Abstract
Scope
Flavones have reported anti-inflammatory activities, but the ability of flavone-rich foods to reduce inflammation is unclear. Here, we report the effect of flavone glycosylation in the regulation of inflammatory mediators in vitro and the absorption of dietary flavones in vivo.
Methods and results
The anti-inflammatory activities of celery extracts, some rich in flavone aglycones and others rich in flavone glycosides, were tested on the inflammatory mediators tumor necrosis factor α (TNF-α) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) in lipopolysaccharide-stimulated macrophages. Pure flavone aglycones and aglycone-rich extracts effectively reduced TNF-α production and inhibited the transcriptional activity of NF-κB, while glycoside-rich extracts showed no significant effects. Deglycosylation of flavones increased cellular uptake and cytoplasmic localization as shown by high-performance liquid chromatography (HPLC) and microscopy using the flavonoid fluorescent dye diphenyl-boric acid 2-aminoethyl ester (DPBA). Celery diets with different glycoside or aglycone contents were formulated and absorption was evaluated in mice fed with 5 or 10% celery diets. Relative absorption in vivo was significantly higher in mice fed with aglycone-rich diets as determined by HPLC-MS/MS (where MS/MS is tandem mass spectrometry).
Conclusion
These results demonstrate that deglycosylation increases absorption of dietary flavones in vivo and modulates inflammation by reducing TNF-α and NF-κB, suggesting the potential use of functional foods rich in flavones for the treatment and prevention of inflammatory diseases.
Keywords: Apigenin, Bioactive food components, Flavones, Inflammation, Macrophages
1 Introduction
The innate immune system provides an important first line of defense to infection by promoting an inflammatory response that mediates pathogen clearance [1]. Thus, inflammation is a normal central mechanism of host immune surveillance in response to infections [2]. Deregulated inflammation is central in sepsis and other acute inflammatory pulmonary diseases [3,4]. In addition, systemic activation of the host immune response has been associated with many chronic diseases, including type 2 diabetes [5], rheumatoid arthritis [6], cancer [7], and atherosclerosis [8]. Several anti-inflammatory oral drug therapies are currently available, but their adverse effects include gastrointestinal side effects, high costs, and the potential for cardiovascular damage [9]. Thus, there is growing interest in using naturally occurring dietary compounds to modulate inflammation.
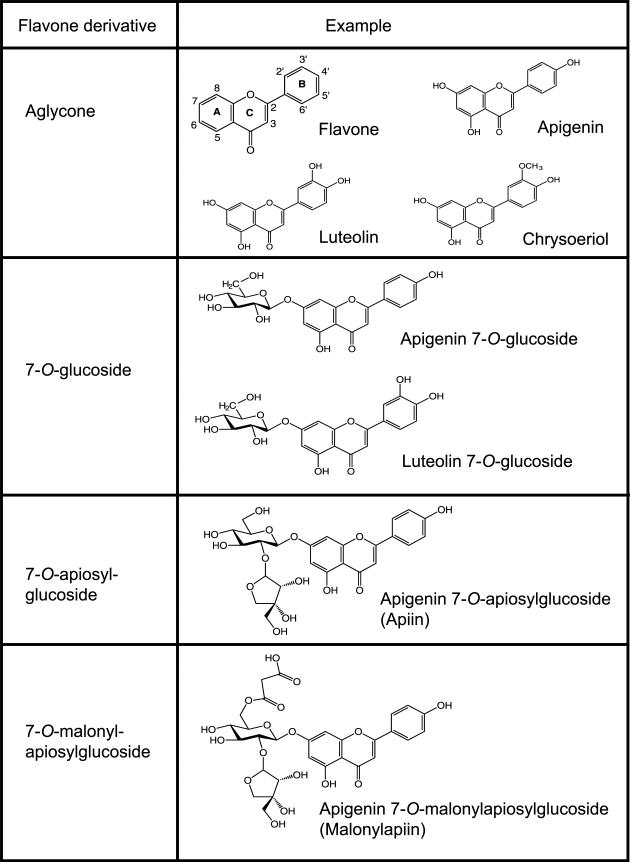
Flavonoids, common in a variety of fruits and vegetables [10], have anti-inflammatory activities and can potentially play a key role as neutraceuticals and in functional foods [11]. Like other flavonoids, flavones are typically found in plants as glycosides [10]. Flavone O-glycosides are composed of the aglycone moiety with one or more sugars attached via a β-linkage (Fig. 1). These compounds may be modified by endogenous enzymes such as malonylesterases (conversion from malonylapiin to apiin) [12], but frequently remain as glycosides after processing such as shredding [13], juicing [14], and heating [15]. For many flavonoid glucosides, brush border β-glucosidase activity in the small intestine is sufficient to hydrolyze the aryl glycosidic bond and allow absorption of the aglycone [16, 17]. Intestinal β-glucosidase, however, cannot hydrolyze oligosaccha-ride moieties [16] and although colonic bacteria can cleave these bonds [18], absorption is reduced. Given that dietary flavones are ingested predominantly as glycosides, their fate and biological effect are determined by how and where they are hydrolyzed, absorbed, metabolized, transported, and excreted. Flavone glycosides in celery occur as apiosylgluco-sides (Fig. 1) and might also be resistant to brush border β-glucosidase action. We therefore hypothesized that convert ing flavone glycosides to aglycones would result in increased absorption.
Figure 1.
Structures of flavone aglycones and glycosides derived from celery.
Although the metabolic fate of flavones such as lute-olin and apigenin has not been studied as extensively as that of other flavonoids [19, 20], flavones have been shown to have antiproliferative and anti-inflammatory properties [21–23]. Treatment of murine macrophages with lipopolysaccharide (LPS), followed by the flavones apigenin, luteolin, or diosmetin, reduced tumor necrosis factor-α (TNF-α) or IL-6 release [24–26]. In addition, we showed that apigenin inhibits LPS-induced TNF-α production in primary human monocytes and macrophages [21]. We also found that when injected, apigenin reduced TNF-α in vivo in a model of acute inflammation [21]. Moreover, mice given purified apigenin orally or in the diet showed lower serum concentrations of the proinflammatory cytokines IL-6 and TNF-α [25]. While it is clear that purified flavone aglycones have anti-inflammatory activities, less apparent is the role of flavone glycosides or flavone-rich foods in modulating inflammation.
Here, we examine the effects of flavone aglycones, glycosides, and flavone-rich food extracts on the immune response. Our results demonstrate that flavone aglycones and celery extracts processed to contain flavone aglycones, unlike flavone glycosides and celery extracts containing native flavone glycosides, inhibit LPS-induced production of TNF-α and reduce the transcriptional activity of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). We also found that targeted formulation of celery-supplemented diets with higher aglycone concentrations resulted in higher relative absorption of flavones in vivo. Collectively, these studies provide evidence that diets crafted to augment agly-cone content increase absorption and might selectively reduce inflammation, uncovering novel aspects for the potential use of naturally occurring dietary compounds to modulate inflammation.
2 Materials and methods
2.1 Plant material, standards, and reagents
Chinese celery (Apium glaveolens) and raw almonds (Prunus dulcis) were purchased from local grocery stores. Chrysoeriol and luteolin 7-O-glucoside (Extrasynthese, Genay, France), apigenin and luteolin (Sigma, St. Louis, MO), and diosmetin and apigenin 7-O-glucoside (Chromadex, Irvine, CA) were dissolved in dimethyl sulfoxide (DMSO) for in vitro experiments. β-glucuronidase/sulfatase from Helix pomatia (lyophilized powder) was obtained from Sigma. Phosphoric acid was obtained from Corco Chemical Corp (Fairless Hills, PA). All other reagents were from Fisher Scientific (Fair Lawn, NJ).
2.2 Preparation of celery extracts and diets
Lyophilized fresh celery leaves, naturally abundant in apigenin 7-O-apiosylglucoside (apiin), were used for extracts and diets. For fresh celery extract (FCE), 200 mg lyophilized celery was extracted with 6 mL 70% ethanol, centrifuged at 450 × g for 10 min at room temperature, and supernatants collected. For acid hydrolyzed celery extracts (ACE), 1 mL of FCE was combined with 0.5 mL 37% HCl and heated for 1 h at 90°C. For enzyme-treated celery extract (ECE), lyophilized fresh celery leaves were first heated in 1.5 N H3PO4 for 90 min at 100°C to convert apiin to apigenin 7-O-glucoside. After neutralization with 10% KOH, the celery leaves were incubated with ground almond (20% dry wt) for 2 h at 50°C to convert apigenin 7-O-glucoside to apigenin aglycone by β-glucosidase present in the almonds. The resulting aglycone-rich mixture was then lyophilized to make enzymatically hydrolyzed celery powder and extracted in 70% ethanol as described above. All extracts were dried under nitrogen gas (N2) and reconstituted in 300 μL DMSO. After determining flavone concentration by high-performance liquid chromatography (HPLC) analysis, samples were diluted in DMSO to 5 mM apigenin equivalents and stored at −20°C.
For preparation of celery-supplemented diets, powdered AIN-93G diet was purchased from Dyets, Inc. (Bethlehem, PA). Lyophilized fresh celery and enzyme-treated celery were combined with AIN-93G powder diet to make 5 or 10% (w/w) celery-supplemented diets. After dry ingredients were mixed, water was added to make a paste and pellets were formed with a Simple Tablet Maker (Wholistic Research Co., Royston, Herts, UK). The same procedure was used to make pellets of the AIN-93G control diet. Pellets were dried at 40°C to a constant weight and stored at −20°C for 1–4 weeks until used for feeding. The following diets were made: AIN-93G pellets supplemented with 5% fresh celery (AIN-5FCP), AIN-93G with 10% fresh celery pellets (AIN-10FCP), AIN-93G with 5% enzyme celery pellets (AIN-5ECP), and AIN-93G with 10% enzyme celery pellets (AIN-10ECP). The flavone contents of the diets were confirmed by HPLC analysis prior to feeding.
2.3 Cell culture
RAW 264.7 murine macrophages from ATCC were cultured as previously described [21]. Briefly, cells were seeded at a density of 5 × 105 cells/mL, incubated for 16 h, and then treated with either 100 ng/mL LPS (Escherichia coli, 0127:B8, Sigma, St. Louis, MO) or DMSO as diluent control in the presence or absence of different concentrations of flavones or food extracts.
2.4 Immunodetection of inflammatory cytokines
TNF-α in cell media was determined by enzyme-linked immunosorbent assay (ELISA) using the DuoSet kit (R&D Systems, Minneapolis, MN) and TNF-α standards provided by the manufacturer as we previously described [21]. Absorbance was measured on an ELX-808 microplate reader with KC Junior software (excitation wavelength 450 nm, emission wavelength 540 nm) and concentrations were determined with a four-parameter standard curve.
2.5 NF-κB activity
Macrophages (6 × 105/mL) were transfected with 0.6 μg NF-κB-secreted human placental alkaline phosphatase (SEAP) or vector control-SEAP (Clontech, Mountain View, CA) and pCMV-β-galactosidase vector using Lipofectamine 2000 in OPTI-MEN (Invitrogen, Carlsbad, CA) according to manufacturer's specifications. Cells were incubated 24 h and treated with LPS, different flavones, celery extracts, or DMSO for 16 h. NF-κB activity in supernatants was determined using a SEAP fluorimetric reporter gene assay (AnaSpec, Fremont, CA) following the manufacturer's instructions and read on a VICTOR X3 multilabel plate reader (PerkinElmer, Waltham, MA). β-galactosidase activity in cell lysates was measured with the β-Gal assay kit (Invitrogen) as previously reported [21]. Relative fluorescent units (RFUs) of NF-κB activity are expressed as relative units of SEAP per mL/β-galactosidase activity per mg protein. All experiments were done in triplicate.
2.6 Subcellular fractionation of macrophages
Macrophage subcellular fractionations were obtained as previously described [22]. Briefly, media was harvested and 5 × 106 cells were lysed in KPM buffer by 5 freeze/thaw cycles and centrifuged at 100 000 × g for 1 h at 4°C to obtain cytoplasmic and membrane fractions. To assess the flavone content in each fraction, the media, cytoplasmic and membrane fractions were extracted twice with equal volume acetonitrile, centrifuged at 2500 × g, and the pellet extracted with 70% acetonitrile. The supernatants were dried under N2 and reconstituted in methanol prior to HPLC analysis.
2.7 DPBA staining and fluorescence microscopy
Cells were treated with flavonoids or DMSO for 3 h at 37°C in supplemented RPMI-1640 (without phenol red). Nuclear staining was done with 1 μg/mL DAPI (4′,6-diamidino-2-phenylindole, Sigma) for 30 min at 37°C. Cells were rinsed three times with phosphate-buffered saline (PBS) and stained with 0.1% (w/v) diphenylboric acid 2-aminoethyl ester (DPBA; Sigma; excitation 490 nm, emission 530 nm) for 1 min as previously described [22]. Fluorescence was detected by microscopy (Olympus IX-81 microscope, Olympus, Melville, NY) and digital images were generated with a QCAM Retiga Exi FAST 1394 camera and Slidebook software (Intelligent Imaging Innovations, Denver, CO).
2.8 Mouse feeding experiments
Male C57BL/6J mice, 6–8 weeks old, were purchased from Harlan (Madison, WI), cared for according to the Ohio State University Institutional Animal Care and Use Committee (IACUC) regulations, and used after 10 days acclimation. For dietary intervention, mice housed into individual cages were fed ad libitum for 7 days with either AIN-93G control or flavone-rich diets. Food weight and consumption were recorded daily. Urine was collected at day 7, immediately prior to euthanasia. Blood samples were obtained by cardiac puncture and serum isolated as previously described [21].
2.9 Flavone extraction and HPLC-MS/MS analysis
HPLC-diode-array detection (DAD) analysis was done with a Waters 2695 separations module with a 2996 detector, Symmetry C18 column (3.5 μm, 4.6 × 75 mm), and Empower software (Waters Corp., Milford, MA). The mobile phase consisted of 0.1% formic acid in water and 0.1% formic acid in ACN, using a gradient method with increasing acetonitrile. Concentrations were measured at 338 nm for apigenin and 350 nm for luteolin and chrysoeriol through external calibration with aglycone standards. Apigenin 7-O-glucoside was identified using a commercial standard, and HPLC-DADMS (QTof Premier; Micromass, UK) was used to identify other flavone glycosides by accurate mass and fragmentation patterns. Urine and serum samples were analyzed by HPLC-MS/MS (where MS/MS is tandem mass spectrometry) (Acquity UPLC, Quattro Ultima; Waters, Milford, MA). Each urine sample (25–100 μL) was combined with twice its volume of sodium acetate buffer (1.4 M, pH 5.5), incubated for 2 h at 37°C with β-glucuronidase/sulfatase to deconjugate flavones as described previously [27]. The resultant flavone aglycones were extracted three times with diethyl ether, supernatants dried under N2, and reconstituted in methanol prior to HPLC-MS/MS analysis. Serum was prepared as previously described [28] with some modifications. Each sample (30–100 μL) was extracted with twice its volume of −20°C ACN, centrifuged, and the supernatant collected. The pellet was extracted in 70% ACN, sonicated, and centrifuged. Super-natants from the two extractions were combined, dried under N2, and reconstituted in sodium acetate buffer (1.4 M, pH 5.5). Samples were digested with β-glucuronidase/sulfatase, extracted four times with diethyl ether, and reconstituted in methanol.
HPLC-MS/MS analysis was done at 0.65 mL/min of 0.1% formic acid versus 0.1% formic acid in ACN with a gradient method, using an Acquity BEH C18 column (2.1 × 50 mm, 1.7 μm) at 40°C. Eluent was interfaced with the MS via an electrospray probe operated in both negative and positive modes after a 1:10 flow splitter. Specific flavones were detected by multiple reaction monitoring (MRM) with an 84 ms dwell time. Source parameters included: capillary voltage 2.8 kV in negative mode and 3.2 kV in positive mode; cone 35 V; source block 110°C, desolvation 500°C; desolvation gas 760 L/h, cone gas 105 L/h; collision energies 20–25 V. Desolvation gas was delivered at 600 L/h and 450°C, cone gas at 100 L/h, collision-induced dissociation at 3 × 10−3 mBar (Argon).
2.10 Statistical analysis and graphical presentation
Statistical analysis was done with R 2.11 for Windows (R Foundation for Statistical Computing, Vienna, Austria) using one-way analysis of variance (ANOVA) for parametric datasets and the Kruskal–Wallis test for nonparametric data. Post hoc comparisons were done with the corresponding parametric or nonparametric Tukey's HSD. When necessary, data were log or square root transformed to achieve a normal distribution and equal variances for the ANOVA tests. p values <0.05 were indicative of significant differences. Chemical structures were produced with ChemDraw Pro 12.0 (CambridgeSoft, Cambridge, MA).
3 Results
3.1 Deglycosylation of flavones determines their ability to inhibit cytokines
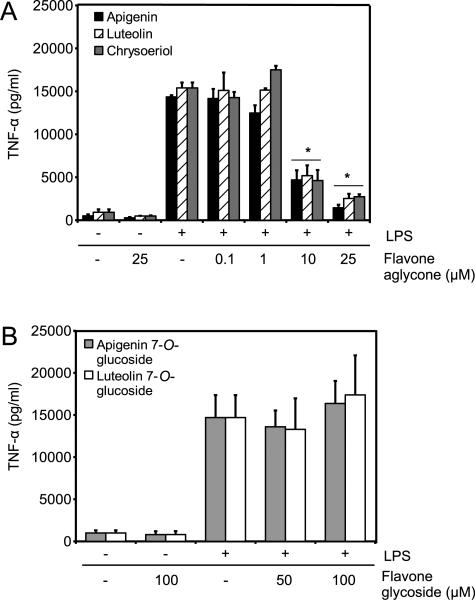
To examine the effect of structural modifications of flavones in their anti-inflammatory activities, macrophages were stimulated with 100 ng/mL LPS in the presence of different doses of the aglycones apigenin, luteolin, and chrysoeriol or diluent control for 8 h. Stimulation with LPS induced the release of TNF-α, undetectable in the presence of diluent controls (Fig. 2A). Stimulation with LPS in the presence of any of the aglycones resulted in a significant reduction of TNF-α at doses as low as 10 μM (Fig. 2A).
Figure 2.
Effects of glycosylated and deglycosylated flavones on TNF-α. Macrophages were treated for 8 h with 100 ng/mL LPS in the presence of flavones or diluent control DMSO. Treatments marked with (−) denote addition of diluent. (A) Deglycosylated flavones used at indicated concentrations were apigenin, luteolin, and chrysoeriol. (B) Glycosylated flavones used at indicated concentrations include apigenin 7-O-glucoside, luteolin 7-O-glucoside. Data are mean ± standard error of the mean (SEM), n = 3, *p < 0.001 versus LPS by ANOVA.
Next, we evaluated the effect of glycosylated flavones on their ability to modulate inflammatory cytokines. Macrophages stimulated with 100 ng/mL LPS in the presence of 10–25 μM of apigenin 7-O-glucoside or luteolin 7-O-glucoside showed no effect on TNF-α (data not shown). To further determine their immunomodulatory activity doses were increased, but even doses as high as 100 μM had no effect on reducing LPS-induced TNF-α (Fig. 2B).
3.2 Deglycosylation of flavones increases their ability to inhibit LPS-induced NF-κB transcriptional activity
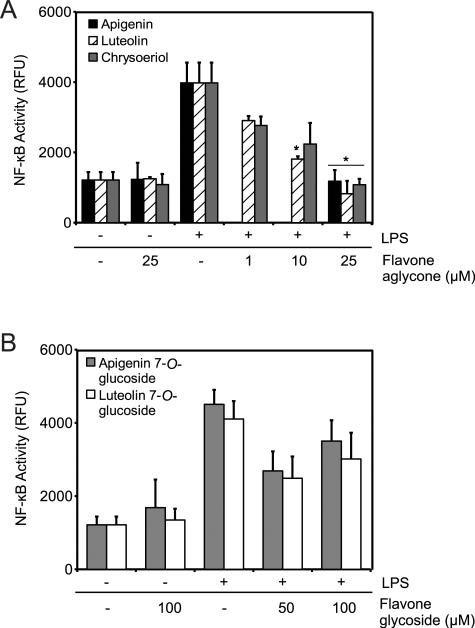
LPS induction of TNF-α is regulated by the activity of the transcription factor NF-κB. To study the effect of flavones in NF-κB activity, macrophages transiently expressing NF-κB SEAP reporter construct were stimulated with 100 ng/mL LPS in the presence of flavone aglycones or diluent control for 16 h. LPS treatment induced NF-κB activity represented by a significant increase in RFUs, while cells treated with diluent or aglycones alone had no effect on NF-κB (Fig. 3A). We found that the treatment of LPS-stimulated cells with different concentrations of the aglycones apigenin, luteolin or chrysoeriol significantly reduced LPS-stimulated NF-κB activity at doses of 25 μM (Fig. 3A).
Figure 3.
Effects of flavones on NF-κB activity after 16 h. (A) Macrophages were treated with LPS and apigenin, luteolin, chrysoeriol, or DMSO or with control (DMSO + PBS: −/−). (B) Macrophages were treated with LPS and apigenin 7-O-glucoside, luteolin 7-O-glucoside, or DMSO or with control (DMSO + PBS: −/−). Data are mean ± SEM, n = 3, *p < 0.05 versus control + LPS.
We next examined the effect of flavone glycosides on LPS stimulation of NF-κB. Macrophages treated with apigenin 7-O-glucoside or luteolin 7-O-glucoside in the presence of diluent control had no effect on NF-κB activity (Fig. 3B). Moreover, macrophages treated with apigenin 7-O-glucoside or luteolin 7-O-glucoside at doses as high as 100 μM had no effect on LPS-induced NF-κB activity (Fig. 3B). Although there was a decrease of NF-κB activity with 50 μM flavone glycosides, the differences were not statistically significant from the control (p = 0.11, 0.21 for apigenin 7-O-glucoside and luteolin 7-O-glucoside, respectively). These results indicate that deglycosylation of flavones increases their ability to modulate NF-κB activity.
3.3 Aglycone content in newly developed flavone-rich food extracts
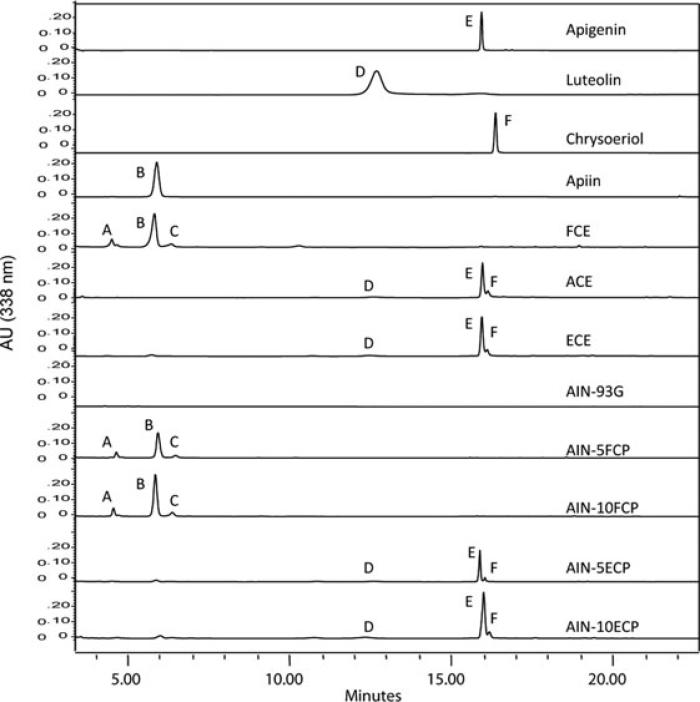
The different ability of pure flavone aglycones and glyco-sides to modulate inflammation suggests that flavone-rich food extracts might also effectively reduce inflammation if their native flavone glycosides are converted to the respective aglycones. To test this hypothesis, we formulated food extracts enriched in aglycone content. Several foods are abundant in flavones, including artichokes, parsley, celery, and kumquat [29–31]. We decided to pursue celery as a flavone source because it is relatively palatable and celery leaves have very high flavone concentrations [29]. Three types of celery extracts: FCE, ACE and ECE were formulated (see material and methods). HPLC analysis showed that the major peaks in FCE corresponded to luteolin apiosylglucoside, apigenin 7-O-apiosylglucoside (apiin), and chrysoeriol apiosylglucoside (Fig. 4, peaks A, B, and C). In contrast, ACE and ECE had almost exclusively the aglycone forms luteolin, apigenin, and chrysoeriol (Fig. 4, peaks D, E, and F, respectively). The celery leaves used in this study had fivefold greater concentrations of apigenin derivatives relative to luteolin or chrysoeriol (Table 1, FCP). Thus, our results found that targeted formulation of diets increased aglycone content.
Figure 4.
Aglycone contents of newly formulated flavone-rich food extracts and diets. HPLC chromatograms of celery food extracts and mouse diets. Peaks represent: (A) luteolin apiosylglucoside, (B) apiin, (C) chrysoeriol apiosylglucoside, (D) luteolin, (E) apigenin, (F) chrysoeriol.
Table 1.
Concentrations of total flavones and aglycones in flavone-rich diets. Flavones were quantified as aglycone equivalents (mg/100 g dry weight)
| Diet | Luteolin |
Apigenin |
Chrysoeriol |
|||
|---|---|---|---|---|---|---|
| Total | Aglycone | Total | Aglycone | Total | Aglycone | |
| AIN-93G | ND | ND | ND | ND | ND | ND |
| FCP | 288 ± 20.3 | ND | 1491 ± 74.6 | ND | 216 ± 14.8 | ND |
| AIN-5FCP | 13.6 ± 0.1 | ND | 62.3 ± 0.3 | ND | 9.0 ± 0.1 | ND |
| AIN-10FCP | 29.2 ± 3.5 | ND | 138 ± 4.4 | 0.2 ± 0.2 | 24.9 ± 0.1 | ND |
| ECP | 143 ± 14.8 | 115 ± 17.6 | 631 ± 9.4 | 551 ± 5.2 | 95.2 ± 0.6 | 95.2 ± 0.6 |
| AIN-5ECP | 5.3 ± 0.4 | 3.4 ± 0.4 | 22.0 ± 0.2 | 18.7 ± 0.1 | 3.7 ± 0.0 | 3.7 ± 0.0 |
| AIN-10ECP | 9.2 ± 0.8 | 6.7 ± 0.6 | 46.9 ± 0.2 | 39.6 ± 0.2 | 7.5 ± 0.1 | 7.5 ± 0.1 |
Data are mean ± SEM, n = 3. ND, not detected. AIN-5FCP, 5% fresh celery pellets; AIN-10FCP, 10% fresh celery pellets; AIN-5ECP, 5% enzyme-treated celery pellets; AIN-10ECP, 10% enzyme-treated celery pellets.
3.4 Effect of food extracts on inflammatory mediators
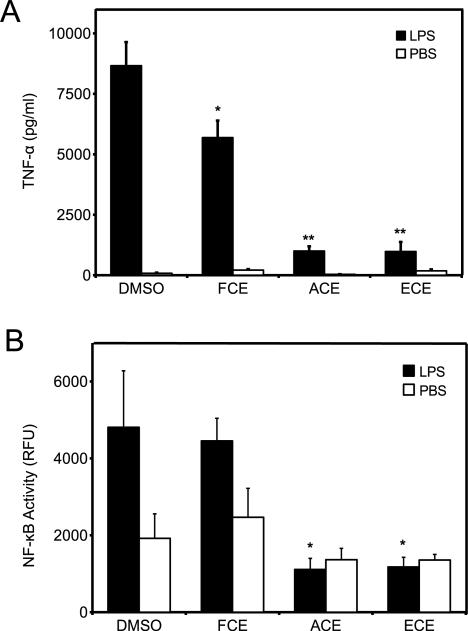
To investigate the ability of flavone-rich celery extracts to reduce inflammation, macrophages were treated with 100 ng/mL LPS or PBS diluent for 8 h in the presence of FCE, ACE, ECE, or DMSO. LPS-treated macrophages released TNF-α (Fig. 5A, black bar LPS+DMSO), while cells treated with food extracts and diluent+PBS did not induce TNF-α release, indicating that the extracts are not proinflammatory (Fig. 5A, white bar). FCE containing predominantly apiin inhibited LPS-induced TNF-α release by only 30%, while ACE and ECE led to approximately 90% reductions (Fig. 5A).
Figure 5.
Effects of flavone-rich celery extracts on inflammation. Macrophages were treated with PBS control or LPS in the presence of DMSO, FCE, ACE, or ECE at 25 μM apigenin equivalents. (A) TNF-α was measured in the media after 8 h. (B) NF-κB activity was measured in the media after 16 h. Data are mean ± SEM, n = 3, *p < 0.05, **p < 0.001 versus DMSO + LPS.
Next, we evaluated the effect of flavone-rich celery extracts on NF-κB transcriptional activity. Macrophages transiently expressing NF-κB SEAP reporter vector were stimulated with 100 ng/mL LPS or PBS control in the presence of FCE, ACE, ECE, or DMSO diluent control for 16 h. Macrophages treated with DMSO and LPS induced NF-κB activity compared to PBS-treated cells (Fig. 5B, DMSO black and white bars, respectively), while cells treated with either food extracts and PBS had no effect on NF-κB (Fig. 5B, FCE, ACE, and ECE white bars). FCE had no effect on LPS-induced NF-κB activity when compared with LPS- and DMSO-treated cells (Fig 5B). In contrast, both ACE and ECE significantly inhibited NF-κB activity to levels found in PBS-treated cells (Fig. 5B, ACE, ECE, black bars). Collectively, these results indicate that the conversion of flavones in food extracts from glycoside to aglycone greatly enhances their ability to modulate inflammatory mediators.
3.5 Cellular uptake of dietary flavones
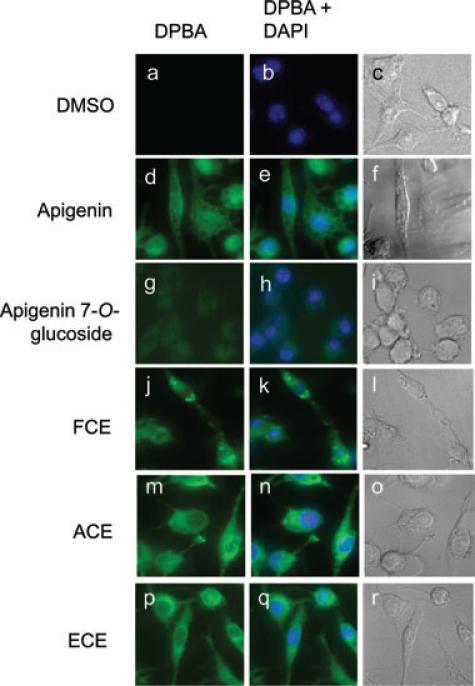
To investigate whether the difference in anti-inflammatory activity of glycoside and aglycone flavones was due to cellular uptake, macrophages were cultured for 3 h in the presence of 25 μM apigenin, apigenin 7-O-glucoside, flavone-rich extracts, or DMSO diluent control and stained with DPBA as previously described [32]. Apigenin-treated, but not control cells showed DPBA staining as previously reported [22] (Fig. 6D–F and 6A–C, respectively). In contrast, apigenin 7-O-glucoside-treated cells had negligible DPBA staining (Fig. 6G–I). In addition, we found that FCE enters cells as suggested by the DPBA staining, but to a lesser extent than ACE and ECE, which showed higher staining (Fig. 6J, 6M, 6P). Nuclear staining with DAPI showed that there were no changes in nuclear morphology associated with any of the treatments (Fig. 6E, 6H, 6K, 6N, 6Q), and identification of nuclei in the DPBA-stained cells showed that apigenin-treated cells accumulated more flavones than ACE- and ECE-treated cells (Fig. 6D, 6M, 6P).
Figure 6.
Cellular localization of dietary flavones in macrophages. Macrophages treated with DMSO, apigenin, apigenin 7-O-glucoside, FCE, ACE, or ECE at 25 μM for 3 h were stained with DPBA and DAPI. Representative of three independent experiments.
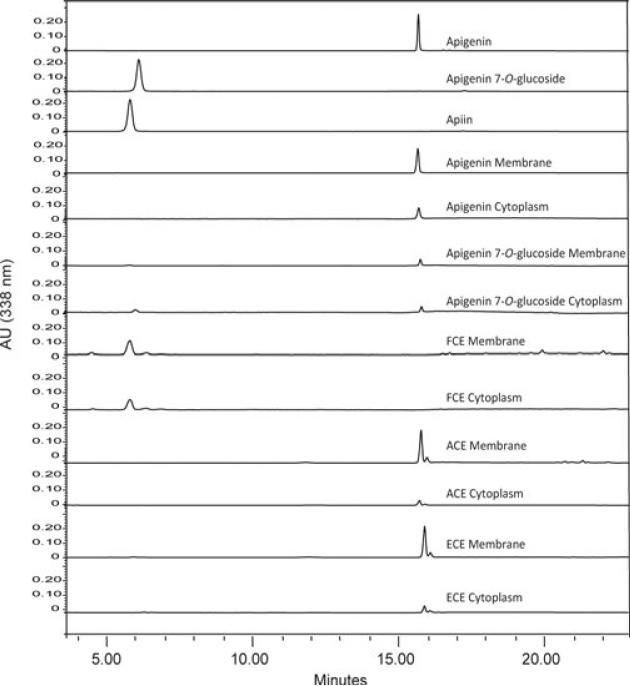
To further study cellular uptake of flavones, subcellular fractionation of cytoplasm and membrane fractions were used to determine the content of flavones by HPLC-DAD in macrophages treated for 3 h with flavone-rich extracts at a 25 μM apigenin equivalent. Consistent with our previous findings in leukemia cells, the apigenin absorbed by macrophages corresponded to 5–15% of the amount added [22], and was primarily associated with the membrane fraction, accounting for 88% of all the apigenin uptake by the cells, and the remaining 12% was found in the cytoplasm fraction. No apigenin 7-O-glucoside was recovered from the cytoplasm or membrane fractions of cells treated with this glucoside. However, apigenin equaling to 0.1% of the dose of 7-O-glucoside used was detected. This percentage is similar to the 0.1% apigenin aglycone contamination found in the apigenin 7-O-glucoside stock (not shown). In cells treated with FCE, apigenin was found as apiin at low levels, reaching only ~0.2–0.3% of the dose used with the majority (62%) in the membrane fraction. In contrast, ACE- and ECE-treated macrophages had up to tenfold higher accumulation of apigenin, with the majority, 62% found in the membrane fraction as apigenin aglycone and proportional to their concentration in the extracts luteolin and chrysoeriol (Figs. 4B and 7). Collectively these results show that flavone aglycones pure or as part of flavones-rich extracts are taken into cells much more efficiently than flavone glycosides and that are abundantly found in the membrane fractions, potentially impacting their biological effects.
Figure 7.
Subcellular distribution of dietary flavones. HPLC analysis of flavone standards, cytoplasmic and membrane fractions of macrophages treated with DMSO, apigenin, apigenin 7-O-glucoside, FCE, ACE, or ECE at 25 μM for 3 h. Representative of three independent experiments.
3.6 Effects of celery-supplemented diets on flavone absorption in mice
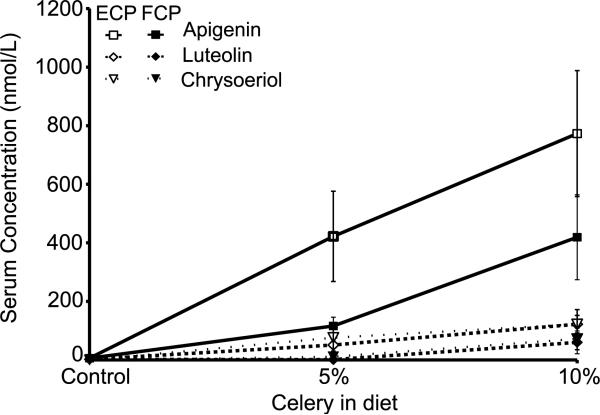
Based on the ability of flavones to counter inflammation, we prepared foods with high flavone concentrations. Mouse diets were made as described in Materials and methods and Table 1. Mice were fed the diets for 7 days, and steady-state levels of flavones in serum were determined by measuring concentrations in urine and serum at sacrifice. For all diets, apigenin, luteolin, and chrysoeriol were detected in urine proportional to their concentration in celery. Mice fed AIN-5ECP had higher urine flavone concentrations than those fed AIN-5FCP; however, no difference was observed between mice fed AIN-10ECP and AIN-10FCP diets (data not shown). Serum luteolin, apigenin, and chrysoeriol concentrations followed a dose-response pattern with each diet formulation at 5 and 10%, and were highest in mice fed AIN-10ECP diet (Fig. 8). After accounting for the lower flavone concentrations in the enzyme-treated diets, relative apigenin and luteolin absorption was significantly higher in enzyme-treated diets relative to their fresh celery counterparts. Relative absorption of chrysoeriol was also higher in AIN-5ECP diets than AIN-5FCP diets. These results indicate that conversion of dietary flavones from glycoside to aglycone forms improves absorption into systemic circulation.
Figure 8.
Concentration of flavones in serum. Flavone concentrations were determined by HPLC-MS/MS in serum of mice fed ad libitum for 7 days with flavone-rich diets: AIN-5FCP, AIN-5ECP, AIN-10FCP, or AIN-10ECP diets. Data are mean ± SEM, n = 4–8.
4 Discussion
Flavones, a class of natural flavonoids with a large number of derivatives in herbs, fruits, and vegetables, are common components of our diet [31, 33]. Potential nutraceutical anti-inflammatory activity for flavones is emerging but the mechanisms responsible for their action remain unclear [34]. Here, we demonstrate using a number of flavones and flavone-rich food extracts that the anti-inflammatory activity is dependent on deglycosylation of the compounds. We further show that flavone glycosylation reduces intestinal absorption into systemic circulation.
Flavones including apigenin, luteolin, and chrysoeriol have been shown to reduce TNF-α secretion in vitro at 10– 50 μM concentrations [24, 26, 35]. In agreement with these studies, we found that as little as 10 μM apigenin, luteolin, or chrysoeriol reduced TNF-α secretion compared to the control (Fig. 2A). We previously reported that apigenin inhibits the phosphorylation of p65NF-κB subunit [21]. This study shows that flavone aglycones at 25 μM concentrations significantly reduce NF-κB activity (Fig. 3A). In contrast, the flavone glycosides apigenin and luteolin 7-O-glucoside had no effect on TNF-α release or NF-κB activity even at concentrations up to 100 μM (Figs. 2B and 3B). The higher efficacy of aglycones rather than glycosides to reduce TNF-α and NF-κB activity is consistent with other studies showing that neither diosmetin 7-O-rutinoside nor apiin reduced nitric oxide (NO) and TNF-α in response to LPS, whereas apigenin effectively decreased these inflammatory mediators [26, 36]. Apiin and FCEs, however, reduced nitrite (NO2) and inducible nitric oxide synthase (iNOS) in macrophages, though under different experimental conditions and at much higher concentrations [37]. Our studies further suggest that the decreased anti-inflammatory activity of the glycosides can be in large part attributed to a lower transport/diffusion through the plasma membrane. Indeed, flavone localization, using fluorescence microscopy, showed a higher cellular uptake of apigenin compared to apigenin 7-O-glucoside (Fig. 6). Consistent with these results, DPBA staining showed higher levels of fluorescence in macrophages treated with ACE and ECE rich in aglycones than in FCE-treated macrophages. Previous studies using fluorescent microscopy or HPLC analysis showed that flavonol aglycones, rather than glycosides, are taken into hepatocytes accumulating in the nucleus [38, 39]. We found that flavones can be nuclear or cytoplasmic but were mainly associated with membrane fractions, reaching quantities ten times higher than in soluble cytoplasmic fractions (Fig. 7). Consistent with results obtained using fluorescent microscopy, HPLC analyses showed higher uptake of apigenin than the glucoside counterpart. Moreover, concentrations of apigenin in macrophage membranes treated with aglycone-rich celery extracts, ECE and ACE, are higher than in FCE-treated cells (Fig. 7). These results indicate that most apigenin is found inside cells associated with organelle membranes and not in the plasma membrane. Although signaling through membrane components cannot be excluded, it has been shown that flavones counter LPS-induced inflammation through several pathways in the cytosol. In addition to inactivating NF-κB through suppression of NF-κB-p65 subunit phosphorylation [21], flavones can reduce TNF-α by interfering with mitogen-activated protein kinases (MAPK). In particular, reductions in extracellular signal-regulated kinase (ERK), p38, and casein kinase 2 (CK2) activation were associated with reduction in TNF-α release from macrophages [40]. Our results indicate that aglycones either pure or as part of a diet can readily be taken up by cells of the immune system and can effectively regulate immune responses. In this context, the mechanisms for cellular influx of flavonoids are not well understood, and they may vary with the type of flavonoid and membrane transporters found in specific cell types. It has also been proposed that flavonoids are transported into cells by passive diffusion [41, 42], which would favor the transport of less polar (e.g., aglycone) molecules through the lipid bilayer membrane. Further studies are needed to understand the mechanisms of cellular uptake of flavonoids.
Adverse effects associated with current anti-inflammatory compounds have prompted the interest in implementing diets with nutraceutical activity for the treatment and prevention of inflammatory diseases [43]. To determine whether flavone-rich foods can effectively attenuate inflammation, we developed food formulations with different levels of aglycone and glycoside flavones. We found that acid- and enzyme-treated celery extracts (rich in aglycones) reduced TNF-α release and NF-κB activity while FCEs (rich in apiin) did not (Fig. 5). These effects are not due to cytotoxicity since cells treated with flavone-rich extracts showed only 10% toxicity, a similar level was observed with aglycones and no cytotoxic effects were reported with glycosides (not shown).
Dietary intervention studies using flavone-rich diets showed increased serum flavone concentrations in mice fed AIN-5ECP and AIN-10ECP diets, averaging nearly twice those of their fresh celery counterparts (Fig. 8) even though they contained approximately one third the concentration of flavones measured as apigenin equivalents (Table 1). After adjusting for these differences in flavone intake, AIN-5ECP and AIN-10ECP diets resulted in significantly greater relative absorption of luteolin and apigenin than their fresh celery counterparts. These data clearly indicate that dietary flavone aglycones are more efficiently absorbed into serum than their corresponding glycosides, which is consistent with observations that flavonoids glycosides must first be deconjugated to aglycones prior to intestinal absorption [44]. Apigenin and luteolin are absorbed primarily in the small intestine [45], and apigenin is more than five times more permeable than apigenin 7-O-glucoside in the Caco-2 model [46].
We found that the flavone-rich diets here resulted in much higher concentrations of apigenin in serum than those previously reported in mice fed pure apigenin. The mice fed AIN-10ECP in our study consumed 0.05% apigenin for 7 days and averaged 773 nM apigenin in serum, compared with 90 nM reported in mouse plasma following a diet containing 0.2% pure apigenin for 7 days [47]. Studies with flavones and flavone-rich diets found much greater absorption in rats, although these reported maximum rather than steady-state concentrations in blood. The AIN-10ECP mice in our study consumed ~68 mg apigenin/kg body weight daily, while rats fed only 14 mg luteolin/kg body weight by gavage had a peak plasma concentration of 6.9 μM after 1 h and those fed lute-olin in the form of peanut hull extract had concentrations four times as high [48]. Rats consuming 10 mg apigenin equivalents/kg body weight in the form of chrysanthemum had 63 μM apigenin in plasma after 4 h [49]. These studies show that flavone concentrations in circulation are much higher within a few hours after consumption, but fall to lower steady-state levels. There may also be differences in intestinal absorption between rats and mice, making comparisons with our study difficult.
In summary, we have demonstrated that deconjugation of flavone glycosides to aglycones determines their anti-inflammatory activity in vitro and their systemic absorption in vivo. Our study also indicates that administration of dietary flavone aglycones rather than glycosides results in more efficient uptake of flavones into serum, which will be important in designing and implementing food-based approaches to reduce chronic inflammation.
Acknowledgments
The authors acknowledge the Ohio Plant Biotechnology Consortium (OPBC) for financial support and the PHPID-Program fellowship to D.A. We thank Ms. Nicholas for assistance and Dr. Grotewold for critical reading of the manuscript. We also thank the Nutrient and Phytochemical Analytical Shared Resource (NPASR) for flavone's identification studies.
Abbreviations
- ACE
acid-treated celery extract
- DPBA
diphenyl-boric acid 2-aminoethyl ester
- ECE
enzyme-treated celery extract
- FCE
fresh celery extract
- LPS
lipopolysaccharide
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- SEAP
secreted human placental alkaline phosphatase
- TNF-α
tumor necrosis factor α
Footnotes
The authors have declared no conflict of interest.
References
- 1.Gorczynski RM. Clinical Immunology. Landes Bioscience; Austin, Texas: 1999. [Google Scholar]
- 2.Henson PM. Dampening inflammation. Nat. Immunol. 2005;6:1179–1181. doi: 10.1038/ni1205-1179. [DOI] [PubMed] [Google Scholar]
- 3.Kellum JA, Kong L, Fink MP, Weissfeld LA, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch. Intern. Med. 2007;167:1655–1663. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gan WQ, Man SFP, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59:574–580. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pradhan AD, Manson JE, Rifai N, Buring JE, et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 6.Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N. Engl. J. Med. 2001;344:907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 7.Lin W-W, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J. Clin. Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 9.Dubois RW, Melmed GY, Henning JM, Laine L. Guidelines for the appropriate use of non-steroidal anti-inflammatory drugs, cyclo-oxygenase-2-specific inhibitors and proton pump inhibitors in patients requiring chronic anti-inflammatory therapy. Aliment. Pharmacol. Ther. 2004;19:197–208. doi: 10.1111/j.0269-2813.2004.01834.x. [DOI] [PubMed] [Google Scholar]
- 10.Manach C, Scalbert A, Morand C, Remesy C, et al. Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 11.García-Lafuente A, Guillamón E, Villares A, Rostagno MA, et al. Flavonoids as anti-inflammatory agents: implications in cancer and cardiovascular disease. Inflamm. Res. 2009;58:537–552. doi: 10.1007/s00011-009-0037-3. [DOI] [PubMed] [Google Scholar]
- 12.Matern U. Acylhydrolases from parsley (Petroselinum hortense). Relative distribution and properties of four esterases hydrolyzing malonic acid hemiesters of flavonoid glucosides. Arch. Biochem. Biophys. 1983;224:261–271. doi: 10.1016/0003-9861(83)90209-6. [DOI] [PubMed] [Google Scholar]
- 13.DuPont MS, Mondin Z, Williamson G, Price KR. Effect of variety, processing, and storage on the flavonoid glycoside content and composition of lettuce and endive. J. Agric. Food Chem. 2000;48:3957–3964. doi: 10.1021/jf0002387. [DOI] [PubMed] [Google Scholar]
- 14.Schutz K, Kammerer D, Carle R, Schieber A. Identification and quantification of caffeoylquinic acids and flavonoids from artichoke (Cynara scolymus L.) heads, juice, and pomace by HPLC-DAD-ESI/MSn. J. Agric. Food Chem. 2004;52:4090–4096. doi: 10.1021/jf049625x. [DOI] [PubMed] [Google Scholar]
- 15.Gil-Izquierdo A, Gil MI, Ferreres F. Effect of processing techniques at industrial scale on orange juice antioxidant and beneficial health compounds. J. Agric. Food Chem. 2002;50:5107–5114. doi: 10.1021/jf020162+. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen ILF, Chee WSS, Poulsen L, Offord-Cavin E, et al. Bioavailability is improved by enzymatic modification of the citrus flavonoid hesperidin in humans: a randomized, double-blind, crossover trial. J. Nutr. 2006;136:404–408. doi: 10.1093/jn/136.2.404. [DOI] [PubMed] [Google Scholar]
- 17.Richelle M, Pridmore-Merten S, Bodenstab S, Enslen M, et al. Hydrolysis of isoflavone glycosides to aglycones by β-glycosidase does not alter plasma and urine isoflavone pharmacokinetics in postmenopausal women. J. Nutr. 2002;132:2587–2592. doi: 10.1093/jn/132.9.2587. [DOI] [PubMed] [Google Scholar]
- 18.Hanske L, Loh G, Sczesny S, Blaut M, et al. The bioavail-ability of apigenin-7-glucoside is influenced by human intestinal microbiota in rats. J. Nutr. 2009;139:1095–1102. doi: 10.3945/jn.108.102814. [DOI] [PubMed] [Google Scholar]
- 19.Manach C, Williamson G, Morand C, Scalbert A, et al. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 20.Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005;81:243S–255S. doi: 10.1093/ajcn/81.1.243S. [DOI] [PubMed] [Google Scholar]
- 21.Nicholas C, Batra S, Vargo MA, Voss OH, et al. Apigenin blocks lipopolysaccharide-induced lethality in vivo and proinflammatory cytokines expression by inactivating NF-κB through the suppression of p65 phosphorylation. J. Immunol. 2007;179:7121–7127. doi: 10.4049/jimmunol.179.10.7121. [DOI] [PubMed] [Google Scholar]
- 22.Vargo MA, Voss OH, Poustka F, Cardounel AJ, et al. Apigenin-induced-apoptosis is mediated by the activation of PKCδ and caspases in leukemia cells. Biochem. Pharmacol. 2006;72:681–692. doi: 10.1016/j.bcp.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Walle T, Ta N, Kawamori T, Wen X, et al. Cancer chemopreventive properties of orally bioavailable flavonoids–methylated versus unmethylated flavones. Biochem. Pharmacol. 2007;73:1288–1296. doi: 10.1016/j.bcp.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Comalada M, Ballester I, Bailón E, Sierra S, et al. Inhibition of pro-inflammatory markers in primary bone marrow-derived mouse macrophages by naturally occurring flavonoids: analysis of the structure-activity relationship. Biochem. Pharmacol. 2006;72:1010–1021. doi: 10.1016/j.bcp.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Smolinski AT, Pestka JJ. Modulation of lipopolysaccharide-induced proinflammatory cytokine production in vitro and in vivo by the herbal constituents apigenin (chamomile), ginsenoside Rb(1) (ginseng) and parthenolide (feverfew). Food Chem. Toxicol. 2003;41:1381–1390. doi: 10.1016/s0278-6915(03)00146-7. [DOI] [PubMed] [Google Scholar]
- 26.Shanmugam K, Holmquist L, Steele M, Stuchbury G, et al. Plant-derived polyphenols attenuate lipopolysaccharide-induced nitric oxide and tumour necrosis factor production in murine microglia and macrophages. Mol. Nutr. Food Res. 2008;52:427–438. doi: 10.1002/mnfr.200700180. [DOI] [PubMed] [Google Scholar]
- 27.Li L-P, Jiang H-D. Determination and assay validation of luteolin and apigenin in human urine after oral administration of tablet of Chrysanthemum morifolium extract by HPLC. J. Pharm. Biomed. Anal. 2006;41:261–265. doi: 10.1016/j.jpba.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 28.Twaddle NC, Churchwell MI, Doerge DR. High-throughput quantification of soy isoflavones in human and rodent blood using liquid chromatography with electrospray mass spectrometry and tandem mass spectrometry detection. J. Chromatogr. B. 2002;777:139–145. doi: 10.1016/s1570-0232(02)00275-1. [DOI] [PubMed] [Google Scholar]
- 29.Justesen U, Knuthsen P, Leth T. Quantitative analysis of flavonols, flavones, and flavanones in fruits, vegetables and beverages by high-performance liquid chromatography with photo-diode array and mass spectrometric detection. J. Chromatogr. A. 1998;799:101–110. doi: 10.1016/s0021-9673(97)01061-3. [DOI] [PubMed] [Google Scholar]
- 30.Azzini E, Bugianesi R, Romano F, Di Venere D, et al. Absorption and metabolism of bioactive molecules after oral consumption of cooked edible heads of Cynara scolymus L. (cultivar Violetto di Provenza) in human subjects: a pilot study. Br. J. Nutr. 2007;97:963–969. doi: 10.1017/S0007114507617218. [DOI] [PubMed] [Google Scholar]
- 31.Sakakibara H, Honda Y, Nakagawa S, Ashida H, et al. Simultaneous determination of all polyphenols in vegetables, fruits, and teas. J. Agric. Food Chem. 2003;51:571–581. doi: 10.1021/jf020926l. [DOI] [PubMed] [Google Scholar]
- 32.Peer WA, Brown DE, Tague BW, Muday GK, et al. Flavonoid accumulation patterns of transparent testa mutants of arabidopsis. Plant Physiol. 2001;126:536–548. doi: 10.1104/pp.126.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wojdylo A, Oszmianski J, Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007;105:940–949. [Google Scholar]
- 34.Rathee P, Chaudhary H, Rathee S, Rathee D, et al. Mechanism of action of flavonoids as anti-inflammatory agents: a review. Inflamm. Allergy Drug Targets. 2009;8:229–235. doi: 10.2174/187152809788681029. [DOI] [PubMed] [Google Scholar]
- 35.Manthey JA, Grohmann K, Montanari A, Ash K, et al. Polymethoxylated flavones derived from citrus suppress tumor necrosis factor-α expression by human monocytes. J. Nat. Prod. 1999;62:441–444. doi: 10.1021/np980431j. [DOI] [PubMed] [Google Scholar]
- 36.Kim HK, Cheon BS, Kim YH, Kim SY, et al. Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW 264.7 and their structure-activity relationships. Biochem. Pharmacol. 1999;58:759–765. doi: 10.1016/s0006-2952(99)00160-4. [DOI] [PubMed] [Google Scholar]
- 37.Mencherini T, Cau A, Bianco G, Della Loggia R, et al. An extract of Apium graveolens var. dulce leaves: structure of the major constituent, apiin, and its anti-inflammatory properties. J. Pharm. Pharmacol. 2007;59:891–897. doi: 10.1211/jpp.59.6.0016. [DOI] [PubMed] [Google Scholar]
- 38.Mukai R, Shirai Y, Saito N, Yoshida K, et al. Subcellular localization of flavonol aglycone in hepatocytes visualized by confocal laser scanning fluorescence microscope. Cytotechnology. 2009;59:177–182. doi: 10.1007/s10616-009-9206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanazawa K, Uehara M, Yanagitani H, Hashimoto T. Bioavailable flavonoids to suppress the formation of 8-OHdG in HepG2 cells. Arch. Biochem. Biophys. 2006;455:197–203. doi: 10.1016/j.abb.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Xagorari A, Roussos C, Papapetropoulos A. Inhibition of LPS-stimulated pathways in macrophages by the flavonoid luteolin. Br. J. Pharmacol. 2002;136:1058–1064. doi: 10.1038/sj.bjp.0704803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gee JM, DuPont MS, Day AJ, Plumb GW, et al. Intestinal transport of quercetin glycosides in rats involves both deglycosylation and interaction with the hexose transport pathway. J. Nutr. 2000;130:2765–2771. doi: 10.1093/jn/130.11.2765. [DOI] [PubMed] [Google Scholar]
- 42.Walgren RA, Walle UK, Walle T. Transport of quercetin and its glucosides across human intestinal epithelial Caco-2 cells. Biochem. Pharmacol. 1998;55:1721–1727. doi: 10.1016/s0006-2952(98)00048-3. [DOI] [PubMed] [Google Scholar]
- 43.Ameye LG, Chee WS. Osteoarthritis and nutrition. From nutraceuticals to functional foods: a systematic review of the scientific evidence. Arthritis Res. Ther. 2006;8:R127–R127. doi: 10.1186/ar2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Németh K, Plumb GW, Berrin J-G, Juge N, et al. Deglycosylation by small intestinal epithelial cell β-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur. J. Nutr. 2003;42:29–42. doi: 10.1007/s00394-003-0397-3. [DOI] [PubMed] [Google Scholar]
- 45.Lu X-Y, Sun D-L, Chen Z-J, Chen T, et al. Relative contribution of small and large intestine to deglycosylation and absorption of flavonoids from Chrysanthemun morifolium extract. J. Agric. Food Chem. 2010;58:10661–10667. doi: 10.1021/jf102992r. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y, Hu M. Absorption and metabolism of flavonoids in the caco-2 cell culture model and a perused rat intestinal model. Drug Metab. Dispos. 2002;30:370–377. doi: 10.1124/dmd.30.4.370. [DOI] [PubMed] [Google Scholar]
- 47.Cai H, Boocock D, Steward W, Gescher A. Tissue distribution in mice and metabolism in murine and human liver of apigenin and tricin, flavones with putative cancer chemopreventive properties. Cancer Chemother. Pharm. 2007;60:257–266. doi: 10.1007/s00280-006-0368-5. [DOI] [PubMed] [Google Scholar]
- 48.Zhou P, Li L-P, Luo S-Q, Jiang H-D, et al. Intestinal absorption of luteolin from peanut hull extract is more efficient than that from individual pure luteolin. J. Agric. Food Chem. 2008;56:296–300. doi: 10.1021/jf072612+. [DOI] [PubMed] [Google Scholar]
- 49.Chen T, Li L-P, Lu X-Y, Jiang H-D, et al. Absorption and excretion of luteolin and apigenin in rats after oral administration of Chrysanthemum morifolium extract. J. Agric. Food Chem. 2007;55:273–277. doi: 10.1021/jf062088r. [DOI] [PubMed] [Google Scholar]