Summary
Background
Angiotensin-receptor blockers (ARBs) are a widely used drug class approved for treatment of hypertension, heart failure, diabetic nephropathy, and, recently, for cardiovascular risk reduction. Experimental studies implicate the renin-angiotensin system, particularly angiotensin II type-1 and type-2 receptors, in the regulation of cell proliferation, angiogenesis, and tumour progression. We assessed whether ARBs affect cancer occurrence with a meta-analysis of randomised controlled trials of these drugs.
Methods
We searched Medline, Scopus (including Embase), Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and the US Food and Drug Administration website for studies published before November, 2009, that included any of the seven currently available ARBs. Randomised controlled trials with an ARB given in at least one group, with a follow-up of at least 1 year, and that enrolled at least 100 patients were included. New-cancer data were available for 61 590 patients from five trials. Data on common types of solid organ cancers were available for 68 402 patients from five trials, and data on cancer deaths were available for 93 515 patients from eight trials.
Findings
Telmisartan was the study drug in 30 014 (85·7%) patients who received ARBs as part of the trials with new cancer data. Patients randomly assigned to receive ARBs had a significantly increased risk of new cancer occurrence compared with patients in control groups (7·2% vs 6·0%, risk ratio [RR] 1·08, 95% CI 1·01–1·15; p=0·016). When analysis was limited to trials where cancer was a prespecified endpoint, the RR was 1·11 (95% CI 1·04–1·18, p=0·001). Among specific solid organ cancers examined, only new lung-cancer occurrence was significantly higher in patients randomly assigned to receive ARBs than in those assigned to receive control (0·9% vs 0·7%, RR 1·25, 1·05–1·49; p=0·01). No statistically significant difference in cancer deaths was observed (1·8% vs1·6%, RR 1·07, 0·97–1·18; p=0·183).
Interpretation
This meta-analysis of randomised controlled trials suggests that ARBs are associated with a modestly increased risk of new cancer diagnosis. Given the limited data, it is not possible to draw conclusions about the exact risk of cancer associated with each particular drug. These findings warrant further investigation.
Introduction
Antagonists of the angiotensin II type-1 receptor (ie, angiotensin-receptor blockers, ARBs) are a widely used class of drugs. The first ARB, losartan, was approved for clinical use in 1995, followed by six other drugs, including valsartan, candesartan, irbesartan, telmisartan, olmesartan, and eprosartan. These drugs are used for treatment of highly prevalent conditions such as hypertension, heart failure, and diabetic nephropathy.1–3 Most recently, telmisartan was approved for lowering the risk of cardiovascular events in high-risk patients who are unable to take angiotensin-converting enzyme (ACE) inhibitors. Many large, randomised controlled trials of ARBs have supported their clinical indications.4–23
Currently, there are no major safety concerns with ARBs, apart from their use in pregnancy, renal-artery stenosis, and chronic kidney disease. Although preclinical carcinogenicity studies in rats and mice have been negative,24–26 experimental studies implicate the reninangiotensin system, particularly angiotensin II type-1 and type-2 receptors (AT1R and AT2R), in the regulation of cellular proliferation, angiogenesis, and tumour progression.27 However, clinical trials of ARBs have mainly assessed their effects on cardiovascular and renal endpoints and have usually not reported incidence of cancers. In 2003, the Candesartan in Heart failure Assessment of Reduction in Mortality and Morbidity (CHARM) programme, assessing ARBs in heart failure, reported an unexpected finding of significantly higher fatal cancers in the candesartan group than with placebo.5 Because the results of several other large ARB trials have become available since the publication of the CHARM trial, we decided to do a meta-analysis of randomised controlled trials of these drugs, to examine their effect on occurrence of new cancers. Our secondary objectives were to determine whether ARBs affect the occurrence of specific solid-organ cancers and cancer deaths.
Methods
Data collection
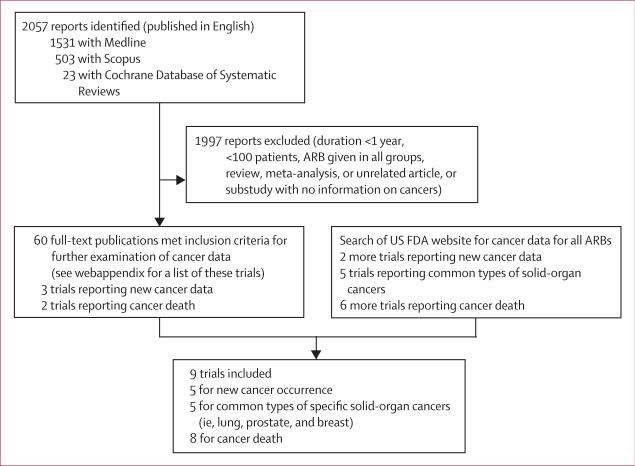
The aim of this meta-analysis was to include all publicly available data for development of cancers from randomised controlled trials of ARBs. We did systematic searches of Medline, Scopus (including Embase and several other journal groups from a wide range of disciplines), Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and the public website of the US Food and Drug Administration (FDA), through November, 2009. The search terms and other search strategies are described in detail for each database in the webappendix. Briefly, Medline searches for randomised controlled trials published in English were done for all seven clinically available ARBs using their generic names as search terms (losartan, valsartan, candesartan, irbesartan, eprosartan, telmisartan, and olmesartan), giving 1531 results (figure 1). Scopus was searched with a similar method and led to 503 additional results. A search of the Cochrane Central Register of Controlled Trials did not identify any new results different from those found with Medline. The Cochrane Database of Systematic Reviews was also searched for all seven ARBs and led to 23 results, resulting in a total of 2057 search results. The FDA website was searched using the generic and brand names of all available ARBs. The links of every search result were examined thoroughly for additional information on cancer in randomised trials of ARBs.
Figure 1. Flow diagram of literature search to identify randomised controlled trials of angiotensin-receptor blockers.
See webappendix for search terms used in the different databases. FDA=Food and Drug Administration. ARB=angiotensin-receptor blocker.
Study selection
All of the search results were assessed for study duration and sample size. Since cancer is an uncommon adverse event with a long latent period, it is rarely reported in small studies with short follow-up periods. Therefore, trials with a median or mean follow-up of less than 12 months, or involving a study population of fewer than 100 patients, were excluded. These cut-off values for study duration and enrolment size are based on a previous meta-analysis examining the effect of statins on incident cancer.28 However, to limit the possibility of missing pertinent information, the abstracts of all smaller trials were screened for the terms “cancer”, “carcinoma”, “malignancy”, “neoplasm”, “neoplasia”, and “tumor”, which did not reveal any relevant information. Trials where all groups received an ARB were also excluded.
60 trials that fulfilled the inclusion criteria were examined for reporting of cancer data (figure 1; webappendix), which was available for five trials: LIFE,4 TROPHY,6 and TRANSCEND7 reported cancer occurrence; CHARM-Overall5 and OPTIMAAL12 reported cancer deaths. The FDA website was searched for additional information, using the search strategy stated in the webappendix. Information on the occurrence of new cancer, solid-organ cancers (lung, breast, and prostate cancer), and cancer death was obtained for two more large trials (ONTARGET and PROFESS).29 Additionally, searches of the FDA website identified data for organ-specific new cancers in LIFE,30 TRANSCEND,29 and CHARM-Overall,31,32 and for cancer deaths in LIFE,30 TRANSCEND,29 VALIANT,33 and VAL-HEFT34 (figure 1). For the CHARM-Overall programme, only the total number of neoplasms and cancer deaths were reported and data for malignant neoplasms (ie, fatal and non-fatal cancers, excluding benign neoplasms) were not described in the peer-reviewed publication or in the FDA website documents.5,31,32 As a result, for the primary objective of assessing the effect of ARBs on new cancer occurrence, a total of five trials were included: LIFE, TROPHY, TRANSCEND, ONTARGET, and PROFESS (n=61 590).
For the secondary outcome of assessing occurrence of specific solid-organ cancers, five trials were included: LIFE, CHARM-Overall, TRANSCEND, ONTARGET, and PROFESS (n=68 402). Data on organ cancers other than lung, prostate, and breast cancer were not uniformly reported and were grouped as other cancers. For the secondary outcome of assessing cancer-related deaths, eight trials were included: LIFE, CHARM-Overall, TRANSCEND, ONTARGET, PROFESS, OPTIMAAL, VALIANT, and VAL-HEFT (n=93 515; figure 1). Therefore, data from a total of nine different trials were used in the analyses. Data on fatal cancers and two organ-specific cancers (lung and prostate, but not breast cancer) were also available for the CHARM-Added component of the CHARM programme, which assessed candesartan plus an ACE inhibitor versus ACE inhibitor alone.31,32,35 This information, and data from the ONTARGET study (telmisatan plus ramipril vs ramipril alone),29 were used for analysis of overall cancer risk and risk of specific solid-organ cancers associated with ARB plus ACE-inhibitor therapy compared with ACE inhibitors alone.
Ascertainment of cancer diagnosis
Cancer was a prespecified endpoint of special interest in three of the five trials that included new-cancer data for analysis of cancer occurrence (LIFE, ONTARGET, and TRANSCEND), corresponding to 66% (40 739 of 61 590) of patients with new-cancer data. Cancer was a prespecified adverse event of special interest in the LIFE trial,30 and adverse experiences were monitored throughout the study and specifically recorded at each visit.36 In the ONTARGET and TRANSCEND trials, information on the occurrence of malignancies was also collected prospectively, in more detail than usual for trials of cardiovascular outcome, according to the FDA briefing document.29 After the last patient visit, the steering and operations committees requested detailed information for each report of cancer, and all reports were reviewed by the adjudication committee of each trial. In the remaining two trials (PROFESS and TROPHY; 34% of patients [20 851 of 61 590]), cancer information was collected as new serious adverse events per routine pharmacovigilance monitoring.6,29
Data extraction
Data extraction from source documents was done independently by two of the investigators (IS and SMD) and verified. Number of cancers, specific organ cancers, cancer deaths, and total number of patients in each trial group were extracted, along with other information including disorders studied, all-cause death and myocardial infarction rates or hazard ratios (HR), trial duration, age, sex, ethnic origin, smoking status, cancer history, medication adherence, and dropout rates. In addition to number of cancers, HR for cancer occurrence were reported in three trials (TRANSCEND, ONTARGET, and PROFESS) and were also extracted.29 There were two cases of discrepancy between the peer-reviewed articles and the FDA documents (356 and 315 new cancers were reported in the LIFE study4 for losartan and atenolol, respectively, vs 358 and 320 in the FDA document;30 86 cancers deaths with candesartan were reported in CHARM-Overall5 vs 84 in the FDA document31,32). In these cases, data from the FDA documents were used in the analyses because they were more recent.
Statistical analysis
Begg's rank-correlation method was used to assess publication bias,37 by testing for Kendall's tau with Wessa software, version 1.0.10 (Free Statistics Software, version 1.1.23-r4),38 and a funnel plot was generated. Statistical heterogeneity across trials was tested by Cochran's Q statistic. An alpha value of 0·10 was taken to indicate heterogeneity among trials for each analysis. Degree of heterogeneity for each analysis was presented with I2 values. Fixed-effect models were used in all analyses unless there was evidence of heterogeneity. A random-effects model was used for a single occasion with evidence of heterogeneity, for assessing risk of new lung cancers in patients not receiving background ACE inhibitors. Risk ratios (RRs) were mainly used as the meta-analytic measure of association, and were calculated using the number of cancers recorded and number of patients in each trial group (ie, 2×2 tables). When the main analysis was limited to three trials with cancer as a prespecified event (ONTARGET, TRASCEND, and LIFE), we used a more robust method of combining the available HR from two of the trials (ONTARGET and TRANSCEND), and the 2×2 table information from the third trial (LIFE) to obtain the meta-analytic RR.
To assess for possible survival bias, a meta-analytic HR for all-cause death was calculated by use of HR for death with ARBs in the included trials. Additional sensitivity analyses were done, where the primary outcome of cancer occurrence was explored with the more conservative random-effects model, the meta-analysis was limited to patients without a history of cancer at baseline, and all neoplasms reported in the CHARM trial were assumed to be malignant. Number needed to harm was calculated using background cancer incidence for people aged 65–69 years (corresponding to the mean age of patients in these trials),39 and the meta-analytic RRs obtained from all trials, and also with the RR obtained from trials where cancer was a prespecified endpoint.40,41 p values less than 0·05 were considered significant, and all reported p values are two-sided. Data were analysed with Comprehensive Meta Analysis version 2.2.048.
Role of funding source
There was no funding source for this study. The corresponding author had full access to all data and had final responsibility for the decision to submit for publication.
Results
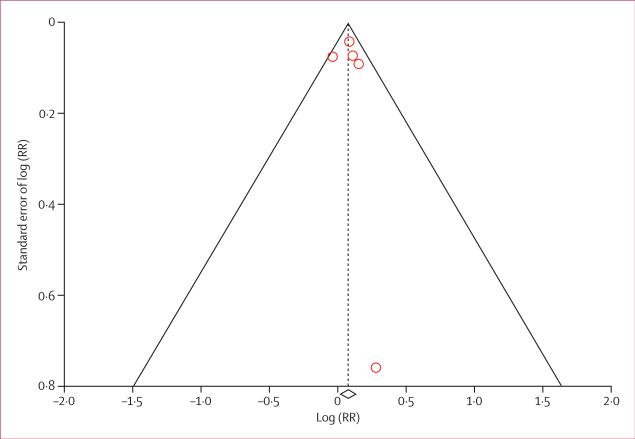
Characteristics of the randomised controlled trials included in the analyses are summarised in table 1. Both patients and investigators were masked to treatment allocation in all trials. For the primary outcome of cancer occurrence, telmisartan was the main ARB used as the study drug (in 30 014 [85·7%] patients). There was no notable imbalance within trial groups with regard to age, sex, ethnic origin, smoking status, and history of previous cancer. Overall, very few patients had a history of cancer at baseline. At trial closure, adherence to ARBs was 77–85% (73–85% with control), and dropouts or loss to follow-up were very low and were comparable in the ARB and control groups (table 2). The test statistic for Begg's rank-correlation method, Kendall's tau, was non-significant (p>0·80) for new cancer occurrence and cancer death, denoting no evidence of publication bias (figure 2).
Table 1.
Randomised controlled trials of angiotensin-receptor blockers that reported cancer data
| Condition studied | Mean or median duration, years | Number of patients | Study drug | Control | Mean age, years |
Men, % |
Black, % |
Current smoker, % |
History of cancer at baseline, % |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study drug | Control | Study drug | Control | Study drug | Control | Study drug | Control | Study drug | Control | ||||||
| Trials with data on new cancer, new specific solid-organ cancers, and cancer death | |||||||||||||||
| LIFE (2002)4,30,36 | Hypertension | 4.8 | 9193 | Losartan up to 100 mg (n=4605) | Atenolol up to 100 mg (n=4588) | 66.9 | 66.9 | 46.0 | 46.0 | 6.0 | 6.0 | 16.0 | 16.0 | NA | NA |
| ONTARGET (2008)8,29 | Cardiovascular disease* or diabetes with end-organ damage | 4.7 | 25 620 | Telmisartan 80 mg (n=8542) or telmisartan 80 mg+ramipril 5 mg (n=8502) | Ramipril 5 mg (n=8576) | 66.5 | 66.4 | 73.6 | 72.8 | NA | NA | 12.7 | 12.4 | 6.3 | 6.3 |
| TRANSCEND (2008)7,29 | ACE-inhibitor-intolerant patients with cardiovascular disease* or diabetes, with end-organ damage | 4.7 | 5926 | Telmisartan 80 mg (n=2954) | Placebo (n=2972) | 66.9 | 66.9 | 56.7 | 57.4 | 1.7 | 1.9 | 9.9 | 9.7 | 4.9 | 4.9 |
| PROFESS (2008)9,29 | Recent (<90 days) ischaemic stroke | 2.5 | 20 332 | Telmisartan 80 mg (n=10 146) | Placebo (n=10 186) | 66.1 | 66.2 | 64.3 | 63.8 | NA | NA | 21.2 | 21.2 | NA | NA |
| Trials with data on new specific solid-organ cancers and cancer death | |||||||||||||||
| CHARM-Overall programme (2003)5,31,32 | Heart failure | 3.1 | 7599 | Candesartan up to 32 mg (n=3803) | Placebo (n=3796) | 65.9 | 66.0 | 69.0 | 68.0 | 4.3 | 4.3 | 14.9 | 14.5 | 7.1 | 6.4 |
| Trial with new-cancer data only | |||||||||||||||
| TROPHY (2006)6 | Prehypertension | 3.6 | 787 | Candesatran 16 mg (n=391) | Placebo (n=381) | 48.6 | 48.3 | 59.1 | 60.1 | 12.3 | 8.1 | NA | NA | NA | NA |
| Trials with cancer-death data only | |||||||||||||||
| VAL-HEFT (2001)34,42 | Heart failure | 1-9 | 5010 | Valsartan up to 120 mg twice daily (n=2511) | Placebo (n=2499) | 62.4 | 63.0 | 79.9 | 80.0 | 7.2 | 6.5 | NA | NA | NA | NA |
| OPTIMAAL (2002)12 | Acute myocardial infarction | 2.7 | 5477 | Losartan up to 50 mg daily (n=2744) | Captopril up to 50 mg three times daily (n=2733) | 67.6 | 67.2 | 71.8 | 70.7 | NA | NA | NA | NA | NA | NA |
| VALIANT (2003)19,33 | Acute myocardial infarction | 2.1 | 14 626 | Valsartan up to 80 mg twice daily (n=4885) or valsartan up to 40 mg twice daily+ captopril up to 25 mg three times daily (n=4862) | Captopril up to 25 mg three times daily (n=4879) | 64.8 | 64.9 | 69.0 | 68.7 | 2.7 | 3.0 | NA | NA | NA | NA |
ACE=angiotensin-converting enzyme. NA=not available.
Includes coronary, peripheral, or cerebrovascular disease.
Table 2.
Adherence and dropout or loss to follow-up in trials included in meta-analysis
| Adherence at end of trial |
Dropout or loss to follow-up |
|||
|---|---|---|---|---|
| ARB | Control | ARB | Control | |
| LIFE30,36 | 77% | 73% | 2.3% | 2.0% |
| ONTARGET8,29 | 85%* | 85% | NA† | NA† |
| TRANSCEND7,29 | 81% | NA | 0.3% | 0.3% |
| PROFESS9,29 | 68% (at 3 years) | 71% (at 3 years) | 0.5% | 0.7% |
| CHARM-Overall5,31,32 | 77% | 81% | 0.2% | 0.1% |
| TROPHY6 | NA | NA | 14% | 14% |
| VAL-HEFT34,42 | NA | NA | NA | NA |
| OPTIMAAL12 | 87% (at 2 years) | 80% (at 2 years) | 0% | 0% |
| VALIANT19,33 | NA | NA | NA | NA |
ARB=angiotensin-receptor blocker. NA=not available.
Average adherence to telmisartan at end of study in telmisartan and telmisartan plus ramipril groups.
Overall, 0.2% of patients were lost to follow-up, data per study group not available.
Figure 2. Funnel plot for assessing publication bias for cancer occurrence.
Diamond represents log risk ratio (RR) for cancer occurrence (0·07, 95% CI 0·01–0·14).
In all trials, use of concomitant ACE inhibitors was determined by the study protocol. LIFE, TROPHY, and TRANSCEND trials did not allow treatment with ACE inhibitors. In the CHARM-Preserved component of CHARM, and in the PROFESS and VAL-HEFT trials, treatment with an ACE inhibitor was allowed in both groups. In the CHARM-Added component, all patients in both candesartan and placebo groups were given a concomitant ACE inhibitor. In the CHARM-Alternative component, patients were not allowed to be on an ACE inhibitor in the placebo or candesartan groups. The ONTARGET and VALIANT studies had three groups (ARB alone, ACE inhibitor alone, and ARB plus ACE inhibitor) that did not allow any other ARB or ACE inhibitor.
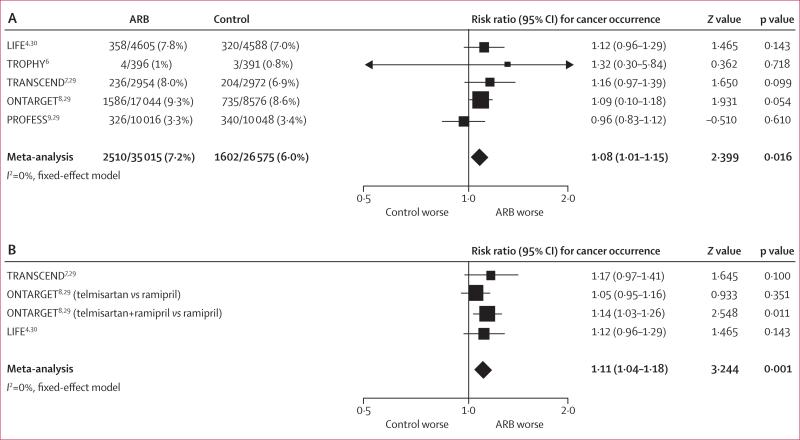
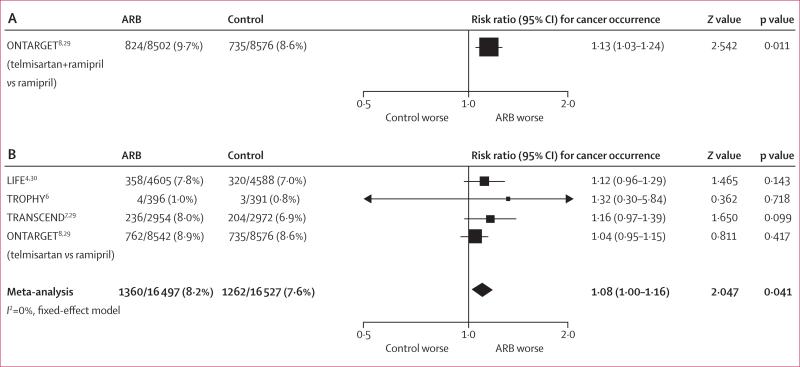
In all trials apart from PROFESS, there was a numerical excess of new cancers with ARBs. Meta-analysis showed a significantly increased risk of new cancer for patients randomised to receive ARBs compared with patients randomised to receive controls (7·2% vs 6·0%, I²=0%, meta-analytic RR 1·08, 95% CI 1·01–1·15; p=0·016; figure 3A). When analysis was limited to the three clinical trials where cancer was a prespecified endpoint and cancer data was rigorously collected (LIFE, ONTARGET, and TRANSCEND) there was also a significant increase in risk of cancer with ARBs, compared with those randomly assigned to control (I2=0%, RR 1·11, 1·04–1·18; p=0·001; figure 3B). Meta-analysis of the three trials of telmisartan again showed an increase in new cancer occurrence with this ARB, compared with control (RR 1·07, 95% CI 1·00–1·14; p=0·05). The occurrence of new cancer was also assessed according to background ACE-inhibitor therapy (figure 4). For patients in both treatment groups receiving a mandatory ACE inhibitor, randomisation to an ARB was associated with a significantly higher occurrence of new cancer than was randomisation to control (9·7% vs 8·6%, RR 1·13, 95% CI 1·03–1·24; p=0·011; figure 4A). In trials where ACE inhibitors were not allowed as concomitant treatment, again there was significant excess of new cancers with ARBs compared with controls (8·2% vs 7·6%, RR 1·08, 95% CI 1·00–1·16; p=0·041; figure 4B).
Figure 3. Cancer occurrence reported in all included trials of angiotensin-receptor blockers (A) and trials in which cancer was a prespecified endpoint (B)*.
ARB=angiotensin-receptor blocker. *To obtain the meta-analytic risk ratio, hazard ratios from the ONTARGET and TRANSCEND trials were combined with the risk ratio from the LIFE trial.
Figure 4. Cancers in randomised controlled trials, in patients with (A) and without (B) background ACE-inhibitor treatment.
ACE=angiotensin-converting enzyme. ARB=angiotensin-receptor blocker.
The effect of ARBs on the occurrence of new lung, prostate, and breast cancers is shown in table 3. There was an excess of new lung cancers in all trials, with a significant excess in the LIFE trial (0·6% with losartan, 0·3% with atenolol, RR 2·41, 95% CI 1·23–4·71; p=0·01). Meta-analysis showed an increase in relative risk for the occurrence of new lung cancer in patients randomised to an ARB compared with control (0·9% vs 0·7%, I2=6·6%, RR 1·25, 1·05–1·49; p=0·01]). This effect was also seen in the subgroup of patients who received background ACE-inhibitor therapy (1·4% vs 1·1%, RR 1·32, 1·03–1·69; p=0·031). For patients not receiving a concomitant ACE inhibitor, the occurrence of new lung cancer was 1·0% in the ARB group and 0·8% in controls (RR 1·50, 0·93–2·41; p=0·097; random-effects model used due to evidence of heterogeneity among trials, I2 65%). In all five trials included, there was an excess of prostate cancer in the ARB groups compared with control, although it was not significant in meta-analysis (1·7% vs 1·3%, RR 1·15, 0·99–1·34; p=0·076). The occurrence of breast cancer and of cancers other than lung, prostate, and breast combined did not differ significantly between ARB and control groups (p=0·74 for breast cancer, p=0·33 for other cancers combined).
Table 3.
Solid-organ cancers reported in randomised controlled trials of angiotensin-receptor blockers
| ARB | Control | RR (95% CI) | I 2 | p value | |
|---|---|---|---|---|---|
| Lung cancer | |||||
| All available trials | |||||
| LIFE30 | 29/4605 (0.6%) | 12/4588 (0.3%) | 2.41 (1.23–4.71) | 0.01 | |
| CHARM-Overall5,31,32 | 31/3803 (0.8%) | 25/3796 (0.7%) | 1.24 (0.73–2.09) | 0.43 | |
| TRANSCEND7,29 | 35/2954 (1.2%) | 27/2972 (0.9%) | 1.30 (0.79–2.15) | 0.30 | |
| ONTARGET8,29 | 229/17 044 (1.3%) | 101/8576 (1.2%) | 1.14 (0.90–1.44) | 0.27 | |
| PROFESS9,29 | 37/10 016 (0.4%) | 30/10 048 (0.3%) | 1.24 (0.77–2.00) | 0.39 | |
| Meta-analysis | 361/38 422 (0.9%) | 195/29 980 (0.7%) | 1.25 (1.05–1.49) | 6.6% | 0.01 |
| With background ACE-inhibitor treatment | |||||
| CHARM-Added31,32,35 | 12/1276 (0.9%) | 7/1272 (0.6%) | 1.71 (0.68–4.33) | 0.26 | |
| ONTARGET8,29 (telmisartan+ramipril vs ramipril) | 129/8502 (1.5%) | 101/8576 (1.2%) | 1.29 (0.99–1.67) | 0.055 | |
| Meta-analysis | 141/9778 (1.4%) | 108/9848 (1.1%) | 1.32 (1.03–1.69) | 0% | 0.031 |
| Without background ACE-inhibitor treatment | |||||
| LIFE30 | 29/4605 (0.6%) | 12/4588 (0.3%) | 2.41 (1.23–4.71) | 0.01 | |
| TRANSCEND7,29 | 35/2954 (1.2%) | 27/2972 (0.9%) | 1.30 (0.79–2.15) | 0.30 | |
| ONTARGET8,29 (telmisartan vs ramipril) | 100/8542 (1.2%) | 101/8576 (1.2%) | 0.99 (0.76–1.31) | 0.97 | |
| CHARM-Alternative31,32,43 | 10/1013 (1.0%) | 3/1015 (0.3%) | 3.34 (0.93–12.10) | 0.066 | |
| Meta-analysis | 174/17 114 (1.0%) | 143/17 151 (0.8%) | 1.50 (0.93–2.41) | 65% | 0.097 |
| Prostate cancer* | |||||
| All available trials | |||||
| LIFE30 | 58/2118 (2.7%) | 42/2112 (2.0%) | 1.38 (0.93–2.04) | 0.11 | |
| CHARM-Overall5,31,32 | 32/2617 (1.2%) | 27/2582 (1.0%) | 1.17 (0.70–1.95) | 0.55 | |
| TRANSCEND7,29 | 35/1674 (2.1%) | 27/1705 (1.6%) | 1.32 (0.80–2.17) | 0.27 | |
| ONTARGET8,29 | 275/12 544 (2.2%) | 128/6245 (2.0%) | 1.07 (0.87–1.32) | 0.53 | |
| PROFESS9,29 | 36/6455 (0.6%) | 32/6418 (0.5%) | 1.12 (0.70–1.80) | 0.64 | |
| Meta-analysis | 436/25 408 (1.7%) | 256/19 062 (1.3%) | 1.15 (0.99–1.34) | 0% | 0.076 |
| With background ACE-inhibitor treatment | |||||
| CHARM-Added31,32,35 | 7/1006 (0.7%) | 9/1000 (0.9%) | 0.77 (0.29–2.07) | 0.61 | |
| ONTARGET8,29 (telmisartan+ramipril vs ramipril) | 141/6252 (2.3%) | 128/6245 (2.0%) | 1.10 (0.87–1.39) | 0.43 | |
| Meta-analysis | 148/7258 (2.0%) | 137/7245 (1.9%) | 1.08 (0.86–1.36) | 0% | 0.52 |
| Without background ACE-inhibitor treatment | |||||
| LIFE30 | 58/2118 (2.7%) | 42/2112 (2.0%) | 1.38 (0.93–2.04) | 0.11 | |
| TRANSCEND7,29 | 35/1674 (2.1%) | 27/1705 (1.6%) | 1.32 (0.80–2.17) | 0.27 | |
| ONTARGET8,29 (telmisartan vs ramipril) | 134/6292 (2.1%) | 128/6245 (2.0%) | 1.04 (0.82–1.32) | 0.75 | |
| CHARM-Alternative31,32,43 | 8/691 (1.2%) | 3/691 (0.4%) | 2.67 (0.71–10.01) | 0.15 | |
| Meta-analysis | 235/10 775 (2.2%) | 200/10 753 (1.9%) | 1.17 (0.97–1.41) | 9.6% | 0.10 |
| Breast cancer† | |||||
| All available trials | |||||
| LIFE30 | 37/2487 (1.5%) | 36/2476 (1.5%) | 1.02 (0.65–1.61) | 0.92 | |
| CHARM-Overall5,31,32 | 17/1186 (1.4%) | 17/1214 (1.4%) | 1.02 (0.52–2.00) | 0.95 | |
| TRANSCEND7,29 | 20/1280 (1.6%) | 17/1267 (1.3%) | 1.16 (0.61–2.21) | 0.64 | |
| ONTARGET8,29 | 60/4500 (1.3%) | 34/2331 (1.5%) | 0.91 (0.60–1.39) | 0.67 | |
| PROFESS9,29 | 20/3561 (0.6%) | 15/3630 (0.4%) | 1.36 (0.70–2.65) | 0.37 | |
| Meta-analysis | 154/13 014 (1.2%) | 119/10 918 (1.1%) | 1.04 (0.82–1.32) | 0% | 0.74 |
| With background ACE-inhibitor treatment‡ | |||||
| ONTARGET8,29 (telmisartan+ramipril vs ramipril) | 33/2250 (1.5%) | 34/2331 (1.5%) | 1.00 (0.61–1.66) | >0.99 | |
| Without background ACE-inhibitor treatment | |||||
| LIFE30 | 37/2487 (1.5%) | 36/2476 (1.5%) | 1.02 (0.65–1.61) | 0.92 | |
| TRANSCEND7,29 | 20/1280 (1.6%) | 17/1267 (1.3%) | 1.16 (0.61–2.21) | 0.64 | |
| ONTARGET8,29 (telmisartan vs ramipril) | 27/2250 (1.2%) | 34/2331 (1.55) | 0.83 (0.50–1.36) | 0.45 | |
| CHARM-Alternative31,32,43 | 5/322 (1.6%) | 4/324 (1.2%) | 1.26 (0.34–4.64) | 0.73 | |
| Meta-analysis | 89/6339 (1.2%) | 91/6398 (1.4%) | 0.99 (0.74–1.32) | 0% | 0.93 |
ARB=angiotensin-receptor blocker. RR=risk ratio. ACE=angiotensin-converting enzyme.
Analysis limited to men.
Analysis limited to women, all breast cancers were assumed to have occurred in women.
Breast-cancer data were not available for the CHARM-Added trial.
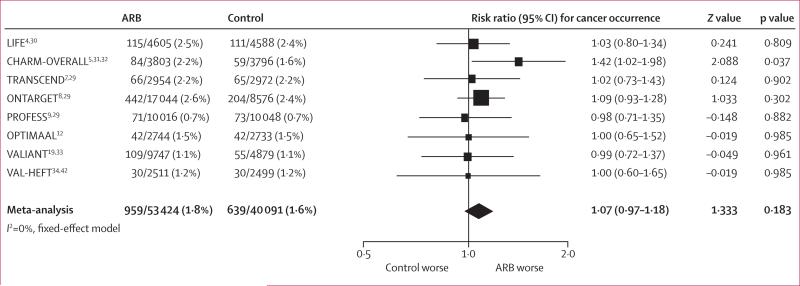
Figure 5 shows the effect of randomisation to an ARB on cancer deaths. Overall, there was no significant difference in cancer deaths between patients randomised to ARBs and those randomised to control for the duration of follow-up (1·8% vs 1·6%, RR 1·07, 95% CI 0·97–1·18; p=0·18).
Figure 5. Cancer deaths reported in randomised controlled trials of angiotensin-receptor blockers.
ARB=angiotensin-receptor blocker.
The meta-analysis findings remained robust to methodological changes in sensitivity analysis. When the primary outcome of cancer occurrence was explored with a random-effects model, the results did not change (RR 1·08, 95% CI 1·01–1·15; p=0·016). Another analysis was done in which patients with a history of cancers at baseline were excluded, since this information was available for the ONTARGET and TRANSCEND trials. Patients with no history of cancer who were randomised to ARBs had a significantly increased risk of new cancer compared with controls (1541 of 18 778 at risk [8·2%] vs 794 of 10 859 [7·3%], I2 17·3%, meta-analytic HR 1·10, 95% CI 1·02–1·18; p=0·01). The CHARM-Overall programme could not be included in the primary analysis of new cancers because of a lack of discrimination between benign and malignant neoplasms. Because the FDA document stated that most neoplasms were malignant, the primary meta-analysis was repeated (total n=69 189) considering all neoplasms in CHARM as malignant (244 neoplasms with candesartan, 230 with placebo in the FDA document). This analysis yielded a similar RR (1·08, 95% CI 1·02–1·15; p=0·014).
To assess for survival bias, risk of all-cause death with ARBs was calculated by including the trials used in the main analysis and the CHARM trial. An HR of 0·99 was found for all-cause death (95% CI 0·93–1·05, p=0·67), suggesting that there was no survival bias (webappendix). Similarly, there was no change in the risk of myocardial infarction with ARBs in these trials (meta-analytic RR 1·00, 0·93–1·08; p=0·96).
Number needed to harm was calculated using background cancer incidence for people aged 65–69 years, corresponding to mean age of patients enrolled in these trials (2240·5 cancers per 100 000 people per year).39 According to the RR for cancer occurrence found from all trials, for one excess cancer diagnosis 143 patients (95% CI 76–793) would need to receive treatment for about 4 years (weighted average duration of follow-up). According to the RR found from trials where cancer was a prespecified outcome, number needed to harm was 105 (63–271) with the same method.
Discussion
In this meta-analysis, we found that ARBs are associated with a modestly increased risk of new cancer occurrence. To the best of our knowledge, this analysis incorporated all publicly available data on new cancer occurrence from randomised trials of ARBs. Information was from peer-reviewed publications and data posted on the FDA website, a valuable source for meta-analyses.44 A strength of this meta-analysis is the inclusion of data from randomised controlled trials only, thereby reducing the possibility of confounding variables. The increased risk of new cancer occurrence is modest but significant. However, the clinical significance of this potential excess cancer risk is unknown. The finding of a 1·2% increase in absolute risk of cancer over an average of 4 years needs to be interpreted in view of the estimated 41% lifetime cancer risk.45 Variation in the magnitude of effect with different durations of exposure, and the significance of these findings at the population level, are also unknown.
The first indication of a possible increase in cancer risk with angiotensin-receptor blockade was noted in the CHARM trial of candesartan in 2003.5 This trial reported a significant increase in risk of fatal cancers in patients randomised to candesartan compared with placebo (86 [2·3%] vs 59 [1·6%], p=0·038). This imbalance was attributed to chance by the investigators and was later reviewed in detail in an FDA document, which noted that “more cancer deaths occurred in the candesartan group, but the investigator-reported rate of nonfatal neoplasms was more equal between treatment groups”.31,32 5 years later, results of the ONTARGET and TRANSCEND trials were published, which investigated another ARB, telmisartan.7,8 In July, 2009, a detailed briefing document on telmisartan was presented to the FDA Cardiovascular and Renal Drugs Advisory Committee,29 noting excesses in malignancies with telmisartan in both ONTARGET and TRANSCEND. In ONTARGET, the hazard for malignancies was significantly higher for telmisartan plus ramipril than ramipril alone, irrespective of the presence of malignancies at baseline (824 [9·7%] vs 735 [8·6%], HR 1·14, 95% CI 1·03–1·26).29 In TRANSCEND, the hazard for malignancies in patients without cancer at baseline (95% of all patients) was significantly higher for telmisartan than placebo (206 [7·3%] vs 169 [6·0%], HR 1·24, 95% CI 1·01–1·52).29 Given these recurring concerns, we decided to do a complete meta-analysis off all available data on cancer occurrence in randomised controlled trials of ARBs.
It is unlikely that the increased occurrence of new cancers in patients given ARBs was a secondary result of the treatments given to the control groups protecting against malignancies. The beta-blocker atenolol was the control drug in one trial, the ACE inhibitor ramipril was the comparator in another trial, and placebo therapy was given in the remaining trials. Meta-analyses of randomised trials with beta-blockers and ACE inhibitors have shown that these drugs have no significant effect on incident cancer.46
New-cancer data were available for three of the seven FDA-approved ARBs; however, most patients in this meta-analysis received telmisartan as the study drug. When analysis was limited to telmisartan, the excess in new cancer occurrence was of borderline significance (p=0·05). The overall risk of cancer with losartan, which had cancer data from a single trial, was not significant (356 [8%] vs 315 [7%], p=0·118), but the drug was associated with a significantly increased risk of lung cancer (RR 2·41, 95% CI 1·23–4·71; p=0·01).4,30 Although data for new cancer occurrence were not available for candesartan, there was a significant excess in fatal cancers with candesartan in the CHARM-Overall programme (p=0·038) and CHARM-Added trial (p=0·01).5,31,32 Nevertheless, given the limited data, it is not possible to draw conclusions about the exact risk of cancer associated with each particular drug. It is also unknown whether the other ARBs (valsartan, irbesartan, olmesartan, and eprosartan) are associated with an increased risk of new cancer occurrence.
In this analysis, the increased cancer occurrence did not result in a significant excess in cancer deaths, although oncogenesis, tumour growth, and treatment failure followed by death is typically a slow process. Therefore, with the present trials, it is not possible to make conclusions regarding the effect of ARBs on cancer-related deaths. However, in trials with an average follow-up shorter than 3 years (PROFESS, OPTIMAAL, VALIANT, and VAL-HEFT), the point estimate of RR for cancer death was very close to 1·0, and in trials longer than 3 years (LIFE, CHARM-Overall, TRANSCEND, and ONTARGET) the point estimate was consistently above 1·0.
The mechanism for the possible increase in the occurrence of new cancer cases associated with ARBs is uncertain. Angiotensin II exerts its actions through AT1R and AT2R. ARB drugs are antagonists of AT1R. Experimental studies using cancer cell lines and mouse models have implicated the renin-angiotensin system in the regulation of cell proliferation, tumour growth, angiogenesis, and metastasis.27,47 Evidence shows that both AT1R blockade with an ARB (which is associated with unopposed AT2R stimulation) and direct stimulation of AT2R are capable of stimulating tumour angiogenesis in vivo.48 However, the relevance of these observations in human malignancy is largely unknown.
Our study has important limitations. We pooled the results of a group of trials that were not designed to explore cancer outcomes as the primary outcome measure. The adjudication of cancer diagnoses was not uniform among the included studies. However, when analysis was limited to the three trials where cancer was a prespecified endpoint and cancer data were collected rigorously, there was a significant increase in risk of cancer with ARBs. Cancer data were not available in peer-reviewed publications or FDA website documents for several randomised trials of ARBs, which raises the concern of publication bias. Moreover, most of these trials, including the large VALUE trial (n=15 245),13 did not collect cancer information. For the meta-analysis, we were still able to include most patients enrolled in the randomised mortality and morbidity trials of ARBs, including the two largest trials ever done (ONTARGET, n=25 620; and PROFESS, n=20 332), and trials with the longest follow-up (LIFE, 4·8 years; ONTARGET and TRANSCEND, 4·7 years). However, we did not have access to individual patient data for any of these trials. Analysis of cancer data was based on summaries of events from peer-reviewed publications or publicly disclosed documents, and the lack of availability of individual data and timing of cancers did not allow a more statistically powerful time-to-event analysis that could be useful for cancer because of the latency period. Notably, the effect of sex, age, and smoking could not be examined because of the lack of patient level data. Finally, meta-analyses are generally considered less convincing than a large prospective trial designed to assess the outcome of interest. Nonetheless, meta-analyses can be useful in providing insights into issues of safety and rare adverse events that might provide the hypothesis for a prospective trial.
In conclusion, this meta-analysis shows that ARBs are associated with a modestly increased risk of new cancer occurrence. Among the solid organ cancers examined, only the risk of lung cancer was significantly increased. Given limited data, it is not possible to draw conclusions about the exact risk of cancer associated with each particular ARB. Our findings warrant further investigation.
Acknowledgments
Funding None.
Footnotes
Contributors
IS was responsible for the study concept, design, and literature search. IS and SMD collected data. IS, SMD, and DYR did the statistical analysis. IS, SMD, and DIS drafted the manuscript. IS, DIS, and JCF supervised the study. All authors participated in analysis and interpretation of data and critical revision of the manuscript.
Conflicts of interest
IS received an educational grant and lecture honoraria from Pfizer, and lecture honoraria from AstraZeneca and Ranbaxy. SMD is a member of the data monitoring committee for Centocor Research & Development, Inc. DIS serves on advisory boards for Cordis/Johnson & Johnson, Daiichi-Sankyo, Medicines Company, Medtronic Vascular, Portola, and Schering-Plough, and has received lecture honoraria from Accumetrics, Cordis/Johnson & Johnson, Daiichi-Sankyo, Eli Lilly, Medicines Company, Sanofi-Aventis, and Schering-Plough. JCF serves on an advisory panel for Novartis and as a consultant for ARCA Biopharma, Inc. DYR declared no conflicts of interest.
References
- 1.Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 2.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (writing committee to update the 2001 guidelines for the evaluation and management of heart failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 3.Standards of medical care in diabetes—2009. Diabetes Care. 2009;32(suppl 1):S13–61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 5.Pfeffer MA, Swedberg K, Granger CB, et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759–66. doi: 10.1016/s0140-6736(03)14282-1. [DOI] [PubMed] [Google Scholar]
- 6.Julius S, Nesbitt SD, Egan BM, et al. Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med. 2006;354:1685–97. doi: 10.1056/NEJMoa060838. [DOI] [PubMed] [Google Scholar]
- 7.Yusuf S, Teo K, Anderson C, et al. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet. 2008;372:1174–83. doi: 10.1016/S0140-6736(08)61242-8. [DOI] [PubMed] [Google Scholar]
- 8.Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–59. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 9.Yusuf S, Diener HC, Sacco RL, et al. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med. 2008;359:1225–37. doi: 10.1056/NEJMoa0804593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–69. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 11.Chaturvedi N, Porta M, Klein R, et al. Effect of candesartan on prevention (DIRECT-Prevent 1) and progression (DIRECT-Protect 1) of retinopathy in type 1 diabetes: randomised, placebo-controlled trials. Lancet. 2008;372:1394–402. doi: 10.1016/S0140-6736(08)61412-9. [DOI] [PubMed] [Google Scholar]
- 12.Dickstein K, Kjekshus J. Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: the OPTIMAAL randomised trial. Optimal Trial in Myocardial Infarction with Angiotensin II Antagonist Losartan. Lancet. 2002;360:752–60. doi: 10.1016/s0140-6736(02)09895-1. [DOI] [PubMed] [Google Scholar]
- 13.Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–31. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 14.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–60. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 15.Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–67. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 16.Ogihara T, Nakao K, Fukui T, et al. Effects of candesartan compared with amlodipine in hypertensive patients with high cardiovascular risks: candesartan antihypertensive survival evaluation in Japan trial. Hypertension. 2008;51:393–98. doi: 10.1161/HYPERTENSIONAHA.107.098475. [DOI] [PubMed] [Google Scholar]
- 17.Lithell H, Hansson L, Skoog I, et al. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens. 2003;21:875–86. doi: 10.1097/00004872-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–78. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 19.Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893–906. doi: 10.1056/NEJMoa032292. [DOI] [PubMed] [Google Scholar]
- 20.Mochizuki S, Dahlof B, Shimizu M, et al. Valsartan in a Japanese population with hypertension and other cardiovascular disease (Jikei Heart Study): a randomised, open-label, blinded endpoint morbidity-mortality study. Lancet. 2007;369:1431–39. doi: 10.1016/S0140-6736(07)60669-2. [DOI] [PubMed] [Google Scholar]
- 21.Schrader J, Luders S, Kulschewski A, et al. Morbidity and mortality after stroke, eprosartan compared with nitrendipine for secondary prevention: principal results of a prospective randomized controlled study (MOSES). Stroke. 2005;36:1218–26. doi: 10.1161/01.STR.0000166048.35740.a9. [DOI] [PubMed] [Google Scholar]
- 22.Sjolie AK, Klein R, Porta M, et al. Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): a randomised placebo-controlled trial. Lancet. 2008;372:1385–93. doi: 10.1016/S0140-6736(08)61411-7. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki H, Kanno Y. Effects of candesartan on cardiovascular outcomes in Japanese hypertensive patients. Hypertens Res. 2005;28:307–14. doi: 10.1291/hypres.28.307. [DOI] [PubMed] [Google Scholar]
- 24.Merck & Co [May 26, 2010];COZAAR: highlights of prescribing information. http://www.merck.com/product/usa/pi_circulars/c/cozaar/cozaar_pi.pdf.
- 25.AstraZeneca [May 26, 2010];ATACAND: highlights of prescribing information. http://www1.astrazeneca-us.com/pi/Atacand.pdf.
- 26.Boehringer Ingelheim [May 26, 2010];MICARDIS: material safety data sheet. http://bi-msds.e3solutionsinc.com/Micardis%20tabletsR2.pdf.
- 27.Deshayes F, Nahmias C. Angiotensin receptors: a new role in cancer? Trends Endocrinol Metab. 2005;16:293–99. doi: 10.1016/j.tem.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Dale KM, Coleman CI, Henyan NN, Kluger J, White CM. Statins and cancer risk: a meta-analysis. JAMA. 2006;295:74–80. doi: 10.1001/jama.295.1.74. [DOI] [PubMed] [Google Scholar]
- 29. [April 13, 2010];Micardis (Telmisartan) Cardiovascular and Renal Drugs Advisory Committee briefing document, July 29, 2009. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/CardiovascularandRenalDrugsAdvisoryCommittee/UCM173529.pdf.
- 30.Marciniak TA. [April 13, 2010];Clinical Review, Supplemental NDA Submission, NDA 20-386, Cozaar Tablets, Merck & Co. http://www.fda.gov/ohrms/dockets/ac/03/briefing/3920B1_02_AFDA-Cozaar%20Clinical%20Review.htm.
- 31.Khin Maung U. [April 13, 2010];Clinical Review, N20-838/SE1-022, Atacand (Candesartan cilexetil) tablets. http://www.fda.gov/ohrms/dockets/ac/05/briefing/2005-4092B1_01_03-FDA-Clinical-Review-S022-02-Pages_100-199.pdf.
- 32.Khin Maung U. Clinical Review, N20-838/SE1-022, Atacand (Candesartan cilexetil) tablets. http://www.fda.gov/ohrms/dockets/ac/05/briefing/2005-4092B1_01_05-FDA-Clinical-Review-S024-03-Pages_200-241.pdf (accessed April 13, 2010) http://www.fda.gov/ohrms/dockets/ac/05/briefing/2005-4092B1_01_05-FDA-Clinical-Review-S024-03-Pages_200-241.pdf
- 33. [April 13, 2010];Center For Drug Evaluation and Research Approval Package For: Application Number NDA 21-283/S011 Combined Medical and Statistical Review. 2005 http://www.accessdata.fda.gov/drugsatfda_ docs/nda/2005/021283_S011_DIOVAN_MEDR%20&%20STATR.pdf.
- 34. [April 13, 2010];Diovan (Valsartan) Congestive Heart Failure Advisory Comittee Briefing Document, 2001. http://www.fda.gov/ohrms/dockets/ac/01/briefing/3793b1_01_NOVARTIS.pdf.
- 35.McMurray JJ, Ostergren J, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767–71. doi: 10.1016/S0140-6736(03)14283-3. [DOI] [PubMed] [Google Scholar]
- 36.Dahlof B, Devereux R, de Faire U, et al. The Losartan Intervention For Endpoint reduction (LIFE) in Hypertension study: rationale, design, and methods. The LIFE study group. Am J Hypertens. 1997;10:705–13. [PubMed] [Google Scholar]
- 37.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 38.Wessa P. [April 13, 2010];Kendall tau Rank Correlation (v1.0.10) in Free Statistics Software (v1.1.23-r4), Office for Research Development and Education. http://www.wessa.net/rwasp_kendall.wasp.
- 39.US Cancer Statistics Working Group . United States Cancer Statistics: 1999–2006 Incidence and Mortality Web-based Report. US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; Atlanta: 2010. [April 13, 2010]. http://www.cdc.gov/uscs. [Google Scholar]
- 40.Altman DG, Deeks JJ. Meta-analysis, Simpson's paradox, and the number needed to treat. BMC Med Res Methodol. 2002;2:3. doi: 10.1186/1471-2288-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smeeth L, Haines A, Ebrahim S. Numbers needed to treat derived from meta-analyses—sometimes informative, usually misleading. BMJ. 1999;318:1548–51. doi: 10.1136/bmj.318.7197.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–75. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 43.Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772–76. doi: 10.1016/S0140-6736(03)14284-5. [DOI] [PubMed] [Google Scholar]
- 44.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–71. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 45.Institute NC. [April 13, 2010];Surveillance Epidemiology and End Results (SEER) Cancer Statistics Review 1975–2006. http://seer.cancer.gov/csr/1975_2006/results_merged/topic_lifetime_risk.pdf.
- 46.Coleman CI, Baker WL, Kluger J, White CM. Antihypertensive medication and their impact on cancer incidence: a mixed treatment comparison meta-analysis of randomized controlled trials. J Hypertens. 2008;26:622–29. doi: 10.1097/HJH.0b013e3282f3ef5e. [DOI] [PubMed] [Google Scholar]
- 47.Soto-Pantoja DR, Menon J, Gallagher PE, Tallant EA. Angiotensin-(1-7) inhibits tumor angiogenesis in human lung cancer xenografts with a reduction in vascular endothelial growth factor. Mol Cancer Ther. 2009;8:1676–83. doi: 10.1158/1535-7163.MCT-09-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walther T, Menrad A, Orzechowski HD, Siemeister G, Paul M, Schirner M. Differential regulation of in vivo angiogenesis by angiotensin II receptors. FASEB J. 2003;17:2061–67. doi: 10.1096/fj.03-0129com. [DOI] [PubMed] [Google Scholar]