Abstract
We present data showing that structures serving as drains and catch basins for stormwater are important sources for production of the mosquito arbovirus vectors Aedes aegypti and Culex quinquefasciatus in Mérida City, México. We examined 1,761 stormwater drains – located in 45 different neighborhoods spread across the city – over dry and wet seasons from March 2012–March 2013. Of the examined stormwater drains, 262 (14.9%) held water at the time they were examined and 123 yielded mosquito immatures. In total, we collected 64,560 immatures representing nine species. The most commonly encountered species were Cx. quinquefasciatus (n=39,269) and Ae. aegypti (n=23,313). Ae. aegypti and Cx. quinquefasciatus were collected during all 11 months when we found water-filled stormwater drains, and both were found in stormwater drains located throughout Mérida City. We also present data for associations between structural characteristics of stormwater drains or water-related characteristics and the abundance of mosquito immatures. In conclusion, stormwater drains produce massive numbers of Ae. aegypti and Cx. quinquefasciatus across Mérida City, both in the wet and dry seasons, and represent non-residential development sites that should be strongly considered for inclusion in the local mosquito surveillance and control program.
Keywords: Aedes aegypti, Culex quinquefasciatus, Catch basin, Drain, Stormwater
1. Introduction
A wide range of water-holding containers are exploited by the dengue virus vector Aedes (Stegomyia) aegypti as sites for oviposition of eggs and development of immatures (Focks and Alexander 2006, WHO 2009). The most important container types for production of this mosquito differ among geographic locations but often include water storage tanks or jars, barrels/drums, buckets, tires, and small trash items (Tun-Lin et al. 2009, Arunachalam et al. 2010). There also is an increasing recognition that atypical development sites may be important contributors to the production of Ae. aegypti immatures, especially after the container types traditionally perceived as being most productive in the local environment have been controlled. Moreover, contrary to early field surveys indicating that Ae. aegypti is absent from water containing sewage (James et al. 1914), there is increasing evidence for production of immatures in water containing a high concentration of decomposing organic matter (Murrell et al. 2011, Nguyen et al. 2012).
A variety of atypical development sites have been incriminated in the production of Ae. aegypti. Some of these are structures that hold relatively clean water, such as drains on residential premises (Morrison et al. 2004, Maciel-de-Freitas et al. 2007, David et al. 2009, Glasser et al. 2011), stormwater drains/catch basins/drain sumps on streets or sidewalks or in other non-residential settings (Tinker 1974; Gonzalez and Suárez 1995; Montgomery et al. 2004; Suárez-Rubio and Suárez 2004; Marquetti et al. 2005; Morrison et al. 2006; Rey et al. 2006; Giraldo-Calderón et al. 2008; Manrique-Saide et al. 2012, 2013), service manholes/pits (Kay et al. 2000a, b), wells (Panicker et al. 1982, Lardeux 1992, Jennings et al. 1995, Tun-Lin et al. 1995, Russell et al. 1996, Nam et al. 1998, Gionar et al. 1999, Kay et al. 2002, Tsuzuki et al. 2009, Surendran et al. 2012), roof gutters (Tinker 1974; Montgomery and Ritchie 2002; Morrison et al. 2004, 2006; Glasser et al. 2011; Pilger et al. 2011) and roofs (Pilger et al. 2011). Other atypical development sites for Ae. aegypti hold water containing a high concentration of decomposing organic matter, such as septic tanks (Chinery 1970, Babu et al. 1983, Hribar et al. 2004, Barrera et al. 2008, MacKay et al. 2009, Burke et al. 2010, Somers et al. 2011) and cesspits or pit latrines (Curtis 1980, Hribar et al. 2004).
We were particularly interested in structures serving as drains and catch basins for stormwater (hereinafter referred to as stormwater drains) because they can be important habitats for Ae. aegypti (Montgomery et al. 2004, Manrique-Saide et al. 2012, 2013), especially during drier parts of the year when they provide sheltered micro-environments where standing water may persist for extended time periods. Moreover, stormwater drains may be overlooked in mosquito control campaigns that focus on residential premises. A field survey in Cairns, Australia suggested that stormwater drains contributed nearly 15% of the standing crop of Ae. aegypti pupae during the dry season (Montgomery et al. 2004), and stormwater drains are considered to be among the most important development sites for Ae. aegypti in Cali, Colombia (González and Suárez 1995, Suárez-Rubio and Suárez 2004, Giraldo-Calderón et al. 2008). It also has been determined in the laboratory that Ae. aegypti females readily oviposit in water from stormwater drains (Chen et al. 2007).
Most recently, the importance of stormwater drains for production of Ae. aegypti was highlighted in two studies focusing on single neighborhoods in Mérida City, México during the rainy and dry seasons in October/November 2011 and March 2012, respectively (Manrique-Saide et al. 2012, 2013). Herein, we expand on these studies by reporting on mosquito collections from stormwater drains located geographically more broadly throughout Mérida City and sampled across dry and rainy seasons from March 2012 - March 2013. Additionally, we examine associations between water characteristics, shading, and physical structure of the stormwater drains with the abundance of immatures of the two most commonly encountered species: Ae. aegypti and Culex quinquefasciatus.
2. Materials and methods
2.1. Area and timing of study
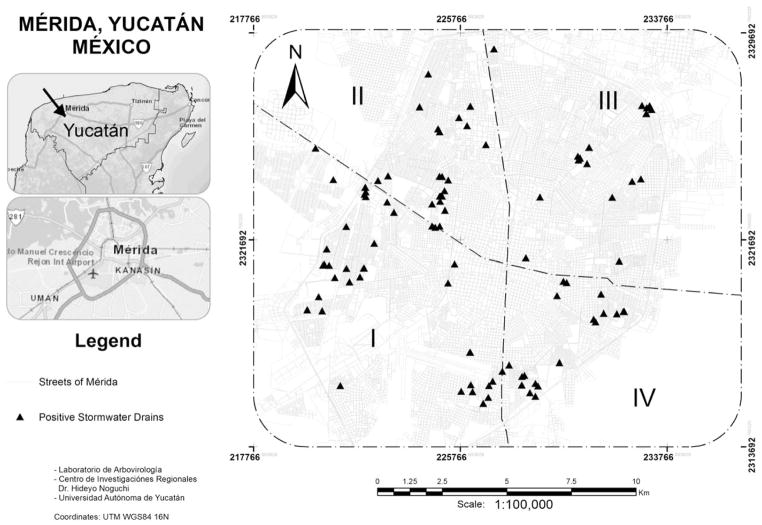
Studies were conducted within Mérida City (population ~ 800,000) in the Yucatán Peninsula of southeastern México. Mean monthly maximum temperatures in Mérida range from 29 °C in December to 34 °C in July, and peak rainfall occurs from June to September (García-Rejón et al. 2008). Collections of mosquito immatures were undertaken from March of 2012 to March of 2013, and included sampling of 1,761 individual stormwater drains located in different parts of Mérida City: I-Southwest, II-Northwest, III-Northeast, and IV-Southeast (Figure 1). Two of these stormwater drains were sampled on two different dates, resulting in a total of 1,763 sampling occasions. Examples of locations for stormwater drains are shown in Figure 2. Based on monthly totals for rainfall during the study period (Table 1), November to March were classified as dry months (range, 1 to 47 mm) and April to October as wet months (range, 52 to 235 mm).
Figure 1.
Figure 2.
Table 1.
Collections of mosquito immatures from stormwater drains and catch basins (SWDCBs) in Mérida City by month from March 2012 to March 2013.
| Month and Yeara | No. SWDCBs sampled | Monthly total rain-fall (mm)b | No. (%) of examined SWDCBs with water present | No. (%) of water-filled SWDCBs with immatures present | No. immatures collected (mean per water-filled and infested SWDCB)
|
||
|---|---|---|---|---|---|---|---|
| Ae. aegypti | Cx. quinquefasciatus | Other species | |||||
| March 2012 | 802 | 2 | 79 (9.9) | 18 (22.8) | 3,543 (196.8) | 6,770 (376.1) | 38 (2.1) |
| April 2012 | 271 | 112 | 25 (9.2) | 17 (68) | 4,241 (249.5) | 9,320 (548.2) | 80 (4.7) |
| May 2012 | 100 | 52 | 34 (34.0) | 14 (41.2) | 1,826 (130.4) | 3,139 (224.2) | 16 (1.1) |
| June 2012 | 111 | 187 | 31 (27.9) | 11 (35.5) | 2,112 (192.0) | 4,903 (445.7) | 3 (0.3) |
| July 2012 | 38 | 100 | 14 (36.8) | 13 (92.9) | 1,087 (83.6) | 4,540 (349.2) | 855 (65.8) |
| September 2012 | 18 | 235 | 15 (83.3) | 6 (40.0) | 496 (82.7) | 2,991 (498.5) | 81 (13.5) |
| October 2012 | 48 | 138 | 20 (41.7) | 16 (80.0) | 4,591 (286.9) | 3,109 (194.3) | 5 (0.3) |
| November 2012 | 87 | 16 | 16 (18.4) | 16 (100) | 4,103 (256.4) | 3,955 (247.2) | 884 (55.3) |
| December 2012 | 122 | 14 | 9 (7.4) | 8 (88.9) | 1,016 (127.0) | 355 (44.4) | 0 (0) |
| January 2013 | 79 | 47 | 0 (0) | (0) | 0 | 0 | 0 |
| February 2013 | 57 | 1 | 18 (31.6) | 3 (16.7) | 177 (59.0) | 115 (38.3) | 0 (0) |
| March 2013 | 28 | 27 | 1 (3.6) | 1 (100) | 121 (121.0) | 72 (72.0) | 16 (16.0) |
| All months | 1,761 | 262 (14.9) | 123 (47.0) | 23,313 (189.5) | 39,269 (319.3) | 1,978 (15.8) | |
No SWDCBs were sampled in August 2012.
Based on data from a weather station at the Mérida airport operated by Comisión Nacional del Agua.
2.2. Characteristics of stormwater drains
Stormwater drains in Mérida City come in a variety of shapes and sizes. The most common type is a rectangular structure, ~ 2.0 m long × 0.5 m wide × 0.7 m deep, covered with a metal grate and often equipped with a drainage pipe that can be connected to a well. Examples of different types of stormwater drains encountered in the study are shown in Figure 3.
Figure 3.
The following data were recorded in the field (between 0900 and 1400 hours) for each occasion of a stormwater drain being examined: 1) date of examination; 2) nearest adjacent street address; 3) number of larvae and pupae collected; 4) size, classified as small (~ 1.0 m length × 0.5 m width × 0.7 m depth), medium (~ 2.0 m length × 0.5 m width × 0.7 m depth) or large (~ 4.0 m length × 0.5 m width × 0.7 m depth); 5) presence or absence and orientation of drainage pipe (lacking, vertical, or horizontal); 6) presence or absence of a well connected to the drainage pipe; 7) status of the walls, classified as finished and impermeable or unfinished and permeable; and 8) presence versus absence of trash items.
For the stormwater drains that contained water, we also noted: 9) water volume (L); 10) water quality (clear versus turbid); 11) water odor (present or absent); 12) water pH (≤ 7 versus > 7); 13) water temperature (≤ 25, 26–27, 28–29, or ≥ 30 °C); 14) percentage of shade, classified as 0, 25, 50 or 100%; 15) types of organic matter present (primarily wood, grass, leaves, fruits, or flowers); and 16) status of the organic matter present (primarily intact versus decomposed). The locations of the examined stormwater drains were recorded using a global positioning system receiver (Garmin, Olathe, KS).
2.3. Collection of mosquito immatures and species identification
To collect mosquito immatures from stormwater drains, we used a long-handled (1.5 m) zooplankton net (35 × 25 cm, 100 μm mesh). A plastic dipper (capacity 0.5 liter) was used to determine the water volume (Strickman and Kittayapong 2003). In this study, we were not able to extract the entire water volume for all examined stormwater drains, leading to potential underestimation of the production of mosquito immatures from stormwater drains holding standing water in excess of 10 L.
Collected larvae and pupae were placed in plastic containers labeled with date of collection and address for the stormwater drain, and transported to the Laboratorio de Arbovirología at Universidad Autónoma de Yucatán. Larval specimens were counted, and then reared in the laboratory (28 ± 1 °C water temperature and a photoperiod of 12 hr light and 12 hr dark) to fourth instar for more accurate species identification. Pupae were allowed to emerge as adults, and the adults were then identified to species. Species identification was done using stereomicroscopes and published keys (Carpenter and LaCasse 1955, Ibañez-Bernal and Martinez-Campos 1994, Darsie and Ward 2005).
2.4. Data presentation and statistical analyses
Summary data for collection of mosquito immatures are shown in Tables 1–3. Statistical analyses were performed using IBM SPSS Statistics version 19 (IBM Corporation, Armonk, NY) and results were considered significant when P < 0.05. We first compared the abundance of mosquito immatures, separately for Ae. aegypti and Cx. quinquefasciatus, in infested stormwater drains between dry months (November–March) and wet months (April–October); because the data did not meet the assumptions of normality and homogeneity of variances, a Mann-Whitney U-test was used. Further univariate tests were based on combined data from wet and dry months, and similarly compared the abundance of mosquito immatures, separately for Ae. aegypti and Cx. quinquefasciatus, for water-filled and infested stormwater drains with different characteristics as listed in Table 3 (using the Mann-Whitney U-test or Kruskal-Wallis test, as appropriate). Finally, we used a principal component analysis to determine associations between the numbers of Ae. aegypti or Cx. quinquefasciatus immatures collected per infested stormwater drain and potentially explanatory independent variables. These included variables related to the stormwater drain itself (size, presence/absence and orientation of the drainage pipe, presence versus absence of a well, status of the walls, and shade percentage class), the water contained in the stormwater drain (volume, quality, odor, pH, and temperature), or trash or organic matter present in the water (presence/absence of trash and primary type and status of the organic matter present). Based on the outcomes, the results of the principal component analysis are presented only for Ae. aegypti.
Table 3.
Selected characteristics of examined stormwater drains and catch basins in Mérida City, for all zones and months combined, in relation to the total number of Ae. aegypti and Cx. quinquefasciatus immatures collected.
| Characteristic of stormwater drain and catch basin (no. examined for specific characteristic) | No. (%) with characteristic | No. (%)Ae. aegypti immatures collected | No. (%) Cx. quinquefasciatus immatures collected |
|---|---|---|---|
| Size (1,761)a | |||
| Small | 19 (1.1) | 344 (1.5) | 418 (1.1) |
| Medium | 1,724 (97.9) | 21,342 (91.5) | 32,957 (83.9) |
| Large | 18 (1.0) | 1,627 (7.0) | 5,894 (15.0) |
| Drainage pipe (1,761) | |||
| Missing | 739 (42.0) | 19,111 (82.0) | 20,011 (51.0) |
| Vertical | 317 (18.0) | 452 (2.0) | 3,313 (8.4) |
| Horizontal | 705 (40.0) | 3,750 (16.0) | 15,945 (40.6) |
| Well (connected to drainage pipe if this is present) (1,761) | |||
| Present | 419 (23.8) | 1,081 (4.6) | 6,839 (17.4) |
| Absent | 1,342 (76.2) | 22,232 (95.4) | 32,430 (82.6) |
| Status of walls (1,761) | |||
| Finished and impermeable | 878 (49.9) | 8,488 (36.4) | 11,929 (30.4) |
| Unfinished and permeable | 883 (50.1) | 14,825 (63.6) | 27,340 (69.6) |
| Trash items (1,761) | |||
| Present | 402 (22.8) | 15,956 (68.4) | 22,401 (57.0) |
| Absent | 1,359 (77.2) | 7,357 (31.6) | 16,868 (43.0) |
| Water quality (262)b | |||
| Clear | 54 (20.6) | 5,503 (23.6) | 8,638 (22.0) |
| Turbid | 208 (79.4) | 17,810 (76.4) | 30,631 (78.0) |
| Water odor (262)b | |||
| Present | 139 (53.0) | 13,717 (58.8) | 19,177 (48.8) |
| Absent | 123 (47.0) | 9,596 (41.2) | 20,092 (51.2) |
| Water volume (262)b | |||
| ≤10 liters | 27 (10.3) | 799 (3.4) | 994 (2.5) |
| >10 liters | 235 (89.7) | 22,514 (96.6) | 38,275 (97.5) |
| Water pH (262)b | |||
| ≤7 | 102 (38.9) | 7,671 (32.9) | 17,306 (44.1) |
| >7 | 160 (61.1) | 15,642 (67.1) | 21,963 (55.9) |
| Water temperature (262)b | |||
| ≤25 °C | 12 (4.6) | 68 (0.3) | 7,343 (18.7) |
| 26–27 °C | 68 (25.9) | 11,325 (48.6) | 11,079 (28.2) |
| 28–29 °C | 153 (58.4) | 11,063 (47.4) | 20,093 (51.2) |
| ≥30 °C | 29 (11.1) | 857 (3.7) | 754 (1.9) |
| Percentage of shade (262)b | |||
| 0% | 217 (82.8) | 18,594 (79.8) | 26,165 (66.7) |
| 25% | 22 (8.4) | 2,270 (9.7) | 4,450 (11.3) |
| 50% | 19 (7.3) | 2,164 (9.3) | 4,554 (11.6) |
| 100% | 4 (1.5) | 285 (1.2) | 4,100 (10.4) |
| Types of organic matter present (262)b | |||
| Primarily wood | 2 (0.7) | 165 (0.7) | 39 (0.1) |
| Primarily grass | 7 (2.7) | 440 (1.9) | 1,179 (3.0) |
| Primarily leaves | 197 (75.2) | 21,202 (90.9) | 33,792 (86.0 ) |
| Primarily fruits | 7 (2.7) | 385 (1.7) | 2,811 (7.2) |
| Primarily flowersUnrecognizable | 6 (2.3)43 (16.4) | 772 (3.3)349 (1.5) | 641 (1.6)806 (2.1) |
| Status of organic matter (262)b | |||
| Primarily intact | 156 (59.5) | 21,668 (92.9) | 27,433 (69.9) |
| Primarily decomposed | 106 (40.5 ) | 1,645 (7.1) | 11,836 (30.1) |
Small: ~1.0 m length × 0.5 m width × 0.7 m depth; Medium: ~ 2.0 m length × 0.5 m width × 0.7 m depth; Large: ~ 4.0 m length × 0.5 m width × 0.7 m depth.
Based on 262 stormwater drains and catch basins that held water at the time they were examined.
3. Results
3.1. Summary of mosquito collections
We examined 1,761 individual stormwater drains, of which 262 (14.9%) held water at the time they were examined and 123 yielded mosquito immatures (7.0% of all examined stormwater drains; 46.9 % of the ones holding water) (Table 1). In total, we collected 64,560 immatures representing nine species. The most commonly encountered species were Cx. quinquefasciatus (n=39,269 specimens) and Ae. aegypti (n=23,313) (Tables 1–2). Other species collectively accounted for only 3.1% of all immatures collected: they included Culex coronator (n=963), Culex lactator (n=898), Culex thriambus (n=58), Culex interrogator (n=42), Culex salinarius (n=11), Culex tarsalis (n=5), and Aedes (Ochlerotatus) taeniorhynchus (n=1).
Table 2.
Collections of immatures of Ae. aegypti and Cx. quinquefasciatus from stormwater drains and catch basins (SWDCBs) in Mérida City, by zone and neighborhood, from March 2012 to March 2013.
| Zone within Mérida City | Neighborhood | Collection month(s) | No. SWDCBs sampled | No.(%) of examined SWDCBs with water present | No.(%) of water-filled SWDCBs with immatures present | No. immatures collected
|
|||
|---|---|---|---|---|---|---|---|---|---|
| Ae. aegypti | Cx. quinquefasciatus | ||||||||
|
| |||||||||
| Larvae | Pupae | Larvae | Pupae | ||||||
| I | San Antonio Xluch | April 2012 | 107 | 3 (2.8) | 3 (100) | 0 | 25 | 4,993 | 1 |
| I | Bosques Mulsay | Jan 2013 | 5 | 0 (0) | ----- | 0 | 0 | 0 | 0 |
| I | Bosques del Poniente | Jan 2013 | 10 | 0 (0) | ----- | 0 | 0 | 0 | 0 |
| I | Castilla Cámara | Oct 2012 | 7 | 7 (100) | 7 (100) | 877 | 98 | 420 | 45 |
| I | Ciudad Caucel | Dec 2012 | 1 | 1 (100) | 1 (100) | 86 | 10 | 0 | 0 |
| I | Centro | March 2012; Feb 2013 | 2 | 2 (100) | 2 (100) | 83 | 15 | 0 | 0 |
| I | Jardines Yucalpetén | Dec 2012; Jan 2013 | 16 | 1 (6.3) | 1 (100) | 241 | 11 | 88 | 2 |
| I | Juan Pablo II | March–May, Oct, Dec 2012; Feb 2013 | 426 | 49 (11.5) | 14 (28.6) | 617 | 143 | 2,335 | 384 |
| I | Mulsay | May 2012 | 5 | 5 (100) | 1 (20.0) | 970 | 280 | 0 | 0 |
| I | Nora Quintana | June, Nov–Dec 2012; Jan 2013 | 38 | 12 (31.6) | 9 (75.0) | 767 | 203 | 3,732 | 279 |
| I | El Roble | June 2012 | 5 | 5 (100) | 1 (20.0) | 86 | 10 | 7 | 7 |
| I | San José Tecóh | April 2012 | 45 | 6 (13.3) | 5 (83.3) | 84 | 10 | 1,327 | 146 |
| I | Tixcacal Opichén | June, Sept 2012; Jan 2013 | 109 | 16 (14.7) | 3 (18.8) | 279 | 94 | 550 | 97 |
| I | Yucalpetén | Jan 2012; Dec 2013 | 14 | 1 (7.1) | 1 (100) | 62 | 12 | 26 | 3 |
| I | All, Zone I | 790 | 108 (13.7) | 48 (44.4) | 4,152 | 911 | 13,478 | 964 | |
| II | Chuburná de Hidalgo | March 2012 | 125 | 9 (7.2) | 3 (33.3) | 72 | 70 | 695 | 255 |
| II | Fovisste | July 2012 | 1 | 1 (100) | 1 (100) | 102 | 7 | 89 | 0 |
| II | Francisco de Montejo | March 2012 | 119 | 8 (6.7) | 1 (12.5) | 18 | 37 | 187 | 0 |
| II | Inalámbrica | Nov 2012 | 2 | 2 (100) | 2 (100) | 59 | 9 | 91 | 9 |
| II | Paseo de las Fuentes | May, Nov 2012 | 6 | 4 (66.7) | 3 (75.0) | 153 | 16 | 1,134 | 113 |
| II | Paseo de Pensiones | July 2012 | 1 | 1 (100) | 0 (0) | 0 | 0 | 0 | 0 |
| II | Pensiones | July 2012 | 2 | 2 (100) | 2 (100) | 28 | 327 | 0 | 0 |
| II | El Porvenir | July, Sept 2012 | 6 | 3 (50.0) | 3 (100) | 105 | 27 | 217 | 214 |
| II | Puesta del Sol | July 2012 | 9 | 2 (22.2) | 2 (100) | 84 | 117 | 67 | 47 |
| II | Roma | Sept, Nov 2012 | 8 | 8 (100) | 5 (62.5) | 380 | 102 | 2,771 | 166 |
| II | San Pedro Uxmal | March 2012 | 154 | 8 (5.2) | 3 (37.5) | 1 | 0 | 1,078 | 12 |
| II | San Damián | July 2012 | 1 | 1 (100) | 1 (100) | 90 | 15 | 1,827 | 0 |
| II | All, Zone II | 434 | 49 (11.3) | 26 (53.1) | 1,092 | 727 | 8,156 | 816 | |
| III | Polígono 108 | March, Nov 2012 | 59 | 7 (11.9) | 1 (14.3) | 48 | 0 | 54 | 0 |
| III | Las Águilas | March–July 2012 | 18 | 9 (50.0) | 6 (66.7) | 223 | 82 | 1,541 | 213 |
| III | Brisas | July 2012 | 5 | 1 (20.0) | 1 (100) | 0 | 9 | 5 | 49 |
| III | Centro | May 2012 | 2 | 2 (100) | 2 (100) | 2 | 0 | 552 | 94 |
| III | Díaz Ordaz | March–Oct, Nov 2012 | 72 | 17 (23.6) | 14 (82.4) | 8,552 | 1,131 | 5,464 | 794 |
| III | Dzozil Norte | Nov 2012 | 1 | 1 (100) | 1 (100) | 165 | 61 | 136 | 19 |
| III | Itzimná | Feb–March 2013 | 32 | 1 (3.1) | 1 (100) | 88 | 33 | 65 | 7 |
| III | Jardines de Mérida | Nov 2012 | 42 | 0 (0) | ----- | 0 | 0 | 0 | 0 |
| III | Leandro Valle | Nov 2012 | 14 | 1 (7.1) | 1 (100) | 76 | 15 | 0 | 0 |
| III | Pacabtún | March 2012 | 50 | 13 (26.0) | 1 (7.7) | 63 | 6 | 21 | 4 |
| III | All, Zone III | 295 | 52 (17.6) | 28 (53.8) | 9,217 | 1,337 | 7,838 | 1,180 | |
| IV | Cinco Colonias | Oct 2012 | 5 | 1 (20.0) | 1 (100) | 34 | 2 | 65 | 7 |
| IV | Fraccionamiento del Sur | April–July 2012 | 64 | 5 (7.8) | 4 (80.0) | 6 | 9 | 2,437 | 124 |
| IV | Miraflores | April 2012 | 37 | 3 (8.1) | 3 (100) | 131 | 1,103 | 621 | 46 |
| IV | Salvador Alvarado Sur | Oct 2012 | 7 | 1 (14.3) | 1 (100) | 15 | 2 | 158 | 27 |
| IV | Vergela | April–June 2012; Feb 2013 | 81 | 32 (39.5) | 7 (21.9) | 3,414 | 170 | 1,548 | 386 |
| IV | San Nicolás del Sur | Jan 2013 | 4 | 0 (0) | ----- | 0 | 0 | 0 | 0 |
| IV | Serapio Rendón | May, Oct 2012; Jan 2013 | 9 | 6 (66.7) | 2 (33.3) | 13 | 100 | 380 | 680 |
| IV | Unidad Morelos | Jan 2013 | 4 | 0 (0) | ----- | 0 | 0 | 0 | 0 |
| IV | Villa Magna | April, June, Oct 2012 | 31 | 5 (16.1) | 3 (60.0) | 328 | 550 | 46 | 312 |
| IV | All, Zone IV | 242 | 53 (21.9) | 21 (39.6) | 3,941 | 1,936 | 5,255 | 1,582 | |
| I–IV | All, Zones 1–IV | 1,761 | 262 (14.9) | 123 (46.9) | 18,402 | 4,911 | 34,727 | 4,542 | |
Including Vergel, I, II, III, and IV.
Both Ae. aegypti and Cx. quinquefasciatus were collected during all 11 months when we found water-filled stormwater drains (Table 1). Based on data for nine months during which at least 10 water-filled stormwater drains were examined in each month, the average number of immatures collected per examined stormwater drain that contained water ranged from 9.8 (February 2013) to 256.4 (November 2012) for Ae. aegypti, and from 6.4 (February 2013) to 372.8 (April 2012) for Cx. quinquefasciatus. The corresponding averages for only those stormwater drains that had mosquito immatures present ranged from 59.0 (February 2013) to 286.9 (October 2012) for Ae. aegypti, and from 38.3 (February 2013) to 548.2 (April 2012) for Cx. quinquefasciatus (Table 1). Over the full study period (March 2012 to March 2013), the average numbers of immatures collected per water-filled, and water-filled and infested, stormwater drain were 89.0 and 189.5, respectively, for Ae. aegypti, and 149.9 and 319.3, respectively, for Cx. quinquefasciatus (Table 1).
The examined stormwater drains were spread across 45 different neighborhoods (Colonias), and >240 (range, 242–792) stormwater drains were examined within each of the four zones of Mérida City (Table 2, Figure 1). Among the four zones, the percentages of water-filled stormwater drains out of those examined ranged from 11.3–21.9%, and the percentages of water-filled stormwater drains with immatures present ranged from 39.6–53.8% (Table 2). Out of 35 neighborhoods within each of which at least five stormwater drains were examined, Ae. aegypti immatures were collected from 32 of the neighborhoods (91.4%) and Cx. quinquefasciatus immatures from 30 of the neighborhoods (85.7%) (Table 2). Statistical analysis revealed no significant difference across zones for the abundance of immatures in infested stormwater drains for either Ae. aegypti (X2 = 6.626, df = 3, P = 0.085) or Cx. quinquefasciatus (X2 = 3.823, df = 3, P = 0.281)
3.2. Characteristics of stormwater drains
Data for selected characteristics of examined stormwater drains, for all zones and months combined, in relation to the total number of Ae. aegypti and Cx. quinquefasciatus immatures collected are shown in Table 3. Of the examined stormwater drains, 42.0% (739/1,761) lacked any drainage pipe and only 23.8% (419/1,761) of those with a drainage pipe present had it connected to a well to facilitate water being removed from the catch basin. For those stormwater drains that contained water when they were examined, the majority (79.4%) held turbid water, the water volume most often (89.7%) exceeded 10 liters, and most (82.8%) had no shade. Water temperatures most commonly (58.4%) were in the 28–29 °C range. The water commonly held leaves (75.2%) or other organic matter, and this often (40.5%) was decomposed.
3.3. Associations of mosquito abundance with rainfall and characteristics of stormwater drains
We found no significant difference among dry and wet months for the abundance of immatures in infested stormwater drains, either for Ae. aegypti (Z = −0.318, P = 0.750) or for Cx. quinquefasciatus (Z = −0.242, P = 0.809). Further analyses therefore were based on data for infested stormwater drains from dry and wet months combined.
Without regard to the number of the examined stormwater drains with a given specific characteristic, such as trash being present or absent, the majority of all recovered Ae. aegypti immatures came from those stormwater drains with the following specific characteristics: medium size (91.5%); lack of a drainage pipe (82.0%); absence of a well to aid in drainage (95.4%); unfinished walls (63.6%); trash items present (68.4%); turbid water (76.4%); water volume >10 liters (96.6%); water pH >7 (67.1%); water temperature in the 26–29 °C range (96.0%); no shade (79.8%); presence of organic matter in the form of leaves (90.9%); and primarily intact organic matter (92.9%) (Table 3). Trends were similar but often less pronounced for Cx. quinquefasciatus immatures (Table 3).
Notable discrepancies for the number of Ae. aegypti immatures collected, in relation to the number of stormwater drains with a given characteristic, include overrepresentation of immatures from stormwater drains that: 1) lacked a drainage pipe (accounting for 42.0% of the examined stormwater drains versus 82.0% of total immatures recovered); 2) had trash present (accounting for 22.8% of examined stormwater drains versus 68.4% of total immatures); and 3) contained primarily intact organic matter (accounting for 59.5% of the water-filled stormwater drains versus 92.9% of total immatures) (Table 3). The only discrepancy of a similar magnitude for Cx. quinquefasciatus immatures occurred for stormwater drains with trash present, which accounted for 22.8% of examined stormwater drains but produced 57.0% of the total immatures recovered (Table 3).
Univariate tests for the variables listed in Table 3 demonstrated significant differences for the abundance of Ae. aegypti immatures in infested stormwater drains for the following variables: 1) water temperature (X2=22.852, df = 3, P < 0.001), with elevated abundance for the 26–27 °C range; 2) well (Z = −2.146, P = 0.032), with elevated abundance when a well was absent; and 3) status of organic matter (Z = −3.303, P = 0.001), with elevated abundance for primarily intact organic matter. The corresponding tests for Cx. quinquefasciatus immatures showed significant differences in abundance in infested stormwater drains for the following variables: 1) water temperature (X2=16.696, df = 3, P = 0.001), again with elevated abundance for the 26–27 °C range; and 2) size (X2=8.413, df = 2, P = 0.015), with elevated abundance for medium-sized stormwater drains.
The principal component analysis produced five factors that collectively explained 61.2% of the variation in abundance of Ae. aegypti immatures in infested stormwater drains, with a significant Bartlett sphericity (P < 0.001) (Table 4). Factors included associations with water characteristics (PC1: water odor, pH, and quality, and presence/absence of trash in the water; PC4: water volume and temperature), organic matter (PC2: primary type and status of organic matter in the water), and the structural components of the stormwater drain (PC3: presence/absence and orientation of drainage pipe, presence/absence of a well; PC5: status of the walls).
Table 4.
Rotated factor pattern scores from 13 principal components relating to characteristics of stormwater drains and catch basins to explain the number of Ae. aegypti immatures in infested stormwater drains and catch basins.
| Variable | PC1a | PC2a | PC3a | PC4a | PC5a |
|---|---|---|---|---|---|
| Water odor classification | 0.758b | 0.046 | −0.002 | 0.071 | −0.148 |
| Water pH | −0.680b | −0.129 | 0.004 | 0.020 | −0.324 |
| Water quality classification | −0.565b | 0.111 | 0.026 | −0.281 | 0.427 |
| Presence/absence of trash | 0.515b | −0.120 | 0.305 | 0.010 | 0.457 |
| Presence/absence of shade | 0.484 | −0.171 | −0.181 | 0.070 | −0.012 |
| Types of organic matter present | −0.137 | 0.882b | −0.004 | −0.076 | −0.044 |
| Status of organic matter | 0.051 | 0.815b | −0.119 | 0.143 | 0.194 |
| Orientation of drainage pipe | −0.213 | −0.053 | 0.793b | −0.101 | −0.147 |
| Presence/absence of well | 0.063 | −0.183 | 0.742b | 0.298 | 0.059 |
| Size | 0.045 | −0.074 | 0.495 | −0.306 | 0.079 |
| Water temperature | −0.021 | −0.074 | −0.088 | 0.820b | 0.040 |
| Water volume | 0.251 | 0.149 | 0.133 | 0.612b | 0.080 |
| Status of walls | −0.035 | 0.126 | −0.099 | 0.142 | 0.813b |
The percentage variation for each component is: PC1 (17.3%), PC2 (14.4%), PC3 (12.2%), PC4 (9.1%), and PC5 (8.1%);
Scores >0.5.
4. Discussion
In agreement with other studies from Latin America (Tinker 1974; González and Suárez 1995; Suárez-Rubio and Suárez 2004; Marquetti et al. 2005; Morrison et al. 2006; Giraldo-Calderón et al. 2008; Manrique-Saide et al. 2012, 2013), our results indicate that stormwater drains are important sources for production of both Ae. aegypti and Cx. quinquefasciatus in Mérida City. Previous studies in Mérida City, limited geographically to single neighborhoods, reported collection from stormwater drains of substantial numbers of immatures of Ae. aegypti and Cx. quinquefasciatus during the rainy season (Manrique-Saide et al. 2012) and of emerging Ae. aegypti adults during the dry season (Manrique-Saide et al. 2013). We expand on these findings by demonstrating that stormwater drains are productive sources for both Ae. aegypti and Cx. quinquefasciatus throughout Mérida City during both wet and dry months (Tables 1–2, Figure 1). Moreover, it has been estimated that Mérida City harbors >20,000 stormwater drains (Manrique-Saide et al. 2012). Consequently, this type of non-residential development site should be strongly considered for inclusion in the local mosquito surveillance and control program.
We demonstrate that stormwater drains in Mérida City contain standing water not only in wet months – 23.7% of examined stormwater drains were found to hold water during April to October 2012 when monthly rainfall consistently exceeded 50 mm – but also in dry months as 10.5% of examined stormwater drains were found to hold water during March 2012 and November 2012 to March 2013 when monthly rainfall typically was <20 mm and never exceeded 50 mm. We speculate that the commonplace structures represented by stormwater drains may serve as particularly important development sites for Ae. aegypti immatures in Mérida City during the dry season, when this mosquito is least abundant and potentially can be severely impacted by interventions targeted to key development sites. Moreover, as suggested previously by Barrera et al. (2008), atypical development sites – such as septic tanks or stormwater drains – may contribute to keep Ae. aegypti populations at high enough levels for dengue virus transmission to persist even during dry months.
As noted above, the stormwater drains yielded large numbers of Cx. quinquefasciatus. This commonly bird-feeding mosquito also is a nuisance biter of humans (Elizondo-Quiroga et al. 2006; García-Rejón et al. 2008, 2010). It is capable of transmitting several arboviruses known to cause human disease, including West Nile virus (Turell et al. 2005). West Nile virus has been detected from Cx. quinquefasciatus in Nuevo Leon State in northern México (Elizondo-Quiroga et al. 2005) but extensive efforts in Mérida City and other locations in Yucatán State have failed to detect West Nile virus in this mosquito species (Farfan-Ale et al. 2009, 2010). However, recent studies from Yucatán State revealed the presence in Cx. quinquefasciatus of insect-only viruses as well as other viruses with unknown pathogenicity to humans, including the T’Ho virus (Farfan-Ale et al. 2009, 2010; Saiyasombat et al. 2010).
In addition to occurring in large numbers throughout Mérida City, stormwater drains have several features that make them potentially very productive sources for Ae. aegypti and Cx. quinquefasciatus. The drains and catch basins provide a subterranean microhabitat that is cooled by the ground itself and also provides partial protection from solar insolation, thus reducing the rate of evaporation of standing water. Some structural features also may promote standing water, such as drainage pipes being absent or not being connected to well (to facilitate removal of water) when they are present. Another important consideration is that stormwater drains receive water not only from rainfall in the wet season but also year-around from run-off resulting from people washing their terraces or vehicles, or shop-keepers hosing down the sidewalks outside their shops. Moreover, proper drainage can be prevented by accumulations of trash or non-decomposed organic matter within the stormwater drains. Several of the aforementioned features (including the lack of a drainage pipe, lack of a well connected to the drainage pipe, and presence of trash or intact organic matter) were associated with elevated numbers of mosquito immatures in our study.
We observed accumulations of organic matter in 87% of examined stormwater drains that held water. Decomposing organic matter can provide nutrients for mosquito larvae and also may provide cues for females searching for suitable oviposition sites (Barrera et al. 2006, Murrell et al. 2011). Previous studies also have shown that gravid females follow visual and olfactory cues when choosing oviposition substrates, and are guided by chemical cues in and physical properties of the water when deciding on whether or not to deposit eggs (Gjullin et al. 1965, Muir 1988, Bentley and Day 1989, Millar et al. 1994, Torres- Estrada et al. 2001, Chen et al. 2007). Such considerations may help to explain why some stormwater drains may be more attractive oviposition sites than others.
The study had some notable weaknesses. Firstly, we were not able to sample the entire water volume for all examined stormwater drains, leading to underestimation of the production of mosquito immatures from stormwater drains holding standing water in excess of 10 L. Secondly, although all four zones were sampled in both dry and wet months, we were not able to conduct sampling in a fully temporally synchronized scheme across the zones. Thirdly, because of unknown mortality of immatures in stormwater drains, collection of emerging adults, rather than immatures, would have provided data of more direct relevance to assess the risk for human-biting. Further studies are needed to quantify the relative production of Ae. aegypti from stormwater drains and other non-residential mosquito production sites – such as vacant lots – versus residential premises in Mérida City, including data not only for average mosquito production in containers found in these respective environments (based on repeated sampling of individual sites across wet and dry seasons) but also for the numbers of stormwater drains, vacant lots and residential premises present within the study area.
Acknowledgments
We thank Maria Puc-Tinal, Victor Rivero-Osorno, Mildred López-Uribe, Genny López-Uribe, and Carlos Coba-Tun of Universidad Autónoma de Yucatán for technical assistance. The study was supported by the National Institutes of Health/National Institute of Allergy and Infectious Diseases (International Collaborations in Infectious Disease Research Program U01-AI-088647). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIAID or NIH.
References
- Arunachalam N, Tana S, Espino F, Kittayapong P, Abeyewickreme W, Wai KT, Tyagi BK, Kroeger A, Sommerfeld J, Petzold M. Eco-bio-social determinants of dengue vector breeding: A multicountry study in urban and periurban Asia. Bull WHO. 2010;88:173–184. doi: 10.2471/BLT.09.067892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu CJ, Panicker KN, Das PK. Breeding of Aedes aegypti in closed septic tanks. Indian J Med Res. 1983;77:637. [PubMed] [Google Scholar]
- Barrera R, Amador M, Diaz A, Smith J, Munoz-Jordan JL, Rosario Y. Unusual productivity of Aedes aegypti in septic tanks and its implications for dengue control. Med Vet Entomol. 2008;22:62–69. doi: 10.1111/j.1365-2915.2008.00720.x. [DOI] [PubMed] [Google Scholar]
- Bentley MD, Day JF. Chemical ecology and behavioral aspects of mosquito oviposition. Annu Rev Entomol. 1989;34:401–421. doi: 10.1146/annurev.en.34.010189.002153. [DOI] [PubMed] [Google Scholar]
- Burke R, Barrera R, Lewis M, Kluchinsky T, Claborn D. Septic tanks as larval habitats for the mosquitoes Aedes aegypti and Culex quinquefasciatus in Playa-Playita, Puerto Rico. Med Vet Entomol. 2010;24:117–123. doi: 10.1111/j.1365-2915.2010.00864.x. [DOI] [PubMed] [Google Scholar]
- Carpenter SJ, LaCasse WJ. Mosquitoes of North America (North of Mexico) University of California Press; Berkeley, CA: 1955. [Google Scholar]
- Chen CD, Nazni WA, Seleena B, Moo JY, Azizah M, Lee HL. Comparative oviposition preferences of Aedes (Stegomyia) aegypti (L.) to water from storm water drains and seasoned tap water. Dengue Bull. 2007;31:124–130. [Google Scholar]
- Chinery WA. A survey of mosquito breeding in Accra, Ghana during a two year period (Sept. 1964 – Aug 1966) of larval mosquito control III The breeding of Aedes (Stegomyia) aegypti, Linnaeus, in Accra. Ghana Med J. 1970;9:197–200. [Google Scholar]
- Curtis CF. Insect traps for pit latrines. Mosq News. 1980;40:626–628. [Google Scholar]
- Darsie RF, Jr, Ward RA. Identification and Geographical Distribution of the Mosquitoes of North America, North of Mexico. Univeristy Press of Florida; Gainesville, FL: 2005. [Google Scholar]
- David MR, Lourenço-de-Oliveira R, Maciel-de-Freitas R. Container productivity, daily survival rates and dispersal of Aedes aegypti mosquitoes in a high income dengue epidemic neighbourhood of Rio de Janeiro: presumed influence of differential urban structure on mosquito biology. Mem Inst Oswaldo Cruz. 2009;104:927–932. doi: 10.1590/s0074-02762009000600019. [DOI] [PubMed] [Google Scholar]
- Elizondo-Quiroga D, Davis CT, Fernandez-Salas I, Escobar-Lopez R, Olmos DV, Gastalum LCS, Acosta MA, Elizondo-Quiroga A, Gonzalez-Rojas JI, Cordero JFC, Guzman H, da Rosa AT, Blitvich BJ, Barrett ADT, Beaty BJ, Tesh RB. West Nile virus isolation in human and mosquitoes, Mexico. Emerg Infect Dis. 2005;11:1449–1452. doi: 10.3201/eid1109.050121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizondo-Quiroga A, Flores-Suarez A, Elizondo-Quiroga D, Ponce-Garcia G, Blitvich BJ, Contreras-Cordero JF, Gonzalez-Rojas JI, Mercado-Hernandez R, Beaty BJ, Fernandez-Salas I. Gonotrophic cycle and survivorship of Culex quinquefasciatus (Diptera: Culicidae) using sticky ovitraps in Monterrey, northeastern Mexico. J Am Mosq Contr Assoc. 2006;22:10–14. doi: 10.2987/8756-971X(2006)22[10:GCASOC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Farfan-Ale JA, Loroño-Pino MA, García-Rejón JE, Hovav E, Powers AM, Lin M, Dorman KS, Platt KB, Bartholomay LC, Soto V, Beaty BJ, Lanciotti RS, Blitvich BJ. Detection of RNA from a novel West Nile-like virus and high prevalence of an insect-specific flavivirus in mosquitoes in the Yucatan Peninsula of Mexico. Am J Trop Med Hyg. 2009;80:85–95. [PMC free article] [PubMed] [Google Scholar]
- Farfan-Ale JA, Loroño-Pino MA, García-Rejón JE, Soto V, Lin M, Staley M, Dorman KS, Bartholomay LC, Hovav E, Blitvich BJ. Detection of flaviviruses and orthobunyaviruses in mosquitoes in the Yucatan Peninsula of Mexico in 2008. Vector-Borne Zoonotic Dis. 2010;10:777–783. doi: 10.1089/vbz.2009.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focks DA, Alexander N. Findings and recommendations. World Health Organization; Geneva, Switzerland: 2006. Multicountry study of Aedes aegypti pupal productivity survey methodology. [Google Scholar]
- García-Rejón JE, Loroño-Pino MA, Farfán-Ale JA, Flores-Flores L, Rosado-Paredes ED, Rivero-Cardenas N, Nájera-Vázquez R, Gomez-Carro S, Lira-Zumbardo V, Gonzalez-Martinez P, Lozano-Fuentes S, Elizondo-Quiroga E, Beaty BJ, Eisen L. Dengue virus-infected Aedes aegypti in the home environment. Am J Trop Med Hyg. 2008;79:940–950. [PubMed] [Google Scholar]
- García-Rejón JE, Blitvich BJ, Farfán-Ale JA, Loroño-Pino MA, Chim WAC, Flores-Flores LF, Rosado-Paredes E, Baak-Baak C, Perez-Mutul J, Suarez-Solis V, Fernandez-Salas I, Beaty BJ. Host-feeding preference of the mosquito, Culex quinquefasciatus, in Yucatan State, Mexico. J Insect Sci. 2010;10:32. doi: 10.1673/031.010.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gionar YR, Rusmiarto S, Susapto D, Bangs MJ. Use of a funnel trap for collecting immature Aedes aegypti and copepods from deep wells in Yogyakarta, Indonesia. J Am Mosq Contr Assoc. 1999;15:576–580. [PubMed] [Google Scholar]
- Giraldo-Calderón GI, Pérez M, Morales CA, Ocampo CB. Evaluación del triflumurón y la mezcla de Bacillus thuringiensis más Bacillus sphaericus para el control de las formas inmaduras de Aedes aegypti y Culex quinquefasciatus en sumideros en Cali, Colombia. Biomédica. 2008;28:224–233. [PubMed] [Google Scholar]
- Gjullin CM, Johnsen JO, Plapp FW., Jr The effect of odors released by various waters on the oviposition sites selected by two species of Culex. Mosq News. 1965;25:268–271. [Google Scholar]
- Glasser CM, Arduino MB, Barbosa GL, Ciaravolo RMC, Domingos MF, Oliveira CD, Pereira M, Silva M, Trevisan AMY. Comportamento de formas imaturas de Aedes aegypti, no litoral do Estado de São Paulo. Rev Soc Bras Med Trop. 2011;44:349–355. doi: 10.1590/s0037-86822011005000042. [DOI] [PubMed] [Google Scholar]
- González R, Suárez MF. Sewers: the principal Aedes aegypti breeding sites in Cali, Colombia. Am J Trop Med Hyg. 1995;53 (Suppl 2):160. [Google Scholar]
- Hribar LJ, Vlach JJ, DeMay DJ, James SS, Fahey JS, Fussell EM. Mosquito larvae (Culicidae) and other Diptera associated with containers, storm drains, and sewage treatment plants in the Florida Keys, Monroe County. Florida Florida Entomol. 2004;87:199–203. [Google Scholar]
- Ibañez-Bernal S, Martinez-Campos C. Clave para la identificación de larvas de mosquitos comunes en las áreas urbanas y suburbanas de la República Mexicana (Díptera: Culicidae) Folia Entomol Mex. 1994;92:43–73. [Google Scholar]
- James SP, da Silva WT, Arndt EW. Report on a Mosquito Survey of Colombo and the Practicability of Reducing Stegomyia and Some Other Mosquitoes in that Seaport. Government Record Office; Colombo, Ceylon: 1914. [Google Scholar]
- Jennings CD, Phommasack B, Sourignadeth B, Kay BH. Aedes aegypti control in the Lao People’s Democratic Republic, with reference to copepods. Am J Trop Med Hyg. 1995;53:324–330. doi: 10.4269/ajtmh.1995.53.324. [DOI] [PubMed] [Google Scholar]
- Kay BH, Ryan PA, Russell BM, Holt JS, Lyons SA, Foley PN. The importance of subterranean mosquito habitat to arbovirus vector control strategies in North Queensland, Australia. J Med Entomol. 2000a;37:846–853. doi: 10.1603/0022-2585-37.6.846. [DOI] [PubMed] [Google Scholar]
- Kay BH, Sutton KA, Russell BM. A sticky entry-exit trap for sampling mosquitoes in subterranean habitats. J Am Mosq Contr Assoc. 2000b;16:262–265. [PubMed] [Google Scholar]
- Kay BH, Ryan PA, Lyons SA, Foley PN, Pandeya N, Purdie D. Winter intervention against Aedes aegypti (Diptera: Culicidae) larvae in subterranean habitats slows surface recolonization in summer. J Med Entomol. 2002;39:356–361. doi: 10.1603/0022-2585-39.2.356. [DOI] [PubMed] [Google Scholar]
- Lardeux FJR. Biological control of Culicidae with the copepod Mesocyclops aspericornis and larvivorous fish (Poeciliidae) in a village of French Polynesia. Med Vet Entomol. 1992;6:9–15. doi: 10.1111/j.1365-2915.1992.tb00028.x. [DOI] [PubMed] [Google Scholar]
- Maciel-de-Freitas R, Marques WA, Peres RC, Cunha SP, Lourenco-de-Oliveira R. Variation in Aedes aegypti (Diptera: Culicidae) container productivity in a slum and a suburban district of Rio de Janeiro during dry and wet seasons. Mem Inst Oswaldo Cruz. 2007;102:489–496. doi: 10.1590/s0074-02762007005000056. [DOI] [PubMed] [Google Scholar]
- MacKay AJ, Amador M, Diaz A, Smith J, Barrera R. Dynamics of Aedes aegypti and Culex quinquefasciatus in septic tanks. J Am Mosq Contr Assoc. 2009;25:409–416. doi: 10.2987/09-5888.1. [DOI] [PubMed] [Google Scholar]
- Manrique-Saide P, Uc V, Prado C, Carmona C, Vadillo J, Chan R, Dzib-Florez S, Che-Mendoza A, Barrera-Perez M, Sanchez EC, Arredondo-Jimenez JI. Storm sewers as larval habitats for Aedes aegypti and Culex spp. in a neighborhood of Merida, Mexico. J Am Mosq Contr Assoc. 2012;28:255–257. doi: 10.2987/12-6244R.1. [DOI] [PubMed] [Google Scholar]
- Manrique-Saide P, Arisqueta-Chable C, Geded-Moreno E, Herrera-Bojorquez J, Uc V, Chable-Santos J, Che-Mendoza A, Sanchez EC, Arredondo-Jimenez JI, Medina-Barreiro A. An assessment of the importance of subsurface catch basins for Aedes aegypti adult production during the dry season in a neighborhood of Merida, Mexico. J Am Mosq Contr Assoc. 2013;29:164–167. doi: 10.2987/12-6320R.1. [DOI] [PubMed] [Google Scholar]
- Marquetti MC, Suárez S, Bisset J, Leyva M. Reporte de hábitats utilizados por Aedes aegypti en Ciudad de La Habana, Cuba. Rev Cubana Med Trop. 2005;57:159–161. [PubMed] [Google Scholar]
- Millar JG, Chaney JD, Beehler JW, Mulla MS. Interaction of the Culex quinquefasciatus egg raft pheromone with a natural chemical associated with oviposition sites. J Am Mosq Contr Assoc. 1994;10:374–379. [PubMed] [Google Scholar]
- Montgomery BL, Ritchie SA. Roof gutters: a key container for Aedes aegypti and Ochlerotatus notoscriptus (Diptera: Culicidae) in Australia. Am J Trop Med Hyg. 2002;67:244–246. doi: 10.4269/ajtmh.2002.67.244. [DOI] [PubMed] [Google Scholar]
- Montgomery BL, Ritchie SA, Hart AJ, Long SA, Walsh ID. Subsoil drain sumps are a key container for Aedes aegypti in Cairns, Australia. J Am Mosq Contr Assoc. 2004;20:365–369. [PubMed] [Google Scholar]
- Morrison AC, Gray K, Getis A, Astete H, Sihuincha M, Focks D, Watts D, Stancil JD, Olson JG, Blair P, Scott TW. Temporal and geographic patterns of Aedes aegypti (Diptera: Culicidae) production in Iquitos, Peru. J Med Entomol. 2004;41:1123–1142. doi: 10.1603/0022-2585-41.6.1123. [DOI] [PubMed] [Google Scholar]
- Morrison AC, Sihuincha M, Stancil JD, Zamora E, Astete H, Olson JG, Vidal-Ore C, Scott TW. Aedes aegypti (Diptera: Culicidae) production from non-residential sites in the Amazonian city of Iquitos, Peru. Ann Trop Med Parasitol. 2006;100(Suppl 1):S73–S86. doi: 10.1179/136485906X105534. [DOI] [PubMed] [Google Scholar]
- Muir DA. Anopheline mosquitoes: vector reproduction, life-cycle and biotope. In: Wernsdorfer WH, McGregor I, editors. Malaria: Principles and Practice of Malariology. Churchill-Livingstone; London, U.K: 1988. pp. 431–451. [Google Scholar]
- Murrell EG, Damal K, Lounibos LP, Juliano SA. Distributions of competing container mosquitoes depend on detritus types, nutrient ratios, and food availability. Ann Entomol Soc Am. 2011;104:688–698. doi: 10.1603/AN10158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam VS, Yen NT, Kay BH, Marten GG, Reid JW. Eradication of Aedes aegypti from a village in Vietnam, using copepods and community participation. Am J Trop Med Hyg. 1998;59:657–660. doi: 10.4269/ajtmh.1998.59.657. [DOI] [PubMed] [Google Scholar]
- Nguyen AT, Williams–Newkirk AJ, Kitron U, Chaves L. Seasonal weather, nutrients, and conspecific presence impacts on the southern house mosquito oviposition dynamics in combined sewage overflows. J Med Entomol. 2012;49:1328–1338. doi: 10.1603/me12090. [DOI] [PubMed] [Google Scholar]
- Panicker KN, Bai MG, Kalyanasundaram M. Well breeding behaviour of Aedes aegypti. Indian J Med Res. 1982;76:689–691. [PubMed] [Google Scholar]
- Pilger D, Lenhart A, Manrique-Saide P, Siqueira JB, da Rocha WT, Kroeger A. Is routine dengue vector surveillance in central Brazil able to accurately monitor the Aedes aegypti population? Results from a pupal productivity survey. Trop Med Int Health. 2011;16:1143–1150. doi: 10.1111/j.1365-3156.2011.02818.x. [DOI] [PubMed] [Google Scholar]
- Rey JR, O’Meara GF, O’Connell SM, Cutwa-Francis MM. Factors affecting mosquito production from stormwater drains and catch basins in two Florida cities. J Vector Ecol. 2006;31:334–343. doi: 10.3376/1081-1710(2006)31[334:fampfs]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Russell BM, Muir LE, Weinstein P, Kay BH. Surveillance of the mosquito Aedes aegypti and its biocontrol with the copepod Mesocyclops aspericornis in Australian wells and gold mines. Med Vet Entomol. 1996;10:155–160. doi: 10.1111/j.1365-2915.1996.tb00722.x. [DOI] [PubMed] [Google Scholar]
- Saiyasombat R, Dorman KS, García-Rejón JE, Loroño-Pino MA, Farfán-Ale JA, Blitvich BJ. Isolation and sequence analysis of Culex flavivirus from Culex interrogator and Culex quinquefasciatus in the Yucatan Peninsula of Mexico. Arch Virol. 2010;155:983–986. doi: 10.1007/s00705-010-0665-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers G, Brown JE, Barrera R, Powell JR. Genetics and morphology of Aedes aegypti (Diptera: Culicidae) in septic tanks in Puerto Rico. J Med Entomol. 2011;48:1095–1102. doi: 10.1603/me11129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickman D, Kittayapong P. Dengue and its vectors in Thailand: calculated transmission risk from total pupal counts of Aedes aegypti and association of wing-length measurements with aspects of the larval habitat. Am J Trop Med Hyg. 2003;68:209–217. [PubMed] [Google Scholar]
- Suárez-Rubio M, Suárez MF. The use of the copepoid Mesocyclops longisetus as a biological control agent for Aedes aegypti in Cali, Colombia. J Am Mosq Contr Assoc. 2004;20:401–404. [PubMed] [Google Scholar]
- Surendran SN, Jude PJ, Thabothiny V, Raveendran S, Ramasamy R. Pre-imaginal development of Aedes aegypti in brackish and fresh water urban domestic wells in Sri Lanka. J Vector Ecol. 2012;37:471–473. doi: 10.1111/j.1948-7134.2012.00254.x. [DOI] [PubMed] [Google Scholar]
- Tinker ME. Aedes aegypti larval habitats in Surinam. PAHO Bull. 1974;8:293–301. [PubMed] [Google Scholar]
- Torres-Estrada JL, Rodriguez MH, Cruz-Lopez L, Arredondo-Jimenez JI. Selective oviposition by Aedes aegypti (Diptera: Culicidae) in response to Mesocyclops longisetus (Copepoda: Cyclopoidea) under laboratory and field conditions. J Med Entomol. 2001;38:188–192. doi: 10.1603/0022-2585-38.2.188. [DOI] [PubMed] [Google Scholar]
- Tsuzuki A, Vu TD, Higa Y, Nguyen TY, Takagi M. Effect of peridomestic environments on repeated infestation by preadult Aedes aegypti in urban premises in Nha Trang City, Vietnam. Am J Trop Med Hyg. 2009;81:645–650. doi: 10.4269/ajtmh.2009.08-0175. [DOI] [PubMed] [Google Scholar]
- Tun-Lin W, Kay BH, Barnes A. Understanding productivity, a key to Aedes aegypti surveillance. Am J Trop Med Hyg. 1995;53:595–601. doi: 10.4269/ajtmh.1995.53.595. [DOI] [PubMed] [Google Scholar]
- Tun-Lin W, Lenhart A, Nam VS, Rebollar-Tellez E, Morrison AC, Barbazan P, Cote M, Midega J, Sanchez F, Manrique-Saide P, Kroeger A, Nathan MB, Meheus F, Petzold M. Reducing costs and operational constraints of dengue vector control by targeting productive breeding places: A multi-country non-inferiority cluster randomized trial. Trop Med Int Health. 2009;14:1143–1153. doi: 10.1111/j.1365-3156.2009.02341.x. [DOI] [PubMed] [Google Scholar]
- Turell MJ, Dohm DJ, Sardelis MR, O’Guinn ML, Andreadis TG, Blow JA. An update on the potential of North American mosquitoes (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol. 2005;42:57–62. doi: 10.1093/jmedent/42.1.57. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization) Dengue: Guidelines for diagnosis, treatment, prevention and control. World Health Organization; Geneva, Switzerland: 2009. [PubMed] [Google Scholar]