Abstract
Host cell invasion is monitored by a series of pattern recognition receptors (PRRs) that activate the innate immune machinery upon detection of a cognate pathogen associated molecular pattern (PAMP). The RIG-I like receptor (RLR) family of PRRs includes three proteins — RIG-I, MDA5, and LGP2 — responsible for the detection of intracellular pathogenic RNA. All RLR proteins are built around an ATPase core homologous to those found in canonical Superfamily 2 (SF2) RNA helicases, which has been modified through the addition of novel accessory domains to recognize duplex RNA. This review focuses on the structural bases for pathogen-specific dsRNA binding and ATPase activation in RLRs, differential RNA recognition by RLR family members, and implications for other duplex RNA activated ATPases, such as Dicer.
Duplex RNA activated ATPases
Viral infection is monitored on the cellular level by several families of pattern recognition receptors (PRRs) that detect and respond to a variety of pathogen-associated molecular patterns (PAMPs). RIG-I-like Receptors (RLRs) are one family of PRR proteins comprised of three homologous SF2 helicases — RIG-I, MDA-5 and LGP2 — that detect and respond to non-self dsRNA [1–3]. RIG-I and MDA5 contain tandem caspase activation and recruitment domains (CARDs) at their N-termini that are normally found in a ‘signaling silent’ conformation. Upon interaction with pathogenic dsRNAs, RIG-I and MDA-5 CARDs become signaling competent, facilitating an interaction with the downstream adaptor protein MAVS [4]. This interaction induces MAVS oligomerization [5], which in turn engages the innate immune machinery resulting in the production of type I interferon and inflammatory cytokines.
In addition to the tandem CARDs found only in RIG-I and MDA5, all RLRs contain a central RNA helicase-like core that has been modified to recognize duplex RNA substrates. This modified helicase domain is similar to the helicase domain found in the Dicer family of proteins, which have also evolved to interact with dsRNA. Early evidence that these enzymes belong to a structurally distinct family of motor proteins came from phylogenetic analyses which demonstrated that these proteins contain unique sequence motifs not found in processive RNA helicases such as the NS3 helicase from hepatitis C virus [6]. Indeed, the closest relatives to RIG-I and Dicer are the DEAD-box proteins, which are multifunctional, nonprocessive chaperones for RNA annealing and remodeling [7]. Further analysis of RIG-I and Dicer sequences showed that these proteins contain novel domain insertions that distinguish them from other SF2 helicase proteins [8]. This was consistent with biochemical studies, which showed that ATPase activity of these proteins is stimulated by double-stranded RNA [9,10], rather than single-stranded RNA as with viral NS3 or DNA as with FANCM-like or SWI/SNF proteins [11–13], and that RIG-I and Dicer do not robustly unwind duplex substrates [10]. On the basis of their shared sequence and functional attributes, RLRs, Dicer and Dicer related helicases (DRHs) have been termed ‘Duplex RNA-activated ATPases’, or DRAs [7].
Given the importance of RIG-I and MDA-5 as pattern recognition receptors (PRRs) in the innate immune system [14] and the significance of Dicer helicase in small RNA metabolism [15], the complete lack of structural information on this SF2 subgroup restricted understanding of biological function. This situation suddenly improved in 2011, when a set of four papers on RIG-I structure appeared almost simultaneously [16,17•• ,18•• ,19••]. The four studies were remarkably complementary because they each revealed different states of the enzyme that contribute to function. This first set of structures was particularly important for defining the basic ‘parts list’ of the multidomain DRA proteins, and for showing how these parts have been combined to create a new type of nanomechanical device for transmitting information in the cell (Figure 1).
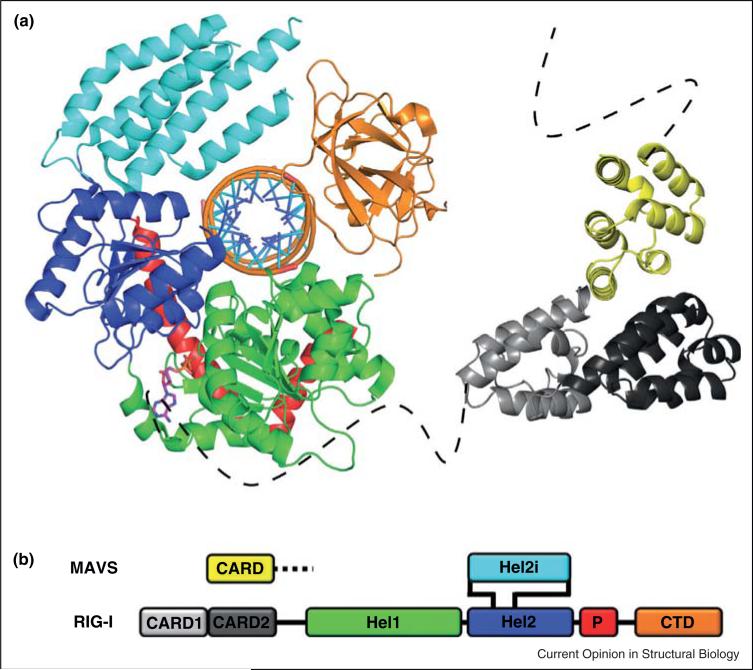
Figure 1.
Architecture of a RIG-I-like receptor. (a) Structural model depicting the RIG-I domain organization and the proposed RIG-I:MAVS interaction. Individual domain components of RIG-I are color coded as follows: CARD1: light gray, CARD2: dark gray, HEL1: Green, HEL2: blue, HEL2i: Cyan, Pincer: Red, and CTD: orange. The CARD domain of MAVS is shown in yellow. The model represents a compilation of several structures of both human and duck RIG-I. The helicase core of duck RIG-I — HEL1, HEL2, HEL2i, and Pincer — is shown bound to a 19mer duplex RNA substrate and ADP-AlF4 (PDB 4A36). The CTD was modeled from a structure of human RIG-I bound to a triphosphorylated 10mer RNA hairpin, and positioned relative to the helicase core by aligning the RNA substrates (PDB 4AY2). The RIG-I CARD domains were taken from an apo structure of duck RIG-I (PDB 4A2W) and positioned relative to the CARD domain of MAVS (PDB 2VGQ) by alignment with the structure of a CARD:CARD interaction reported between Apaf-1 and procaspase-9 (PDB 3YGS). Dashed lines represent the presumed unstructured residues tethering the CARD domains to the remainder of their respective polypeptides. (b) Schematic diagram showing the domain organization of RIG-I primary structure. The MAVS CARD domain is shown above the proposed interaction site with RIG-I. The color scheme is consistent with (a). CARD: caspase activation and recruitment domain, CTD: C-terminal domain.
The parts list and assembly scheme for RIG-I and other DRA proteins
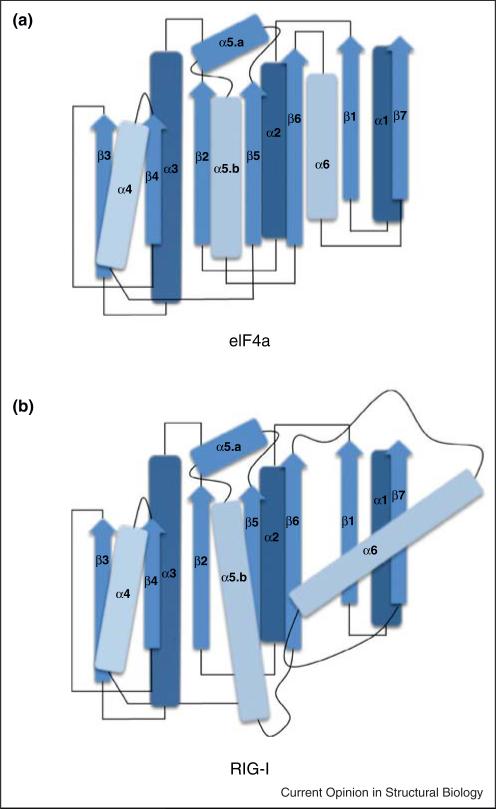
The most immediately recognizable domain in RIG-I is the ATPase core that is shared with all other SF2 proteins [20]. This core is composed of two ‘RecA fold’ domains (HEL1 and HEL2) that form a cleft for binding ATP and a shared interface for binding RNA. However, the ATPase core of RIG-I and MDA-5 deviates significantly from the cognate domains in other SF2 proteins [17•• ,18•• ,19•• ,21••]. An immediately striking feature is that the ATPase cleft is unusually ‘open’ in RIG-I, and that even when bound to RNA in the presence or absence of ADP HEL1 and HEL2 are spaced far apart [18•• ,19•• ,22••]. Furthermore, the domain topology of HEL1 and HEL2 is fundamentally different from the RecA folds in any other SF2 protein or helicase. For example, in DEAD box proteins such as eIF4A, the parallel beta sheet of each RecA fold is buttressed by three alpha helices on each face that form a collinear array of hydrogen bonds with partner beta strands (Figure 2) [23,24]. In HEL1 of RIG-I and related proteins, two of these alpha helices have been elongated, and one has disengaged from the RecA core, protruding at an acute angle from the otherwise parallel array of secondary structures. This alpha helix projects from HEL1 and serves as a contact point for the novel Pincer domain observed in RIG-I and MDA5 [17•• ,18•• ,19•• ,21••]. These structural features explain why, despite a common ancestry with DEAD-box proteins, RIG-I is not a heli-case, but rather has evolved into an entirely different type of motor.
Figure 2.
Architectural comparison of the N-terminal RecA domains of eIF4a and RIG-I. (a) Schematic diagram depicting the relative orientation of secondary structures in the N-terminal RecA domain of the canonical DEAD box protein eIF4a from Saccharomyces cerevisiae (PDB 1FUU). Secondary structures are numbered based on primary sequence, and colored to represent relative proximity to the reader in 3-dimensional space, with lighter objects being closer. β-Strands are denoted with arrows, α-helices are denoted with rods and loop regions are denoted with lines. In eIF4a and similar DEAD-box proteins, the secondary structures comprising the core are aligned in a generally parallel fashion. (b) In RIG-I and other RLRs, helices α5.b and α6 are elongated, and helix a6 has been rotated out of register with the remainder of the RecA core, facilitating an interaction with the novel Pincer domain. RLR: RIG-I like receptor.
In addition to the ATPase core, RIG-I and related proteins contain a distinctive set of accessory domains. The C-terminal domain (CTD) is a small beta sheet core that is stabilized by a coordinated zinc ion. The RIG-I CTD was first visualized in isolation where, remarkably, it bound to RNA duplexes in the same way as observed later for the full-length protein [22•• ,25•• ,26•• ,27•• ,28]. The CTD is connected to HEL2 via a nanomechanical innovation called the Pincer (or Bridging) domain [17•• ,19••]. This V-shaped pair of long alpha helices is pinned in place by the modified alpha-helix that emerges from the face of HEL1. In this configuration, the Pincer transmits information on RNA binding between the CTD and the ATPase core formed by HEL1 and HEL2, thus coordinating ligand recognition with motor function [19•• ,29]. Another domain appended to the helicase core is the large alpha-helical bundle known as HEL2i. This structure facilitates dsRNA binding within the DRA family [17•• ,18•• ,19•• ,21•• ,30,31]. Structural similarity of these domains with the Dicer helicase domain is underscored by the successful construction of a Dicer homology model using crystal structures of RIG-I [32].
In RIG-I and MDA-5, the N-terminus of HEL1 is grafted to two sequential CARD domains [20,33,34], belonging to the Death Domain superfamily that has been studied extensively in signaling [35]. The CARD domains of RIG-I and MDA5 are thought to interact with the CARD domain of the MAVS protein, a receptor on the mitochondrial surface that initiates a series of signal cascades upon activation [36]. It is noteworthy that later stages of RIG-I activation and receptor binding are not well understood from a structural perspective, and they are likely to involve the binding of other ligands — such as polyubiquitin chains — and post translational modifications [5,37,38]. Having defined the ‘parts list,’ one can describe the ways in which these components function together in ligand recognition and activation. Recent crystallo-graphic, solution biophysical and biochemical studies allow us to visualize discrete states along the pathway to RIG-I signaling.
The ligand-free state: profile of an inactivated biosensor
Solution structural methods such as small angle X-ray scattering (SAXS) and analytical ultracentrifugation (AU) have indicated that, in the absence of RNA, RIG-I and MDA-5 are structurally extended monomers in which the individual protein domains sample multiple conformations as these PRRs survey the cytoplasm for viral RNA [18•• ,31]. That this state is not entirely disorganized, however, is indicated by the results of crystallographic studies on ligand-free states. For example, in the absence of RNA, certain RIG-I constructs reveal a partially ordered ATPase core, composed of HEL1, HEL2, HEL2i and the Pincer [16,17••]. In an example that is particularly germane to the regulation of RIG-I signaling, Kowalinski and colleagues obtained a structure of RIG-I in which the CARD domains are present in the context of the ATPase core [17••]. Intriguingly, the CARDs in this RNA-free state are well-ordered alpha-helical bundles that adopt a single conformation in the crystal. In RIG-I, CARD2 tucks against the surface of the HEL2i domain, where it forms an extensive interface that is likely to impede interactions with other ligands. These findings suggest that, while much of RIG-I remains dynamic in the absence of RNA, accessibility of the CARDS is carefully controlled through specific interactions with HEL2i that likely prevent constitutive activation of the protein. Presumably the CARDS are disengaged from HEL2i and presented for signaling only after RIG-I undergoes conformational changes upon ligand-induced activation.
Whether MDA5 uses a similar mode of CARD sequestration is not yet fully understood, as no structure of the CARD domains in the context of the MDA5 helicase core is available. Indeed, two alpha helices of HEL2i that engage in interactions with the CARDs are shortened in MDA5, and a conserved phenylalanine residue important for maintaining this interaction in RIG-I is absent [31].
The RNA-bound state: adaptations for recognizing viral RNA
Upon binding an RNA duplex, RIG-I undergoes dramatic conformational changes that are evident from both solution structural data and from crystallographic studies. Crystal structures from multiple labs, on RIG-I variants from diverse vertebrates, reveal an almost identical structural organization for the complex between duplex RNA and RIG-I [17•• ,18•• ,19••]. In each case, HEL1, HEL2, HEL2i and the CTD completely encircle the A-form RNA duplex, enveloping the undistorted duplex in a network of interactions that engage both strands of the RNA backbone. In a series of adaptations for binding double-stranded RNA, HEL1 interacts with both strands, using motif IIa to bind the top-strand of the substrate [19••], while HEL2 makes contacts with the top strand of the dsRNA via motif Vc [7,18••]. HEL2i plays a key role in duplex RNA recognition, which is consistent with its high degree of conservation within the DRA family [7,19••]. The CTD caps the terminus of the RNA duplex, interacting with the terminal base pair like a ‘bookend’ that establishes a polarity in molecular orientation. Intriguingly for a PRR that has specifically evolved to sense duplex RNA and not DNA (another family of proteins serves as cytoplasmic DNA sensors), the interaction interface between RIG-I and RNA is composed primarily of glutamine: 2′-hydroxyl contacts [17•• ,18•• ,19••], resulting in shape-selective, RNA-specific detection.
RIG-I — and potentially other DRA's — recognize not just the surface features, but also the length of their targets, which is partially evident from the similar footprint of approximately 10 base pairs in each complex visualized to date. Most viral RNAs that are recognized by RIG-I contain blunt terminal duplexes that represent the presumed interaction site [39,40]. This is consistent with solution biophysical, enzymological and cell culture work showing that a 10-base pair duplex is the minimal, high affinity interaction site for functional RIG-I proteins, which bind as monomers to the terminus of an RNA target [30]. A recent set of crystal structures in different nucleotide-bound states suggests that this site size is defined, at least in part, via a scanning mechanism in which the HEL2i domain sweeps along the backbone of the RNA, where it samples contacts from 8 to 10 base pairs as it transits along the backbone [30].
Short RNA duplexes are readily recognized by RIG-I, but the highest affinity ligands are RNA duplexes containing a 5′-triphosphate [41,42], which are typical products of viral RNA replication. The strongest interactions within the entire complex are observed between the CTD and the triphosphosphorylated duplex terminus [43]. This binding is mediated by stacking interactions and contacts with the terminal base pair, along with interactions between conserved lysines and the α-phosphate and β-phosphate [22•]. Remarkably, the CTD occupies almost the same position whether a duplex terminus contains a 5′-triphosphate or not [22•]. However, the CTD exhibits a greater affinity for a triphosphorylated RNA terminus as an isolated domain, which may explain why the energy of RIG-I binding to RNA targets, while very strong, is weaker than the sum of its parts [41]. Intriguingly, the CTD of MDA-5 appears to serve a different function (vida supra), consistent with the lack of a triphosphate requirement in its viral RNA targets [21•• ,44•].
Nucleotide binding and the role of ATP
Activation of RIG-I depends not just on binding of target RNA, but on the binding of ATP. Mutations within the motifs that govern ATP binding and hydrolysis (located in the cleft between HEL1 and HEL2) suggest that both are important for the function of RIG-I and related proteins [2,45,46]. However, the structural basis for ATP activation and its subsequent role in signaling remain elusive. Unlike DEAD-box proteins and other SF2 members, RNA binding by RIG-I does not cause the ATP-binding cleft to become fully ordered (Figure 3a,c) [7,20]. In RIG-I complexes, the HEL2 domain remains partially disordered and the ATPase cleft between HEL1 and HEL2 is unusually wide and open in all structures captured to date, with the exception of one (vida infra). This unusually expanded ATPase cleft adopts the same open conformation in the absence of nucleotide as it does in the presence of ADP, where the nucleotide diphosphate binds almost exclusively on one side of the cleft, interacting directly with the Q motif and motif I, and indirectly with motif II through a coordinated magnesium ion in the well-ordered HEL1 [18•• ,22•].
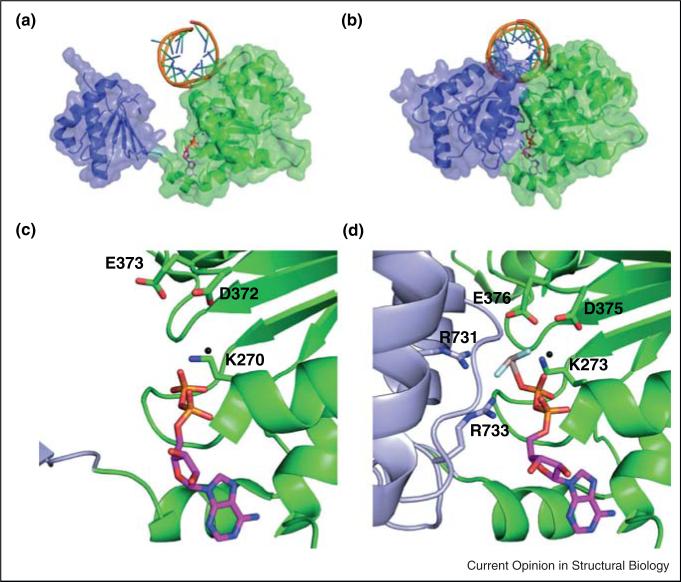
Figure 3.
The RIG-I helicase domain compacts upon interaction with ADP-AlF4. (a) The ‘open state’ structure of the HEL1 and HEL2 domains of RIG-I bound to a 10mer RNA hairpin and an ADP substrate (PDB 4A2Y). The nucleotide diphosphate interacts exclusively with residues in HEL1 and the RecA domains are well separated. (b) The ‘close state’ structure of RIG-I bound to a 19mer duplex RNA and ADP-AlF4 (PDB 4A36). In the presence of the ATP analog, the RecA domains compact, with HEL2 pivoting toward the RNA duplex to make several interactions with the triphosphate mimetic in the binding pocket. (C-D) Close up view of the ATP binding pockets from (a) and (b), respectively. Residues involved in ATP binding are labeled.
The closed state of RIG-I has only been visualized in a construct lacking both the CARD domains and the CTD. Upon binding ADP-AlFx, HEL1 and 2 close around the nucleotide [17••], just as observed for the closed state of other SF2 proteins upon binding ATP (Figure 3b,d) [47]. This closure results in significant compaction of the protein observable by analytical ultracentrifugation [22•]. Indeed, AU experiments show that the RIG-I/ RNA complex has a similar volume in the presence and absence of ADP [22•], but that it compresses upon binding of ADP-AlFx, a nucleotide-triphosphate analog which may more closely resemble the hydrolytic transition state based on the planar geometry of the γ-phosphate mimetic (AlFx).
A two-trigger motor and the mechanism for signaling
The compaction observed for the closed, ADP-AlFx state of RIG-I has clear implications for the mechanism of RIG-I signaling (Figure 3a,b). That the RIG-I ATPase domains can open and close like SF2 helicases suggests that RIG-I might use ATP-dependent motion to power translocation along RNA. Indeed, RIG-I translocation was previously reported using a short-range fluorescence enhancement (PIFE) assay [10], and it has been suggested that this motion might play a role in loading multiple RIG-I molecules on long viral RNAs, thereby facilitating signaling [48]. However, the fact that very short RNAs (minimally 10 base pairs in length) are sufficient for stimulating signaling by RIG-I suggests any translocative behavior by RIG-I is not required for its function in surveillance. In addition, translocation by SF2 proteins requires progressive RNA contacts by conserved side-chains within the ATPase core in both the open and closed states [49,50]. Given the unusual structure of HEL1 and HEL2, particularly in the open state, the ‘motor’ amino acids are not positioned in a manner that would be expected to power translocation.
However, RNA-dependent ATPase activity of RIG-I, and ATP-dependent closure of the HEL1-HEL2 cleft may be coupled to other processes that contribute to signaling. One model for ATPase function is that it catalyzes expulsion of the CARD domains, suggesting that RIG-I behaves more like a G-protein or Hsp70 chaperone than a conventional helicase/translocase (Figure 4, left panel). Analysis of existing crystal structures suggests that, in the open state, all RIG-I domains (including the CARDs) can readily enfold the RNA duplex without experiencing any steric clashes [7]. However, upon ATP binding (as represented in the ADP-AlFx structure), RIG-I squeezes shut, and the CTD crashes directly into the position occupied by the CARDs [7,22•]. Given that the affinity of the CTD for the RNA terminus is extremely high, this motion would be expected to dislodge the CARDS from their docking site on HEL2i, presenting them for interaction with MAVS and activation of downstream signaling (Figure 4).
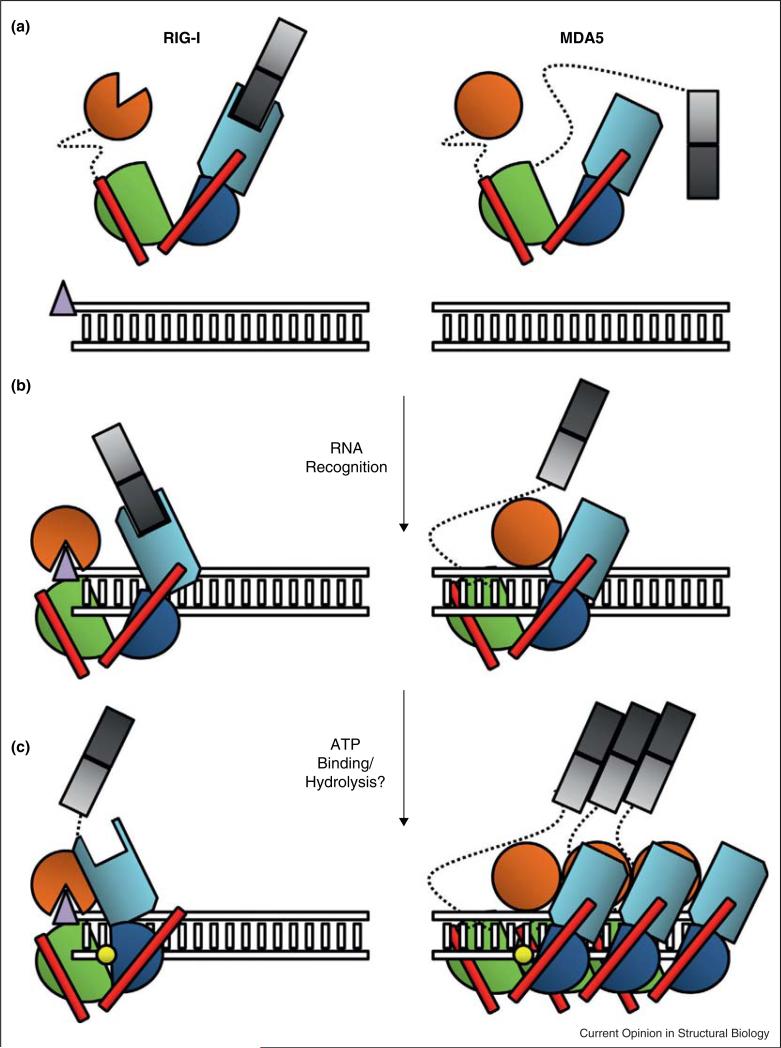
Figure 4.
Schematic representing the stages of RLR activation. RLR proteins RIG-I and MDA5 are shown color coded by domain; CARD1: light gray, CARD2: dark gray, HEL1: Green, HEL2: blue, HEL2i: Cyan, Pincer: Red, and CTD: orange. Duplex RNA is represented as a ladder, with the triphosphate moiety depicted as a purple triangle at the terminus. ATP is depicted as a yellow circle. Dotted lines represent flexible linker regions between domains. (a) RIG-I surveys the cell in a tightly regulated ‘signal off’ conformation, with the CARD domains packed tightly against HEL2i, and the CTD sampling greater conformational space. MDA5 regulation may be more attributable to its requirement for oligomerization than CARD sequestration, although the details of this hypothesis remain unclear. (b) The RIG-I CTD has an exceptionally high affinity for terminal triphosphates, thus the protein binds most strongly to the dsRNA end. The CTD caps the terminus as it interacts with the triphosphate, while the HEL1, HEL2 and HEL2i domains wrap around the duplex forming a ring. In this state, the CARDs of RIG-I can theoretically still be accommodated in a HEL2i-sequestered state. The MDA5 CTD recognizes and binds internal RNA duplexes, in conjunction with the HEL1, Hel2 and HEL2i domains. Notably, the CTD resides closer to the HEL2i domain in MDA5 than in RIG-I, resulting in a more C-shaped than O-shaped conformation. (c) Upon interaction with ATP, the RIG-I helicase domain further compacts, potentially ejecting the CARDs, which become available for interaction with other protein factors and subsequent signaling through MAVS. MDA5 forms a cooperative filament along the RNA substrate, bringing the CARD domains in sufficient proximity to multimerize and promote signaling. The role of ATP in this process remains unclear. CARD: Caspase Activation and Recruitment Domain, CTD: C-terminal Domain.
Protein–protein interactions and induction of the signaling cascade
It is well accepted that activated RIG-I and MDA-5 initiate the β-interferon signaling cascade by interacting with and inducing oligomerization of an array of MAVS adapter proteins, which are located on the surface of the mitochondria [5]. MAVS interacts with PRRs through CARD-CARD interactions, and while the MAVS/RIG-I complex has not yet been visualized crystallographically, the interaction interface is likely to resemble the contacts seen between other Death Domain proteins [51].
Maximal activation of the MAVS array requires that multiple PRRs assemble along its surface. This is readily accomplished by MDA-5, which forms cooperative filaments along its long, duplex RNA targets, thereby presenting numerous CARDS to MAVS (Figure 4). Structural and functional studies have shown that MDA-5 has a domain structure similar to RIG-I, but its CTD is used for cooperative oligomerization rather than 5′-triphosphate recognition. It has been proposed that RIG-I must also form oligomers on RNA in order to activate MAVS, and there are multiple models by which this might occur [20]. RIG-I might oligomerize on RNA [48,52], or 1:1 RIG-I/RNA complexes may join with other proteins to oligomerize on the mitochondrion [20,30]. The latter would be consistent with the finding that polyubiquitin chains associate with RIG-I complexes and that these play a key role in signaling [53].
Perspectives
Superfamily 2 helicases are ubiquitous proteins involved in nearly every aspect of RNA metabolism in the cell. Over the past decade, the structural and functional diversity of these proteins has become increasingly evident as we have achieved a better understanding of their distinct molecular mechanisms. Duplex RNA activated ATPases are fascinating examples of molecular engineering that has evolved a complex, highly regulated signaling system from the foundation of a simpler yet effective core.
Structural biology continues to prove invaluable as a tool for understanding the precise mechanics of RLR activity. Structural work on these proteins has provided insights into how these proteins discriminate pathogenic from native RNA, how they interact with their targets, and how these interactions may promote signaling. Further, structural understanding of the function of RIG-I and MDA5 can provide a conceptual basis from which to study viral mechanisms for immune evasion [54] and develop therapeutic strategies targeting these proteins [55]. Recent work has greatly improved our understanding of the similarities and differences among RLRs, and provided a foundation for understanding other DRA proteins.
Acknowledgements
The authors thank Andrew Kohlway for stimulating discussion and editorial contributions in preparing the manuscript. This research was funded by Howard Hughes Medical Institute.
Footnotes
Conflict of Interest
The authors declare no competing financial interests.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Kang DC, Gopalkrishnan RV, Wu Q, Jankowsky E, Pyle AM, Fisher PB. mda-5: an interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc Natl Acad Sci USA. 2002;99:637–642. doi: 10.1073/pnas.022637199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S. Fujita T: The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 3.Yoneyama M, Kikuchi M, Matsumoto K, Imaizumi T, Miyagishi M, Taira K, Foy E, Loo YM, Gale M, Jr, Akira S, et al. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol. 2005;175:2851–2858. doi: 10.4049/jimmunol.175.5.2851. [DOI] [PubMed] [Google Scholar]
- 4.Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Hou F, Sun L, Zheng H, Skaug B, Jiang QX, Chen ZJ. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell. 2011;146:448–461. doi: 10.1016/j.cell.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jankowsky E. RNA helicases at work: binding and rearranging. Trends Biochem Sci. 2011;36:19–29. doi: 10.1016/j.tibs.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo D, Kohlway A, Pyle AM. Duplex RNA Activated ATPases (DRAs): platforms for RNA sensing, signaling and processing. RNA Biol. 2012;10 doi: 10.4161/rna.22706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jankowsky E, Fairman ME. RNA helicases—one fold for many functions. Curr Opin Struct Biol. 2007;17:316–324. doi: 10.1016/j.sbi.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Matranga C, Pyle AM. Double-stranded RNA-dependent ATPase DRH-3: insight into its role in RNAsilencing in Caenorhabditis elegans. J Biol Chem. 2010;285:25363–25371. doi: 10.1074/jbc.M110.117010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myong S, Cui S, Cornish PV, Kirchhofer A, Gack MU, Jung JU, Hopfner KP, Ha T. Cytosolic viral sensor RIG-I is a 5′-triphosphate-dependent translocase on double-stranded RNA. Science. 2009;323:1070–1074. doi: 10.1126/science.1168352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beran RK, Serebrov V, Pyle AM. The serine protease domain of hepatitis C viral NS3 activates RNA helicase activity by promoting the binding of RNA substrate. J Biol Chem. 2007;282:34913–34920. doi: 10.1074/jbc.M707165200. [DOI] [PubMed] [Google Scholar]
- 12.Whitby MC. The FANCM family of DNA helicases/translocases. DNA Repair (Amst) 2010;9:224–236. doi: 10.1016/j.dnarep.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Lewis R, Durr H, Hopfner KP, Michaelis J. Conformational changes of a Swi2/Snf2 ATPase during its mechano-chemical cycle. Nucleic Acids Res. 2008;36:1881–1890. doi: 10.1093/nar/gkn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ireton RC, Gale M., Jr RIG-I like receptors in antiviral immunity and therapeutic applications. Viruses. 2011;3:906–919. doi: 10.3390/v3060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welker NC, Maity TS, Ye X, Aruscavage PJ, Krauchuk AA, Liu Q, Bass BL. Dicer's helicase domain discriminates dsRNA termini to promote an altered reaction mode. Mol Cell. 2011;41:589–599. doi: 10.1016/j.molcel.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Civril F, Bennett M, Moldt M, Deimling T, Witte G, Schiesser S, Carell T, Hopfner KP. The RIG-I ATPase domain structure reveals insights into ATP-dependent antiviral signalling. EMBO Rep. 2011;12:1127–1134. doi: 10.1038/embor.2011.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17••.Kowalinski E, Lunardi T, McCarthy AA, Louber J, Brunel J, Grigorov B, Gerlier D, Cusack S. Structural basis for the activation of innate immune pattern-recognition receptor RIG-I by viral RNA. Cell. 2011;147:423–435. doi: 10.1016/j.cell.2011.09.039. [One of the initial structural reports of dsRNA bound RIG-I; includes a structure showing CARD domain packing on HEL2i and an ADP-AlFx-bound helicase domain.] [DOI] [PubMed] [Google Scholar]
- 18••.Jiang F, Ramanathan A, Miller MT, Tang GQ, Gale M, Jr, Patel SS, Marcotrigiano J. Structural basis of RNA recognition and activation by innate immune receptor RIG-I. Nature. 2011;479:423–427. doi: 10.1038/nature10537. [One of the three initial structural reports of dsRNA recognition by RIG-I, the authors compare this structure with that of the processive NS3 viral helicase.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19••.Luo D, Ding SC, Vela A, Kohlway A, Lindenbach BD, Pyle AM. Structural insights into RNA recognition by RIG-I. Cell. 2011;147:409–422. doi: 10.1016/j.cell.2011.09.023. [One of the initial structural reports of dsRNA bound RIG-I; includes biochemical analyses relating the novel pincer/linker and conserved helicase motifs to RIG-I signaling.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolakofsky D, Kowalinski E, Cusack S. A structure-based model of RIG-I activation. RNA. 2012;18:2118–2127. doi: 10.1261/rna.035949.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21••.Wu B, Peisley A, Richards C, Yao H, Zeng X, Lin C, Chu F, Walz T, Hur S. Structural basis for dsRNA recognition, filament formation, and antiviral signal activation by MDA5. Cell. 2013;152:276–289. doi: 10.1016/j.cell.2012.11.048. [Contains the first and currently only high resolution crystal structure of MDA5 bound to dsRNA.] [DOI] [PubMed] [Google Scholar]
- 22•.Luo D, Kohlway A, Vela A, Pyle AM. Visualizing the determinants of viral RNA recognition by innate immune sensor RIG-I. Structure. 2012;20:1983–1988. doi: 10.1016/j.str.2012.08.029. [Contains structure of RIG-I bound to a minimal RNA substrate in the form of a short triphosphorylated hairpin.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caruthers JM, Johnson ER, McKay DB. Crystal structure of yeast initiation factor 4A, a DEAD-box RNA helicase. Proc Natl Acad Sci USA. 2000;97:13080–13085. doi: 10.1073/pnas.97.24.13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caruthers JM, McKay DB. Helicase structure and mechanism. Curr Opin Struct Biol. 2002;12:123–133. doi: 10.1016/s0959-440x(02)00298-1. [DOI] [PubMed] [Google Scholar]
- 25•.Lu C, Xu H, Ranjith-Kumar CT, Brooks MT, Hou TY, Hu F, Herr AB, Strong RK, Kao CC, Li P. The structural basis of 5′ triphosphate double-stranded RNA recognition by RIG-I C-terminal domain. Structure. 2010;18:1032–1043. doi: 10.1016/j.str.2010.05.007. [The authors report structures of the RIG-I CTD bound to RNA duplexes containing both a 50 triphosphate and 5′ hydroxyl and compare the modes of interaction.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26•.Wang Y, Ludwig J, Schuberth C, Goldeck M, Schlee M, Li H, Juranek S, Sheng G, Micura R, Tuschl T, et al. Structural and functional insights into 5′-ppp RNA pattern recognition by the innate immune receptor RIG-I. Nat Struct Mol Biol. 2010;17:781–787. doi: 10.1038/nsmb.1863. [Contains a structure of the CTD bound to triphosphorylated RNA and makes a nice comparison between the RIG-I, MDA5, and Lgp2 CTDs.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Cui S, Eisenacher K, Kirchhofer A, Brzozka K, Lammens A, Lammens K, Fujita T, Conzelmann KK, Krug A, Hopfner KP. The C-terminal regulatory domain is the RNA 5′-triphosphate sensor of RIG-I. Mol Cell. 2008;29:169–179. doi: 10.1016/j.molcel.2007.10.032. [Structural and biochemical analyses of triphosphate recognition by the RIG-I CTD.] [DOI] [PubMed] [Google Scholar]
- 28.Takahasi K, Yoneyama M, Nishihori T, Hirai R, Kumeta H, Narita R, Gale M, Jr, Inagaki F, Fujita T. Nonself RNA-sensing mechanism of RIG-I helicase and activation of antiviral immune responses. Mol Cell. 2008;29:428–440. doi: 10.1016/j.molcel.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 29.Kageyama M, Takahasi K, Narita R, Hirai R, Yoneyama M, Kato H, Fujita T. 55 Amino acid linker between helicase and carboxyl terminal domains of RIG-I functions as a critical repression domain and determines inter-domain conformation. Biochem Biophys Res Commun. 2011;415:75–81. doi: 10.1016/j.bbrc.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 30.Kohlway A, Luo D, Rawling DC, Ding SC, Pyle AM. Defining the functional determinants for RNA surveillance by RIG-I. EMBO Rep. 2013;14:772–779. doi: 10.1038/embor.2013.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berke IC, Modis Y. MDA5 cooperatively forms dimers and ATP-sensitive filaments upon binding double-stranded RNA. EMBO J. 2012;31:1714–1726. doi: 10.1038/emboj.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor DW, Ma E, Shigematsu H, Cianfrocco MA, Noland CL, Nagayama K, Nogales E, Doudna JA, Wang HW. Substrate-specific structural rearrangements of human Dicer. Nat Struct Mol Biol. 2013;20:662–670. doi: 10.1038/nsmb.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarkar D, Desalle R, Fisher PB. Evolution of MDA-5/RIG-I-dependent innate immunity: independent evolution by domain grafting. Proc Natl Acad Sci USA. 2008;105:17040–17045. doi: 10.1073/pnas.0804956105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berke IC, Li Y, Modis Y. Structural basis of innate immune recognition of viral RNA. Cell Microbiol. 2013;15:386–394. doi: 10.1111/cmi.12061. [DOI] [PubMed] [Google Scholar]
- 35.Ferrao R, Wu H. Helical assembly in the death domain (DD) superfamily. Curr Opin Struct Biol. 2012;22:241–247. doi: 10.1016/j.sbi.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacobs JL, Coyne CB. Mechanisms of MAVS regulation at the mitochondrial membrane. J Mol Biol. 2013;425:5009–50019. doi: 10.1016/j.jmb.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang X, Kinch LN, Brautigam CA, Chen X, Du F, Grishin NV, Chen ZJ. Ubiquitin-induced oligomerization of the RNA sensors RIG-I and MDA5 activates antiviral innate immune response. Immunity. 2012;36:959–973. doi: 10.1016/j.immuni.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wallach D, Kovalenko A. Phosphorylation and dephosphorylation of the RIG-I-like receptors: a safety latch on a fateful pathway. Immunity. 2013;38:402–403. doi: 10.1016/j.immuni.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 39.Rehwinkel J, Tan CP, Goubau D, Schulz O, Pichlmair A, Bier K, Robb N, Vreede F, Barclay W, Fodor E, et al. RIG-I detects viral genomic RNA during negative-strand RNA virus infection. Cell. 2010;140:397–408. doi: 10.1016/j.cell.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 40.Baum A, Garcia-Sastre A. Differential recognition of viral RNA by RIG-I. Virulence. 2011;2:166–169. doi: 10.4161/viru.2.2.15481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vela A, Fedorova O, Ding SC, Pyle AM. The thermodynamic basis for viral RNA detection by the RIG-I innate immune sensor. J Biol Chem. 2012;287:42564–42573. doi: 10.1074/jbc.M112.385146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schlee M, Roth A, Hornung V, Hagmann CA, Wimmenauer V, Barchet W, Coch C, Janke M, Mihailovic A, Wardle G, et al. Recognition of 50 triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative-strand virus. Immunity. 2009;31:25–34. doi: 10.1016/j.immuni.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng L, Huang Z, Zhou W, Wu Q, Donnola S, Liu JK, Fang X, Sloan AE, Mao Y, Lathia JD, et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell. 2013;153:139–152. doi: 10.1016/j.cell.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Berke IC, Yu X, Modis Y, Egelman EH. MDA5 assembles into a polar helical filament on dsRNA. Proc Natl Acad Sci USA. 2012;109:18437–18441. doi: 10.1073/pnas.1212186109. [An excellent report on the structural basis for long filament assembly by MDA5 using electron microscopy.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bamming D, Horvath CM. Regulation of signal transduction by enzymatically inactive antiviral RNA helicase proteins MDA5, RIG-I, and LGP2. J Biol Chem. 2009;284:9700–9712. doi: 10.1074/jbc.M807365200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gee P, Chua PK, Gevorkyan J, Klumpp K, Najera I, Swinney DC, Deval J. Essential role of the N-terminal domain in the regulation of RIG-I ATPase activity. J Biol Chem. 2008;283:9488–9496. doi: 10.1074/jbc.M706777200. [DOI] [PubMed] [Google Scholar]
- 47.Hilbert M, Karow AR, Klostermeier D. The mechanism of ATP-dependent RNA unwinding by DEAD box proteins. Biol Chem. 2009;390:1237–1250. doi: 10.1515/BC.2009.135. [DOI] [PubMed] [Google Scholar]
- 48.Patel JR, Jain A, Chou YY, Baum A, Ha T, Garcia-Sastre A. ATPase-driven oligomerization of RIG-I on RNA allows optimal activation of type-I interferon. EMBO Rep. 2013;14:780–787. doi: 10.1038/embor.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Myong S, Bruno MM, Pyle AM, Ha T. Spring-loaded mechanism of DNA unwinding by hepatitis C virus NS3 helicase. Science. 2007;317:513–516. doi: 10.1126/science.1144130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buttner K, Nehring S, Hopfner KP. Structural basis for DNA duplex separation by a superfamily-2 helicase. Nat Struct Mol Biol. 2007;14:647–652. doi: 10.1038/nsmb1246. [DOI] [PubMed] [Google Scholar]
- 51.Qin H, Srinivasula SM, Wu G, Fernandes-Alnemri T, Alnemri ES, Shi Y. Structural basis of procaspase-9 recruitment by the apoptotic protease-activating factor 1. Nature. 1999;399:549–557. doi: 10.1038/21124. [DOI] [PubMed] [Google Scholar]
- 52.Peisley A, Wu B, Yao H, Walz T, Hur S. RIG-I forms signaling-competent filaments in an ATP-dependent, ubiquitin-independent manner. Mol Cell. 2013;51:573–583. doi: 10.1016/j.molcel.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 53.Zeng W, Sun L, Jiang X, Chen X, Hou F, Adhikari A, Xu M, Chen ZJ. Reconstitution of the RIG-I pathway reveals a signaling role of unanchored polyubiquitin chains in innate immunity. Cell. 2010;141:315–330. doi: 10.1016/j.cell.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Motz C, Schuhmann KM, Kirchhofer A, Moldt M, Witte G, Conzelmann KK, Hopfner KP. Paramyxovirus V proteins disrupt the fold of the RNA sensor MDA5 to inhibit antiviral signaling. Science. 2013;339:690–693. doi: 10.1126/science.1230949. [DOI] [PubMed] [Google Scholar]
- 55.Martinez-Gil L, Goff PH, Hai R, Garcia-Sastre A, Shaw ML, Palese P. A Sendai virus-derived RNA agonist of RIG-I as a virus vaccine adjuvant. J Virol. 2013;87:1290–1300. doi: 10.1128/JVI.02338-12. [DOI] [PMC free article] [PubMed] [Google Scholar]