Abstract
Objective
Generalized anxiety disorder (GAD) is a prevalent psychiatric condition in older adults with deleterious effects on health and cognition. Although selective serotonin reuptake inhibitor (SSRI) medications have some efficacy as acute treatments for geriatric GAD, incomplete response is the most common outcome of monotherapy. We therefore developed a novel sequential treatment strategy, using personalized, modular cognitive-behavioral therapy (mCBT) to augment SSRI medication.
Method
In an open label pilot study (N =10), subjects received a sequenced trial of 12 weeks of escitalopram followed by 16 weeks of escitalopram augmented with mCBT. We also examined the maintenance effects of mCBT over a 28-week follow-up period following drug discontinuation and termination of psychotherapy.
Results
Results suggest that (1) adding mCBT to escitalopram significantly reduced anxiety symptoms and pathological worry, resulting in full remission for most patients and (2) some patients maintained response after all treatments were withdrawn.
Conclusion
Findings suggest that mCBT may be an effective augmentation strategy when added to SSRI medication and provide limited support for the long-term benefit of mCBT after discontinuation of pharmacotherapy.
Keywords: aged, elderly, cognitive therapy, behavior therapy, drug therapy, psychotherapy, selective serotonin reuptake inhibitor
Introduction
Generalized anxiety disorder (GAD) is defined by excessive, uncontrollable worry accompanied by somatic and mental symptoms such as restlessness and muscle tension (Association, 2000). The disorder tends to be chronic in the absence of effective treatment, with most patients reporting an unremitting course of illness (Rubio and Lopez-Ibor, 2007).
Several studies in elderly populations have found that the point prevalence of GAD in community-living elderly is high, with estimates ranging from 1.2 to 7.3% (Beekman et al., 1998; Schoevers et al., 2003; Trollor et al., 2007; Gum et al., 2009). Younger people often continue to have GAD as they enter the ranks of the aged, and many people develop it late in life (Le Roux et al., 2005; Lenze et al., 2005; Chou, 2009).
Compared to older adults without psychiatric illness, individuals with late-life GAD have a threefold increased risk of health-related activity limitation and similar decrements in health-related quality of life (de Beurs et al., 1999; Wetherell et al., 2004; Porensky et al., 2009). Older adults are also more vulnerable to worsening health and cognition from chronic anxiety (Lenze and Wetherell, 2009), which may be the result of CNS damage (Sinoff and Werner, 2003) due to chronically elevated cortisol (Mantella et al., 2008) or blood pressure (Paterniti et al., 1999). Overall, these increased disabilities are on par with those associated with late-life major depressive disorder (MDD) (Beekman et al., 1997; Wetherell et al., 2004).
Of the few prospective controlled trials for late-life GAD, benzodiazepines, selective serotonin reuptake inhibitors (SSRIs), and to a lesser extent, cognitive-behavioral therapy (CBT), have been shown efficacious acutely (Koepke et al., 1982; Schuurmans et al., 2006; Lenze et al., 2009; Schuurmans et al., 2009; Stanley et al., 2009). Benzodiazepines, commonly prescribed for late-life GAD, increase risk for falls, fractures, and cognitive impairment, and may accelerate cognitive decline; thus their use as long-term monotherapy is not recommended (Gray et al., 2006; Benitez et al., 2008; Pariente et al., 2008; Wright et al., 2009). Moreover, even with an adequate course of SSRI or CBT treatment, most participants fail to achieve high end state functioning (Lenze et al., 2009; Stanley et al., 2009). In sum, many older adults with GAD will not achieve adequate short-term outcomes with a monotherapy (SSRI or CBT) approach, and little is known about optimal long-term strategies for this chronic disorder.
When monotherapy is inadequate, combination treatments may be more efficacious, particularly SSRIs and CBT (Fava et al., 2005), which have different mechanisms and may be able to treat different components of the illness (McNally, 2007; Arce et al., 2008). A recent meta-analysis of studies with younger and middle-aged adults indicates that a combination of pharmacotherapy and CBT may be more effective than CBT alone as an acute treatment for anxiety disorders (Hofmann et al., 2009).
Combination treatment may be introduced sequentially or simultaneously (Fava et al., 2006). Potential advantages of a sequential approach of SSRI and CBT are (1) It reflects real-world clinical practice, in which most older adults who receive mental health treatment do so initially in the form of pharmacotherapy; (2) it allows the patient and provider to focus sequentially on different aspects of treatment, rather than divide focus among multiple treatments and components of illness at once; and (3) it allows for one treatment to be a catalyst for the next. In GAD, for example, SSRI treatment can reduce acute distress and somatic symptoms, paving the way for CBT to address underlying worry control, engage cognitive distortions, and improve coping skills. Thus, by targeting different facets of the illness, the two treatments may lead to optimal acute outcomes and greater long-term reductions in persistent symptoms and risk of relapse.
Adding psychotherapy that targets underlying worry control to medication treatment may provide durable even if medication is eventually discontinued. A recent review of meta-analyses of studies conducted with younger and middle-aged adults concluded that psychotherapies involving cognitive and behavioral strategies for GAD are superior to nondirective therapy and pill placebo and equivalent to pharmacotherapy in the acute phase of treatment, with robust effects extending as far as 10 years following discontinuation of treatment (Butler et al., 2006). In MDD, adding CBT to antidepressants reduces relapse (Blackburn and Moore, 1997; Ma and Teasdale, 2004; Bockting et al., 2005; Paykel et al., 2005), extending years after stopping medication (Fava et al., 2006). A recent pilot study, however, suggested that the relapse prevention benefit of CBT in depressed older adults taking antidepressant medication diminished over time (Wilkinson et al., 2009). Because GAD has a chronic course, and many older adults do not wish to remain on medication indefinitely, an investigation of the maintenance effects of CBT for late-life GAD is warranted.
In older adults, as in younger adults, GAD is heterogeneous, commonly co-occurring with depression and other anxiety disorders (Schoevers et al., 2003). Moreover, efforts to disseminate empirically supported psychotherapies increasingly emphasize the use of flexible treatments that can be individually tailored to meet the needs of diverse patients (McHugh and Barlow, 2010). For these reasons, a modular CBT (mCBT) approach may offer benefits as a treatment for geriatric GAD (Wetherell et al., 2009).
The present pilot study examined a sequenced combination therapy strategy for the treatment of GAD in a sample of older adults. The protocol consisted of 12 weeks open-label escitalopram followed by 16 weeks of escitalopram plus mCBT; this active treatment phase was followed by a 28-week maintenance phase after termination of psychotherapy and drug discontinuation.
Method
Participants and procedure
Participants were 10 adults at least 60 years old who were recruited from medical practices at one of three sites: Pittsburgh, PA, San Diego, CA, or St. Louis, MO. All were Caucasian; other demographic information is presented in Table 1. The patients recruited from the Pittsburgh site had previously participated in a randomized, placebo-controlled trial of escitalopram (Lenze et al., 2009). All participants met Diagnostic and Statistical Manual of Mental Disorders (DSM-IV (First et al., 1996; Association, 2000)) criteria for a principal diagnosis of GAD of at least moderate intensity (Hamilton Anxiety Rating Scale (Hamilton, 1959)) 17 or greater; note that the Ham-A score of one pilot patient had decreased to 14 by the time of first administration of medication). The Structured Clinical Interview for DSM-IV (SCID-IV (First et al., 1996)) and Mini-Mental Status Examination (MMSE (Folstein et al., 1975)) were administered for diagnostic purposes and to determine study eligibility. Medical comorbidity was quantified using the Cumulative Illness Rating Scale for Geriatrics (CIRS-G (Miller et al., 1992)). On this physician-rated scale, higher scores indicate more medical burden, and a score of 10 represents the average for an elderly primary care population. Participants were excluded for conditions that might threaten their safety or limit their ability to participate in the study, including psychosis or substance abuse/dependence in the past 6 months, current suicidal ideation, and cognitive impairment as defined by a score less than 25 on the MMSE. All participants were tapered off other psychotropic medications for at least 2 weeks before enrolling in the study, and all provided written informed consent.
Table 1.
Participant demographics
| ID | Gender | Age | Education (years) | Age of onset of GAD | Axis I comorbidity | Health status (CIRS-G) | Cognitive status (MMSE) |
|---|---|---|---|---|---|---|---|
| 1 | F | 75 | 15 | 75 | None | 9 | Missing |
| 2 | M | 65 | 19 | 65 | None | 10 | 30 |
| 3 | M | 61 | 12 | 5 | Current major depression, agoraphobia, specific phobia, obsessive-compulsive disorder | 7 | 28 |
| 4 | M | 78 | 17 | 5 | None | 15 | 30 |
| 5 | F | 87 | 10 | 87 | Current panic disorder, social phobia; past major depression | 7 | 27 |
| 6 | M | 67 | 19 | 66 | Past major depression and alcohol abuse | 13 | 30 |
| 7 | F | 65 | 12 | 10 | Current panic disorder | 9 | 28 |
| 8 | F | 64 | 19 | 5 | Current specific phobia; past major depression and alcohol abuse | 9 | 29 |
| 9 | F | 61 | 14 | 55 | None | 10 | 30 |
| 10 | F | 63 | 12 | 5 | Current social phobia | 13 | 30 |
GAD, generalized anxiety disorder; CIRS-G, Cumulative Illness Rating Scale—Geriatrics; MMSE, Mini Mental State Examination.
The three phases of study treatment were 12 weeks of open-label escitalopram (acute phase), followed by 16 weeks of continued escitalopram supplemented with 16 individual sessions of mCBT (augmentation phase), after which patients were tapered off the medication and followed for an additional 28 weeks (maintenance phase). The 12 week duration of acute medication was selected because the investigators found a response rate of 69% at 12 weeks in one study of SSRI treatment of geriatric GAD and 60% response at 32 weeks in another, suggesting that most of the benefit occurs within the first 12 weeks (Blank et al., 2006; Lenze et al., 2009). The duration of CBT was selected to allow adequate coverage of both required and supplemental modules plus sufficient time for older adults to practice new skills. During the 8-week period in which they were tapering off the medication, patients received three booster sessions, intended to help them cope with emergent anxiety symptoms. Patients were started on a dose of 10 mg of escitalopram increased to 20 mg as tolerated after 4 weeks if substantial improvement was not noted. None of the patients took concurrent benzodiazepines. Seven participants were prescribed 20 mg escitalopram, and the remaining three were prescribed 10 mg.
Participants were required to demonstrate at least 20% improvement on the Ham-A to receive psychotherapy; all met this requirement. Psychotherapy, which targeted reduction of persistent symptoms of worry and anxiety and prevention of relapse, was conducted according to a manualized protocol (Wetherell et al., 2009). The protocol consisted of modules devoted to education/monitoring, relaxation training, problem-solving skills training, cognitive therapy, and skills practice. To provide personalized, algorithm-driven treatment, patients could receive several supplemental modules based on criteria drawn from patients’ baseline SCID and Ham-A data: Patients who reported a major depressive episode within 1 year prior to enrollment based on the SCID or who scored a 3 or 4 on the Ham-A Depressed Mood item for two consecutive assessments during the open-label acute phase received a behavioral activation module; patients who reported specific or social phobia, panic disorder, agoraphobia, or post-traumatic stress disorder within the past year, or who scored a 3 or 4 on the Ham-A Fears item for two consecutive assessments during acute treatment, received a module on exposure therapy; and patients who scored a 3 or 4 on the Ham-A Insomnia item for two consecutive assessments during the acute phase received the Sleep Hygiene module. Finally, because working with the family is a key component of good geriatric mental health practice, patients with supportive local family members (spouses, adult children) brought a family member to one session that focused on educating the family and making them an ally in the treatment.
Seven doctoral-level therapists were trained at the three sites by Dr Wetherell, who held 2-day inservices at each site with didactic material, videotaped demonstrations of the protocol, and role plays to familiarize therapists with the manual and procedures. They attended weekly meetings at their local site and weekly across-site meetings chaired by Dr Wetherell and conducted by teleconference. An external expert in CBT for geriatric anxiety rated four randomly selected tapes from each therapist for adherence and competence overall and on several specific components. Average overall adherence on a scale of 0 =unsatisfactory, 1 =adequate, 2 =good, 3 =very good, and 4 =excellent ranged from 2.6 to 4.0 across therapists, and overall competence, also rated from 0 to 4.0, ranged from 2.5 to 4.0.
After the augmentation phase, patients were slowly tapered off the medication according to the following schedule: 10 mg/d × 2 wk, 5 mg/d × 2 wk, 2.5 mg/d × 2 wk, then no medication. During this 6-week taper, subjects also received three booster CBT sessions, in person or by phone, to reinforce their coping skills while coming off of the medication. Clinical management and assessment visits took place biweekly to every 4 weeks throughout the acute, augmentation, and maintenance phases. At each visit, clinicians asked about compliance during the interval between visits and collected unused pills. They also administered a battery of self-report and clinician-administered measures described in more detail below.
Relapse was defined as a Ham-A score increased to at least 14, plus a clinically significant increase (defined as at least five points higher than the lowest point in the augmentation phase) on two consecutive assessments, plus meeting GAD criteria (i.e., hard to control worry with at least three associated symptoms more days than not) for three or more weeks duration. Development of MDD was also considered relapse, regardless of Ham-A score or GAD status. We measured symptomatic improvement and remission during the augmentation phase, and maintenance of improvement and relapse rate during the maintenance phase, using the Ham-A and the Penn State Worry Questionnaire (PSWQ (Meyer et al., 1990)). We examined two definitions of remission: Ham-A score of 8 or less, and PSWQ score of 40 or less, based on data from studies in geriatric GAD patients and normal older controls (Hamilton, 1959; Association, 2000; Diefenbach et al., 2001; Schoevers et al., 2003; Sinoff and Werner, 2003; Hollon et al., 2005; Schuurmans et al., 2006; Rubio and Lopez-Ibor, 2007).
Analyses
In addition to examining the data on a patient-by-patient basis, we computed paired t-tests to compare change on Ham-A and PSWQ scores from baseline to end of acute phase, from end of acute to end of augmentation, and, for the Ham-A, from end of augmentation to end of maintenance, with end of maintenance defined as completion of the study or time of relapse. Because we did not collect PSWQ data at time of relapse for two patients, we did not make statistical comparisons from end of augmentation through end of maintenance.
Results
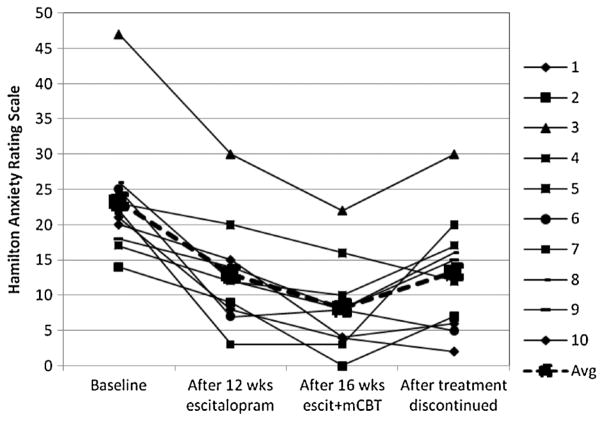
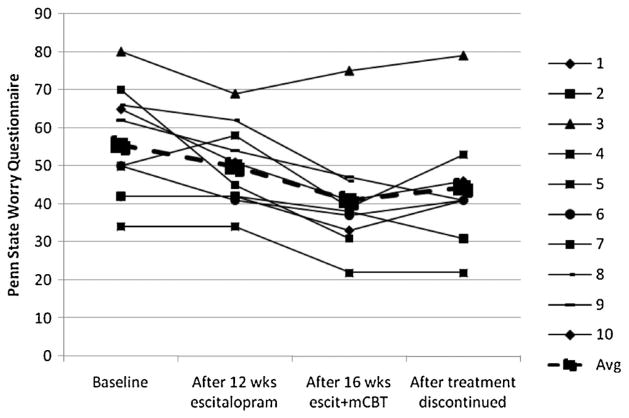
Changes over time for each patient are displayed in Figures 1 and 2. Over the acute escitalopram phase, the mean HAMA score decreased from 23.3 (SD =9.1) to 13.0 (SD =13.0), t(9) =5.01, p <0.001, an effect size of d =0.93; the mean PSWQ decreased from 55.5 (15.8) to 49.8 (10.9), t(9) =1.82, p >0.10, d =0.43. Three of the 10 patients achieved remission according to the Ham-A criteria of 8 or less. One patient scored in the remission range on the PSWQ at baseline; no additional patients reached remission as defined by this measure with escitalopram monotherapy.
Figure 1.
Effects of escitalopram augmented with modular cognitive-behavioral therapy (mCBT) on anxiety symptoms in geriatric generalized anxiety disorder (N=10).
Figure 2.
Effects of escitalopram augmented with modular cognitive-behavioral therapy (mCBT) on worry severity in geriatric generalized anxiety disorder (N=10).
Following 16 sessions of CBT in conjunction with continued escitalopram, the mean Ham-A decreased further to 8.2 (6.4), t(9) =3.77, p =0.004, d =0.49, with seven patients achieving remission. The mean PSWQ decreased to 40.9 (14.0), t(9) =3.92, p =0.003, d =0.71, with six patients achieving remission according to this measure.
Three patients relapsed during the maintenance phase, one each at weeks 10, 16, and 20. Two of them met criteria for MDD in addition to GAD. After discontinuation of the medication, combining the non-relapsers’ scores from the end of the maintenance phase and the relapsers’ scores from the time of relapse, the mean Ham-A was 13.3 (8.6), which represents a marginally significant increase, t(9) =2.19, p =0.06, relative to the score at the end of the augmentation phase. Four patients remained in remission according to Ham-A criteria. According to PSWQ criteria, only two patients remained in remission at the end of maintenance.
The three pilot patients who relapsed were followed after they were put back on the medication. After resumption of the medication, Ham-A scores declined to 28, 2, and 13, all of which were clinically improved from scores at time of relapse. PSWQ scores for two of the patients remained elevated after resumption of escitalopram monotherapy: 65, 34, and 54.
Discussion
In this pilot study of 10 older adults with GAD, we found support for a planned sequential treatment approach of SSRI augmented by modular CBT. Following SSRI monotherapy, subjects showed significant reductions in anxiety symptoms but evidenced less reduction in worry severity, and few patients achieved full remission status. Further reductions in anxiety symptoms and particularly worry severity followed augmentation with mCBT. Most individuals achieved full remission with this sequential SSRI/mCBT approach. Moreover, the finding that anxiety symptoms as measured by the Ham-A appeared to respond well to medication whereas worry as measured by the PSWQ appeared to respond to mCBT is consistent with the model of sequential treatment targeting different aspects of the illness.
We found partial evidence for the maintenance effects of mCBT following drug discontinuation: Although only three of 10 relapsed, and symptom scores remained below pre-treatment levels, few patients remained at the level of remission. By comparison, the only existing study of maintenance pharmacotherapy in GAD (in younger adults) found a relapse rate of 56% for patients following discontinuation of escitalopram (Allgulander et al., 2006). Preliminarily, it appears that some but not all patients can be tapered from SSRI after receiving augmentation CBT.
One important feature of the protocol was the integration of psychotherapy with pharmacotherapy. We emphasized open communication between pharmacotherapists and psychotherapists, with weekly consultations to ensure optimal care. This experience led to the qualitative realization that the treatments did not seem to interfere with each other, and in fact many patients were appreciative of the opportunity to benefit from two different types of therapy. This collaborative approach to care does not often occur in usual clinical practice at present, but success with this model in treating geriatric depression (Unutzer et al., 2002) suggests that it may represent a promising strategy in the management of anxiety as well as mood disorders.
In terms of optimizing relapse prevention, we found that patients did not typically experience increased distress during the 6-week medication taper immediately after mCBT augmentation but rather appeared to be at risk for relapse months later. Furthermore, we learned that some patients stopped engaging in the skills taught in mCBT following termination of treatment. These patients were at particularly high risk for return of symptoms in the face of stressful life events. Thus, going forward, one enhancement to the treatment strategy is using the booster sessions when they are needed during maintenance treatment, as triggered by elevations in anxiety symptom scores or self-reported distress.
Limitations of this study include its small size and the open-label treatment design, in which raters as well as patients were aware of patients’ treatment status. Furthermore, this design cannot rule out the possibility that improvement after 12 weeks was due to the continued administration of escitalopram rather than to mCBT, or that patients who remained well during the maintenance phase would have done so absent psychotherapy. Controlled research with blind raters is needed to more definitively examine the augmentation and/or relapse prevention benefits of mCBT added to SSRI treatment in late-life GAD; a randomized, controlled trial of this strategy is underway.
In conclusion, we found preliminary evidence for the benefits of modular CBT as augmentation treatment with SSRI for geriatric GAD, particularly in the short term. Given the aging of the global population, such treatments will be increasingly needed to avoid the detrimental effects of anxiety disorders in this age group.
Key Points.
In a pilot sample of older adults with generalized anxiety disorder, adding a modular form of cognitive-behavioral therapy to escitalopram significantly reduced anxiety symptoms and pathological worry, resulting in full remission for most patients.
Some patients maintained response after all treatments were withdrawn, suggesting the possibility that behavioral therapy may have relapse prevention benefits for some patients.
Acknowledgments
The authors acknowledge Caroline Ciliberti, Michelle Costantino, M.P.A., Gretchen Diefenbach, Ph.D., Sean Engelkemeyer, Ph.D., Larry Glanz, Ph.D., Debora Goodman, Deborah Greenwald, Ph.D., Kathleen McChesney, Psy.D., Sara Parent, M.A., Andrew Petkus, M.A., Rachel Scullin, M. Katherine Shear, M.D., Grace Snell, M.S.W., Sara Snyder, Chelsea Toner, M.A., and Melinda Stanley, Ph.D. This research was supported by R34 MH080151 (Wetherell) and Forest Laboratories (Wetherell and Lenze).
References
- Allgulander C, Florea I, Huusom AK. Prevention of relapse in generalized anxiety disorder by escitalopram treatment. Int J Neuropsychopharmacol. 2006;9:495–505. doi: 10.1017/S1461145705005973. [DOI] [PubMed] [Google Scholar]
- Arce E, Simmons AN, Lovero KL, Stein MB, Paulus MP. Escitalopram effects on insula and amygdala BOLD activation during emotional processing. Psychopharmacology (Berl) 2008;196:661–672. doi: 10.1007/s00213-007-1004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association AP. Diagnostic and Statistical Manual for Mental Disorders. 4. American Psychiatric Association; Washinton DC: 2000. test revision. [Google Scholar]
- Beekman AT, Bremmer MA, Deeg DJ, et al. Anxiety disorders in later life: a report from the Longitudinal Aging Study Amsterdam. Int J Geriatr Psychiatry. 1998;13:717–726. doi: 10.1002/(sici)1099-1166(1998100)13:10<717::aid-gps857>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Beekman AT, Deeg DJ, Braam AW, Smit JH, Van Tilburg W. Consequences of major and minor depression in later life: a study of disability, well-being and service utilization. Psychol Med. 1997;27:1397–1409. doi: 10.1017/s0033291797005734. [DOI] [PubMed] [Google Scholar]
- Benitez CI, Smith K, Vasile RG, et al. Use of benzodiazepines and selective serotonin reuptake inhibitors in middle-aged and older adults with anxiety disorders: a longitudinal and prospective study. Am J Geriatr Psychiatry. 2008;16:5–13. doi: 10.1097/JGP.0b013e31815aff5c. [DOI] [PubMed] [Google Scholar]
- Blackburn IM, Moore RG. Controlled acute and follow-up trial of cognitive therapy and pharmacotherapy in out-patients with recurrent depression. Br J Psychiatry. 1997;171:328–334. doi: 10.1192/bjp.171.4.328. [DOI] [PubMed] [Google Scholar]
- Blank S, Lenze EJ, Mulsant BH, et al. Outcomes of late-life anxiety disorders during 32 weeks of citalopram treatment. J Clin Psychiatry. 2006;67:468–472. doi: 10.4088/jcp.v67n0319. [DOI] [PubMed] [Google Scholar]
- Bockting CL, Schene AH, Spinhoven P, et al. Preventing relapse/recurrence in recurrent depression with cognitive therapy: a randomized controlled trial. J Consult Clin Psychol. 2005;73:647–657. doi: 10.1037/0022-006X.73.4.647. [DOI] [PubMed] [Google Scholar]
- Butler AC, Chapman JE, Forman EM, Beck AT. The empirical status of cognitive-behavioral therapy: a review of meta-analyses. Clin Psychol Rev. 2006;26:17–31. doi: 10.1016/j.cpr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Chou KL. Age at onset of generalized anxiety disorder in older adults. Am J Geriatr Psychiatry. 2009;17:455–464. doi: 10.1097/jgp.0b013e31818f3a93. [DOI] [PubMed] [Google Scholar]
- de Beurs E, Beekman AT, van Balkom AJ, et al. Consequences of anxiety in older persons: its effect on disability, well-being and use of health services. Psychol Med. 1999;29:583–593. doi: 10.1017/s0033291799008351. [DOI] [PubMed] [Google Scholar]
- Diefenbach GJ, Stanley MA, beck JG, Novy DM, Averill P. Examination of the Hamilton Scales in assessment of anxious older adults: a replication and extension. J Psychopathol Behav Assess. 2001;23:117–124. [Google Scholar]
- Fava GA, Park SK, Sonino N. Treatment of recurrent depression. Expert Rev Neurother. 2006;6:1735–1740. doi: 10.1586/14737175.6.11.1735. [DOI] [PubMed] [Google Scholar]
- Fava GA, Ruini C, Rafanelli C. Sequential treatment of mood and anxiety disorders. J Clin Psychiatry. 2005;66:1392–1400. doi: 10.4088/jcp.v66n1108. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, editors. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID), Clinician Version: Administration Booklet. American Psychiatric Press; Washington, DC: 1996. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gray SL, LaCroix AZ, Hanlon JT, et al. Benzodiazepine use and physical disability in community-dwelling older adults. J Am Geriatr Soc. 2006;54:224–230. doi: 10.1111/j.1532-5415.2005.00571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gum AM, King-Kallimanis B, Kohn R. Prevalence of mood, anxiety, and substance-abuse disorders for older Americans in the national comorbidity survey-replication. Am J Geriatr Psychiatry. 2009;17:769–781. doi: 10.1097/JGP.0b013e3181ad4f5a. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Korte KJ, Smits JA. Is it beneficial to add pharmacotherapy to cognitive-behavioral therapy when treating anxiety disorders? A meta-analytic review. Int J Cogn Ther. 2009;2:160–175. doi: 10.1521/ijct.2009.2.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollon SD, DeRubeis RJ, Shelton RC, et al. Prevention of relapse following cognitive therapy vs medications in moderate to severe depression. Arch Gen Psychiatry. 2005;62:417–422. doi: 10.1001/archpsyc.62.4.417. [DOI] [PubMed] [Google Scholar]
- Koepke HH, Gold RL, Linden ME, Lion JR, Rickels K. Multicenter controlled study of oxazepam in anxious elderly outpatients. Psychosomatics. 1982;23:641–645. doi: 10.1016/S0033-3182(82)73363-8. [DOI] [PubMed] [Google Scholar]
- Le Roux H, Gatz M, Wetherell JL. Age at onset of generalized anxiety disorder in older adults. Am J Geriatr Psychiatry. 2005;13:23–30. doi: 10.1176/appi.ajgp.13.1.23. [DOI] [PubMed] [Google Scholar]
- Lenze EJ, Mulsant BH, Mohlman J, et al. Generalized anxiety disorder in late life: lifetime course and comorbidity with major depressive disorder. Am J Geriatr Psychiatry. 2005;13:77–80. doi: 10.1176/appi.ajgp.13.1.77. [DOI] [PubMed] [Google Scholar]
- Lenze EJ, Rollman BL, Shear MK, et al. Escitalopram for older adults with generalized anxiety disorder: a randomized controlled trial. JAMA. 2009;301:295–303. doi: 10.1001/jama.2008.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenze EJ, Wetherell JL. Bringing the bedside to the bench, and then to the community: a prospectus for intervention research in late-life anxiety disorders. Int J Geriatr Psychiatry. 2009;24:1–14. doi: 10.1002/gps.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SH, Teasdale JD. Mindfulness-based cognitive therapy for depression: replication and exploration of differential relapse prevention effects. J Consult Clin Psychol. 2004;72:31–40. doi: 10.1037/0022-006X.72.1.31. [DOI] [PubMed] [Google Scholar]
- Mantella RC, Butters MA, Amico JA, et al. Salivary cortisol is associated with diagnosis and severity of late-life generalized anxiety disorder. Psychoneuroendocrinology. 2008;33:773–781. doi: 10.1016/j.psyneuen.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Barlow DH. The dissemination and implementation of evidence-based psychological treatments. A review of current efforts. Am Psychol. 2010;65:73–84. doi: 10.1037/a0018121. [DOI] [PubMed] [Google Scholar]
- McNally RJ. Mechanisms of exposure therapy: how neuroscience can improve psychological treatments for anxiety disorders. Clin Psychol Rev. 2007;27:750–759. doi: 10.1016/j.cpr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behav Res Ther. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41:237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- Pariente A, Dartigues JF, Benichou J, et al. Benzodiazepines and injurious falls in community dwelling elders. Drugs Aging. 2008;25:61–70. doi: 10.2165/00002512-200825010-00007. [DOI] [PubMed] [Google Scholar]
- Paterniti S, Alperovitch A, Ducimetiere P, et al. Anxiety but not depression is associated with elevated blood pressure in a community group of French elderly. Psychosom Med. 1999;61:77–83. doi: 10.1097/00006842-199901000-00013. [DOI] [PubMed] [Google Scholar]
- Paykel ES, Scott J, Cornwall PL, et al. Duration of relapse prevention after cognitive therapy in residual depression: follow-up of controlled trial. Psychol Med. 2005;35:59–68. doi: 10.1017/s003329170400282x. [DOI] [PubMed] [Google Scholar]
- Porensky EK, Dew MA, Karp JF, et al. The burden of late-life generalized anxiety disorder: effects on disability, health-related quality of life, and healthcare utilization. Am J Geriatr Psychiatry. 2009;17:473–482. doi: 10.1097/jgp.0b013e31819b87b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio G, Lopez-Ibor JJ. Generalized anxiety disorder: a 40-year follow-up study. Acta Psychiatr Scand. 2007;115:372–379. doi: 10.1111/j.1600-0447.2006.00896.x. [DOI] [PubMed] [Google Scholar]
- Schoevers RA, Beekman AT, Deeg DJ, Jonker C, van Tilburg W. Comorbidity and risk-patterns of depression, generalised anxiety disorder and mixed anxiety-depression in later life: results from the AMSTEL study. Int J Geriatr Psychiatry. 2003;18:994–1001. doi: 10.1002/gps.1001. [DOI] [PubMed] [Google Scholar]
- Schuurmans J, Comijs H, Emmelkamp PM, et al. A randomized, controlled trial of the effectiveness of cognitive-behavioral therapy and sertraline versus a waitlist control group for anxiety disorders in older adults. Am J Geriatr Psychiatry. 2006;14:255–263. doi: 10.1097/01.JGP.0000196629.19634.00. [DOI] [PubMed] [Google Scholar]
- Schuurmans J, Comijs H, Emmelkamp PM, et al. Long-term effectiveness and prediction of treatment outcome in cognitive behavioral therapy and sertraline for late-life anxiety disorders. Int Psychogeriatr. 2009;21:1148–1159. doi: 10.1017/S1041610209990536. [DOI] [PubMed] [Google Scholar]
- Sinoff G, Werner P. Anxiety disorder and accompanying subjective memory loss in the elderly as a predictor of future cognitive decline. Int J Geriatr Psychiatry. 2003;18:951–959. doi: 10.1002/gps.1004. [DOI] [PubMed] [Google Scholar]
- Stanley MA, Wilson NL, Novy DM, et al. Cognitive behavior therapy for generalized anxiety disorder among older adults in primary care: a randomized clinical trial. JAMA. 2009;301:1460–1467. doi: 10.1001/jama.2009.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trollor JN, Anderson TM, Sachdev PS, Brodaty H, Andrews G. Prevalence of mental disorders in the elderly: the Australian National Mental Health and Well-Being Survey. Am J Geriatr Psychiatry. 2007;15:455–466. doi: 10.1097/JGP.0b013e3180590ba9. [DOI] [PubMed] [Google Scholar]
- Unutzer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 2002;288:2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- Wetherell JL, Ayers CR, Sorrell JT, et al. Modular psychotherapy for anxiety in older primary care patients. Am J Geriatr Psychiatry. 2009;17:483–492. doi: 10.1097/JGP.0b013e3181a31fb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherell JL, Thorp SR, Patterson TL, et al. Quality of life in geriatric generalized anxiety disorder: a preliminary investigation. J Psychiatr Res. 2004;38:305–312. doi: 10.1016/j.jpsychires.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Wilkinson P, Alder N, Juszczak E, et al. A pilot randomised controlled trial of a brief cognitive behavioural group intervention to reduce recurrence rates in late life depression. Int J Geriatr Psychiatry. 2009;24:68–75. doi: 10.1002/gps.2076. [DOI] [PubMed] [Google Scholar]
- Wright RM, Roumani YF, Boudreau R, et al. Effect of central nervous system medication use on decline in cognition in community-dwelling older adults: findings from the Health, Aging And Body Composition Study. J Am Geriatr Soc. 2009;57:243–250. doi: 10.1111/j.1532-5415.2008.02127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]