aging Ment Health. 2003;7:376–382. doi: 10.1080/1360786031000150649. [DOI] [PubMed] [Google Scholar]
- 13.Garlo K, O’Leary JR, Van Ness PH, et al. Burden in caregivers of older adults with advanced illness. J Am Geriatr Soc. 2010;58:2315–2322. doi: 10.1111/j.1532-5415.2010.03177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.George LK, Gwyther LP. Caregiver well-being: A multidimensional examination of family caregivers of demented adults. Gerontologist. 1986;26:253–259. doi: 10.1093/geront/26.3.253. [DOI] [PubMed] [Google Scholar]
- 15.Burton AM, Sautter JM, Tulsky JA, et al. Burden and well-being among a diverse sample of cancer, CHF, and COPD caregivers. J Pain Symptom Manage. 2012;44:410–420. doi: 10.1016/j.jpainsymman.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luttik ML, Jaarsma T, Veeger N, et al. Caregiver burden in partners of heart failure patients; limited influence of disease severity. Eur J Heart Failure. 2007;9:695–701. doi: 10.1016/j.ejheart.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Pinto RA, Holanda MA, Medeiros MMC, et al. Assessment of the burden of caregiving for patients with chronic obstructive pulmonary disease. Respir Med. 2007;101:2402–2408. doi: 10.1016/j.rmed.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Daly BJ, Douglas S, Lipson A, et al. Needs of older caregivers of patients with advanced cancer. J Am Geriatr Soc. 2009;57:S293–S295. doi: 10.1111/j.1532-5415.2009.02516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nijboer C, Triemstra M, Tempelaar R, et al. Patterns of caregiver experiences among partners of cancer patients. Gerontologist. 2000;40:738–746. doi: 10.1093/geront/40.6.738. [DOI] [PubMed] [Google Scholar]
- 20.Clipp EC, George LK. Dementia and cancer: A comparison of spouse caregivers. Gerontologist. 1993;33:534–541. doi: 10.1093/geront/33.4.534. [DOI] [PubMed] [Google Scholar]
- 21.Lunney JR, Lynn J, Hogan C. Profiles of older Medicare decedents. J Am Geriatr Soc. 2002;50:1108–1112. doi: 10.1046/j.1532-5415.2002.50268.x. [DOI] [PubMed] [Google Scholar]
- 22.Bergman LR, Trost K. The person-oriented versus the variable-oriented approach: Are they complementary, opposites, or exploring different worlds? Merrill-Palmer Quart. 2006;52:601–632. [Google Scholar]
- 23.Raudenbush S. Comparing personal trajectories and drawing causal inferences from longitudinal data. Annu Rev Psychol. 2001;52:501–525. doi: 10.1146/annurev.psych.52.1.501. [DOI] [PubMed] [Google Scholar]
- 24.Steinhauser KE, Clipp EC, Hays JC, et al. Identifying, recruiting, and retaining seriously-ill patients and their caregivers in longitudinal research. Palliat Med. 2006;20:745–754. doi: 10.1177/0269216306073112. [DOI] [PubMed] [Google Scholar]
- 25.Given C, Given B, Stommel M, et al. The Caregiver Reaction Assessment (CRA) for caregivers to persons with chronic physical and mental impairments. Res Nurs Health. 1992;15:271–283. doi: 10.1002/nur.4770150406. [DOI] [PubMed] [Google Scholar]
- 26.Nijboer C, Triemstra M, Tempelaar R, et al. Determinants of caregiving experiences and mental health of partners of cancer patients. Cancer. 1999;86:577–588. doi: 10.1002/(sici)1097-0142(19990815)86:4<577::aid-cncr6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 27.Grov EK, Fossa SD, Sorebo O, et al. Primary caregivers of cancer patients in the palliative phase: A path analysis of variables influencing their burden. Soc Sci Med. 2006;63:2429–2439. doi: 10.1016/j.socscimed.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Landerman R, George LK, Campbell RT, et al. Alternative models of the stress buffering hypothesis. Am J Community Psychol. 1989;17:625–642. doi: 10.1007/BF00922639. [DOI] [PubMed] [Google Scholar]
- 29.Watson M, Law M, Santos MD, et al. The Mini-MAC: Further development of the Mental Adjustment to Cancer scale. J Psychosoc Oncol. 1994;12:33–46. [Google Scholar]
- 30.Johansson M, Ryden A, Finizia C. Mental adjustment to cancer and its relation to anxiety, depression, HRQL and survival in patients with laryngeal cancer -- A longitudinal study. BMC Cancer. 2011;11:1–9. doi: 10.1186/1471-2407-11-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in the aged: The index of ADL, a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 32.Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 33.Rosow I, Breslau N. A Guttman health scale for the aged. J Gerontol. 1966;21:556–559. doi: 10.1093/geronj/21.4.556. [DOI] [PubMed] [Google Scholar]
- 34.Steinhauser KE, Arnold RM, Olsen MK, et al. Comparing three life-limiting diseases: Does diagnosis matter or is sick, sick? J Pain Symptom Manage. 2011;42:331–341. doi: 10.1016/j.jpainsymman.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peduzzi P, Concato J, Kemper E, et al. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 36.Breslow NE, Day NE. Statistical Methods in Cancer Research, Volume I: The Analysis of Case-Control Studies: Lyon, International Agency for Research on Cancer. 1980 [PubMed] [Google Scholar]
- 37.Lynn J, Teno JM, Harrell FE. Accurate prognostications of death: Opportunities and challenges for clinicians. Western J Med. 1995;163:250–257. [PMC free article] [PubMed] [Google Scholar]
- 38.Grov EK, Valeberg BT. Does the cancer patient's disease stage matter? A comparative study of caregivers’ mental health and health related quality of life. Palliat Support Care. 2012;10:189–196. doi: 10.1017/S1478951511000873. [DOI] [PubMed] [Google Scholar]
- 39.Sepulveda C, Marlin A, Yoshida T, et al. Palliative care: The World Health Organization’s global perspective. J Pain Symptom Manage. 2002;24:91–96. doi: 10.1016/s0885-3924(02)00440-2. [DOI] [PubMed] [Google Scholar]
- 40.Nagin DS. Analyzing developmental trajectories: A semiparametric, group-based approach. Psychol Methods. 1999;4:139–157. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- 41.Kurtz ME, Kurtz JC, Given CW, et al. Relationship of caregiver reactions and depression to cancer patients' symptoms, functional states and depression -- A longitudinal view. Soc Sci Med. 1995;40:837–846. doi: 10.1016/0277-9536(94)00249-s. [DOI] [PubMed] [Google Scholar]
- 42.Post MWM, Festen H, Port I, et al. Reproducibility of the Caregiver Strain Index and the Caregiver Reaction Assessment in partners of stroke patients living in the Dutch community. Clin Rehabil. 2007;21:1050–1065. doi: 10.1177/0269215507079140. [DOI] [PubMed] [Google Scholar]
- 43.Honea NJ, Brintnall R, Given B, et al. Putting evidence into practice: Nursing assessment and interventions to reduce family caregiver strain and burden. Clin J Oncol Nurs. 2008;12:507–516. doi: 10.1188/08.CJON.507-516. [DOI] [PubMed] [Google Scholar]
- 44.Kingston A, Collerton J, Davies K, et al. Losing the ability in activities of daily living in the oldest old: A hierarchic disability scale from the Newcastle 85+ Study. PLoS ONE. 2012;7:e31665. doi: 10.1371/journal.pone.0031665. [DOI] [PMC free article] [PubMed] [Google Scholar]