Abstract
Chronic hepatitis B affects more than a million people in the U.S. and causes 4,000 deaths each year, yet the costs and benefits of treatment have not been fully evaluated. Using a model that simulates disease progression, we compare treatment programs for hepatitis B that start at an early versus late stage of disease. Early care is shown to improve health, reduce premature deaths, and prevent expensive complications, making it highly cost-effective. Our results demonstrate the importance of linking hepatitis B screening to treatment, and illustrate how predictive models can be used to evaluate strategies for improving access to care.
Introduction
Chronic hepatitis B infection is a major cause of cirrhosis of the liver and primary liver cancer worldwide that infects between 800,000 and 2 million individuals in the U.S.(1-4). Infection is especially concentrated among persons born in countries in Asia, sub-Saharan Africa, Eastern Europe, South America and the Caribbean. This disparity is particularly severe in Asian populations within the U.S., as many as 10-15% may be infected, compared to the general population infection rate of less than 0.5%(5,6). Though universal infant vaccination has reduced the number of new infections occurring in the U.S., the actual number of cases of chronic hepatitis B has increased in recent years due to immigration from countries where the virus is endemic(5,7).
Chronic hepatitis B infection has a long latent (clinically silent) phase, during which it causes few or no symptoms, and it is frequently undiagnosed until the development of symptoms of late-stage complications many years later. Fifteen to forty percent of infected individuals will eventually develop complications, the most common (and deadliest) of which are primary liver cancer (hepatocellular carcinoma) and end-stage liver disease(6). When these complications are diagnosed, they require very expensive treatments and are most often fatal. Hepatitis B is responsible for 4,000 deaths in the U.S. each year and total direct and indirect cost burden of the disease in the U.S. is estimated to be as high as one billion dollars per year(4,8).
Hepatitis B infection thus represents a serious public health issue, even as new treatments have become available that reduce the amount of virus in the blood (“viral load”) more effectively, can be administered for a longer period without the development of resistance, and could avert serious outcomes(9-14). In current clinical practice guidelines, viral load is used as a predictor of health outcome, a criterion for starting antiviral treatment and a marker of treatment effectiveness(15). The risk for cirrhosis and primary liver cancer increases significantly with increasing viral load levels(15-17). Therefore monitoring of infected persons for early cirrhosis or hepatocellular carcinoma, along with intervention as needed to keep hepatitis B virus DNA levels low or undetectable, could reduce illness and death(18-21).
Recent assessments of best practices in the clinical management of hepatitis B have emphasized the need for appropriate treatment as a key mechanism for preventing long-term complications(18,19). In January 2010, the Institute of Medicine recommended working to improve access to screening and treatment, highlighting the lack of access to care among key at-risk groups and the need for more investigation of costs and cost-effectiveness of treatment programs(22).
As yet, there has been no systematic assessment of whether providing care would be cost-effective. In light of these improvements in clinical management, we believe it is timely to examine the costs and benefits of providing comprehensive care for this illness. This paper describes a model that simulates a group of individuals with chronic hepatitis B infection over twenty years, whose health status depends on the timing of medical intervention they receive. Our analysis compares the health outcomes, costs and cost-effectiveness of providing comprehensive early treatment and care for individuals with chronic hepatitis B in comparison to a standard population that only receive care at late stages of disease.
Given that access to appropriate early treatment and care for chronic hepatitis B is likely to correlate with the availability of comprehensive health insurance, we also examine strategies for providing expanded care to populations that cannot currently access it. Many infected people have little or no health insurance and are ineligible for publicly-subsidized insurance such as Medicaid. Though this problem will be somewhat alleviated by the implementation of new healthcare reform legislation, care for some of these individuals will remain fragmented and difficult to obtain due to underinsurance or sociocultural barriers to care. Improving access for this population could effectively prevent disability and premature death and could provide an important policy model for future efforts to improve health and reduce costs by providing early-stage care for chronic diseases.
Methods
We constructed a model of chronic hepatitis B care that is based on current clinical decision-making algorithms in a fixed population of persons with chronic hepatitis B in a finite number of discrete health states, called Markov states. Markov state transition models provide a more realistic approximation of outcomes and costs than static models. Transition rates from one state to another were informed by a literature search on the natural history, epidemiology and treatment of hepatitis B(6,16-20,23-28). The model projects and compares outcomes, costs, and quality-of-life estimates over 20 years by running repeated simulations that are randomly selected through the model of a population of individuals chronically infected with hepatitis B for two different scenarios:
Late Care group: Individuals receive no treatment (and accrue no costs) until they become seriously ill with hepatocellular carcinoma or decompensated cirrhosis (a condition in which liver scarring is severe enough to result in serious symptoms, including liver failure).
Early Care group: All individuals are monitored, and any individuals meeting criteria for drug treatment receive it. All hepatitis-B-related costs are included.
More detailed information on model assumptions and specifications can be found in the Technical Appendix.
Initial health states
Individuals in the model are initially divided into one of several discrete health states based on actual baseline health states found among newly diagnosed persons with chronic hepatitis B infection from large-scale community screening campaigns that more accurately reflect the conditions in the population than those from cases referred to specialists (29,30). The health states are categorized by four specific viral load ranges (largely asymptomatic states) and four major complications. The complications we consider are compensated cirrhosis (in which the individual has sustained liver damage, but does not yet show symptoms and can recover with treatment), decompensated cirrhosis, hepatocellular carcinoma (liver cancer), and “post-liver transplant,” representing the lifetime management required by individuals who have received a new liver.
Treatment assumptions and transition probabilities
Individuals in the model move among the health states each year according to probabilities of transition that are drawn from published reports. Transition probabilities depend on whether they are receiving treatment. Individuals in higher viral-load health states are more likely to transition to complications(16). Individuals also progress to death from non-hepatitis B causes according to published death rates by age(31).
In the Early Care scenario, individuals receive treatment with antivirals in accordance with a simplified program of treatment that combines recommendations from several recently published guidelines(19,32). Individuals can transition to healthier states, and they are much more likely to do so when undergoing drug treatment(15,16,25,26). Because of the wide variety of antiviral drugs currently used to treat chronic hepatitis B, we use theoretical drugs with rates of response that could be reasonably expected from the best treatment available today. This theoretical treatment profile includes a first-line drug with a high barrier to resistance and a second drug for those who do not respond(33-36). It is assumed that no individuals have received prior treatment for their infection at the outset of the simulation. Drug resistance is assumed to be limited to those who fail the first-line treatment. We also assume that those eligible will receive the indicated care, although this is a necessary simplification since availability is no guarantee of adherence to interventions, even among individuals who have adequate health coverage and particularly those with chronic, often asymptomatic conditions like hepatitis B.
Early diagnosis of hepatocellular carcinoma increases one's eligibility for life-extending treatments and procedures(20,27,37), so the model assumes that individuals with hepatocellular carcinoma in the Early Care group are more likely to be diagnosed early and to qualify for either a transplant or for one of these other measures(38). Individuals whose immune systems successfully clear their infection, permanently eliminating the hepatitis B surface antigen from their blood, and individuals who die remain permanently in those states for the rest of the time the model runs.
Cost-Effectiveness Assumptions
Costs, as shown in Exhibit 1, were estimated based on current wholesale costs and Medicaid reimbursement rates (for drugs) and a literature review (for other costs)(20,21,24,37-42). The perspective of the cost analysis is that of the U.S. public health system. We note that many uninsured individuals have some access to medical services and some costs of major complications would likely be covered by the individuals themselves. These out-of-pocket costs may be considered a part of the societal perspective of our analysis. On the other hand, this evaluation does not incorporate indirect costs, such as lost wages due to illness or death, even though each healthy life-year gained is associated with an economic gain to society as a whole. All costs were adjusted to 2008 dollars where possible. Cost estimates were discounted at 3% per year(43).
Exhibit 1. Costs used in model calculations (base case).
| Item | Cost (range in sensitivity analysis), $ | |
|---|---|---|
|
| ||
| Outpatient visit | 80 | 60-100 |
|
| ||
| Diagnostic and monitoring tests | ||
| HBV virology | 178 | 100-250 |
| alpha-Fetoprotein | 20 | 10-30 |
| Standard lab tests | 30 | 15-45 |
| Detailed lab tests | 250 | 150-350 |
| Abdominal ultrasound | 200 | 100-300 |
|
| ||
| Drug treatment (per year) | ||
| Initial treatment | 5,000 | 1,000-7,000 |
| Salvage treatment | 6,000 | 1,500-8,000 |
|
| ||
| Decompensated cirrhosis (per year) | 30,571 | 10,000-50,000 |
|
| ||
| HCC procedures (composite) | 11,175 | 8,500-20,000 |
|
| ||
| HCC remission monitoring (per year) | 4,500 | 3,000-6,000 |
|
| ||
| HCC relapse/terminal care (per year) | 45,323 | 30,000-60,000 |
|
| ||
| Liver transplant | 137,918 | 100,000-150,000 |
|
| ||
| Post-liver transplant care (per year) | 24,065 | 10,000-40,000 |
HBV: hepatitis B virus
HCC: hepatocellular carcinoma
Sources: Barazani Y, Hiatt JR, Tong MJ, Busuttil RW. Chronic viral hepatitis and hepatocellular carcinoma. World J.Surg. 2007 Jun;31(6):1243-1248.
Bennett WG, Inoue Y, Beck JR, Wong JB, Pauker SG, Davis GL. Estimates of the cost-effectiveness of a single course of interferon-alpha 2b in patients with histologically mild chronic hepatitis C. Ann.Intern.Med. 1997 Nov 15;127(10):855-865.
De Simone P, Vignali C, Petruccelli S, Carrai P, Coletti L, Montin U, et al. Cost analysis of tumor downsizing for hepatocellular carcinoma liver transplant candidates. Transplant.Proc. 2006 Dec;38(10):3561-3563.
Jing Y, Klein P, Kelton CM, Li X, Guo JJ. Utilization and Spending Trends for Antiretroviral Medications in the U.S. Medicaid Program from 1991 to 2005. AIDS Research and Therapy 2007 Oct 16;4:22.
Kanwal F, Farid M, Martin P, Chen G, Gralnek IM, Dulai GS, et al. Treatment alternatives for hepatitis B cirrhosis: a cost-effectiveness analysis. Am.J.Gastroenterol. 2006 Sep;101(9):2076-2089.
Sarasin FP, Giostra E, Mentha G, Hadengue A. Partial hepatectomy or orthotopic liver transplantation for the treatment of resectable hepatocellular carcinoma? A cost-effectiveness perspective. Hepatology 1998 Aug;28(2):436-442.
Wong JB, Koff RS, Tine F, Pauker SG. Cost-effectiveness of interferon-alpha 2b treatment for hepatitis B e antigen-positive chronic hepatitis B. Ann.Intern.Med. 1995 May 1;122(9):664-675.
The effectiveness of providing Early Care was measured in quality-adjusted-life-years (QALYs), a unit that captures both the life-years gained by a particular intervention and evaluates the quality of life experienced in each disease state. Perfect health is a given a value of 1, death is 0, and each disease state in the model has been assigned a QALY value between 0 and 1 (as displayed in Exhibit 2) based on values obtained by Standard Gamble methodology in hepatitis C patients(24,44). A gain in QALYs therefore represents reduction in both the morbidity and mortality associated with a disease.
Exhibit 2. Annual utility assigned for each health state.
| Health state | Quality-of-life value (range in Sensitivity Analysis) |
|---|---|
| s-antigen-negative, e-antigen-negative, or treatment response | 1 (0.85-1) |
| HBV infection, no cirrhosis | 0.99 (0.6-1) |
| Compensated cirrhosis (CC) | 0.8 (0.7-0.9) |
| Decompensated cirrhosis (DC) | 0.6 (0.5-0.7) |
| Hepatocellular carcinoma (HCC) remission | 0.73 (0.6-0.8) |
| HCC failure | 0.6 (0.5-0.7) |
| Post-liver-transplant | 0.86 (0.8-0.9) |
| Death | 0 |
Note: A value of 1 represents a state equivalent to full health (and full quality-of-life), and a state of 0 represents death. All intervening values are estimates of the quality of life associated with a given health state.
Source: Chong CA, Gulamhussein A, Heathcote EJ, Lilly L, Sherman M, Naglie G, et al. Health-state Utilities and Quality of Life in Hepatitis C Patients. American Journal of Gastroenterology 2003 Mar;98(3):630-8.
Average QALYs were determined for both scenarios at 5, 10, 15, and 20 years from initial diagnosis. While cost-effectiveness models typically include health and cost effects that accumulate after the intervention is stopped, our approach allows us to report what can be achieved at the end of each five-year period. Indirect costs, such as those associated with wage losses due to illness or death, were not calculated. The QALYs gained by coverage were not assigned a specific dollar value; results are presented as health-system dollars per QALY gained and were also discounted at 3% per year.
Results
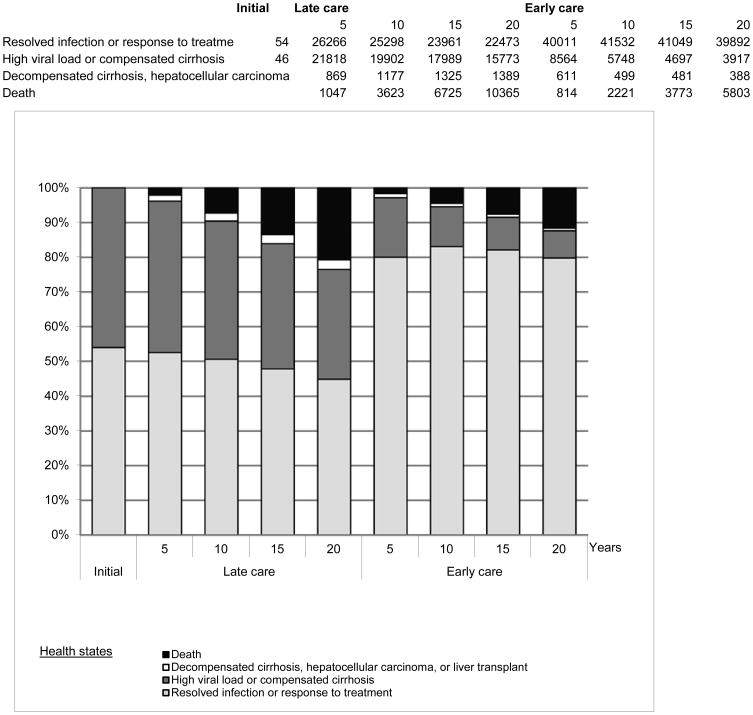
The model shows that providing early care helps prevent long-term health problems even within a short time frame. Since most serious outcomes of hepatitis B infection usually take years to manifest, the health benefits associated with averting these problems increase over time. As shown in Exhibit 3, the proportion of individuals with resolved infections or in low viral-load health states is 52.5% in the Late Care scenario and 80.0% in the Early Care scenario after just five years of treatment (or lack of treatment) according to the rules of the scenario. Meanwhile, the proportion of individuals facing serious complications (decompensated cirrhosis, hepatocellular carcinoma, or transplant) falls from 1.2% at three years to 0.7% after twenty years in Early Care; this proportion is higher in Late Care than Early Care after just three years and grows continually, despite extensive attrition from mortality.
Exhibit 3.
Health state distributions.
Source: Model results
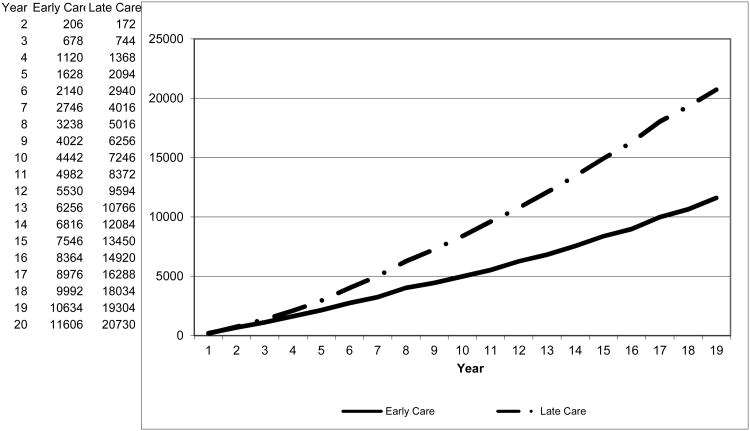
Over twenty years, the average annual incidence (new cases) of hepatocellular carcinoma, one of the most severe and expensive complications of chronic hepatitis B, is 572 per 100,000 chronically infected persons in the Late Care scenario versus just 194 per 100,000 in Early Care. Providing early care also greatly reduces mortality rates, illustrated in Exhibit 4. After just five years in the model, cumulative mortality for these identical initial populations diverges substantially: rates per 100,000 are 2,094 in Late Care, versus 1,628 in Early Care. After twenty years the cumulative mortality rates are, respectively, 20,730 and 11,606 per 100,000.
Exhibit 4.
Cumulative deaths per 100,000 individuals.
Source: Model results
Costs and cost-effectiveness
Costs for Late Care increase steadily from a cumulative average of $1,100 per person after three years to $10,735 after twenty years (see Technical Appendix). Early Care does not become cost-saving over this time frame, but the annual cost of Early Care decreases from year to year, whereas it increases in the Late Care scenario.
Cost-effectiveness analysis was used to evaluate the health gains resulting from the additional spending associated with expanding coverage. For Early Care, the cost per QALY gained versus Late Care decreases dramatically from $61,780 after five years to $5,184 after twenty years. In general, interventions that cost less than $50,000 per QALY gained are considered cost-effective in the U.S., so even after roughly five years the Early Care scenario could be considered quite cost-effective. Cost-effectiveness results are summarized in Exhibit 5.
Exhibit 5. Incremental cost-effectiveness ratios.
| Model Run Time (Years) | Average QALYs gained per person | Additional cost per person ($) | Incremental cost-effectiveness ratio ($ per QALY) |
|---|---|---|---|
| 5 | 0.05 | 3,415 | 61,780 |
| 10 | 0.22 | 4,291 | 20,700 |
| 15 | 0.47 | 4,495 | 12,221 |
| 20 | 0.79 | 4,096 | 5,184 |
Note: Values represent additional costs and gained QALYs in Early Care vs Late Care at four different time endpoints. Incremental cost-effectiveness is shown to improve over time.
Source: Model results
Sensitivity analysis
To test the sensitivity of the results to key assumptions made in the model, including those for which evidence was mixed or controversial, a one-way sensitivity analysis was performed. Only four variables led to a significant (more than 30%) change in the baseline cost-effectiveness, but none changed the final cost-effectiveness result by more than a few thousand dollars, and all values comparing Early Care and Late Care remain highly cost-effective. These results suggest that the model, while somewhat sensitive to certain inputs, robustly captures a rough measure of overall cost-effectiveness. More details on the sensitivity analysis are available in the Technical Appendix.
Discussion
This study demonstrates that the implementation of current clinical guidelines for the treatment of chronic hepatitis B infection (prior to the manifestation of late-stage complications) would be expensive but would decrease morbidity, save lives and be cost-effective over as little as ten years. In addition to generating substantial health status gains, our results indicate that providing appropriate early care for chronic hepatitis B can be highly cost-effective compared to providing treatment only for serious hepatitis B-related illnesses, as money spent on early-stage treatment helps prevent expensive complications. This investment in early-stage treatment likely would have substantially greater cost-effectiveness or even cost savings, given that each healthy life-year gained is associated with a substantial indirect benefit to society as a whole in the form of increased social and economic productivity.
By making it possible to evaluate a wide variety of treatment scenarios among different target populations, this model provides a tool for evaluating both changes in treatment recommendations and healthcare coverage policy options. We believe it could serve as a useful tool for investigating the potential impact of future hepatitis-B-related coverage policy decisions by public and private entities. The model's dynamic nature means it can be easily updated with data on the epidemiological impact and costs of interventions for early-stage disease or for major complications, as new information becomes available.
Ensuring true universal access to care would be the most effective and efficient way to improve long-term health outcomes of individuals with chronic hepatitis B, yet even with the March 2010 passage of the Obama Administration's healthcare reform legislation, access to adequate chronic disease management may remain fragmented and challenging for the population most affected by chronic hepatitis B, which includes many recent immigrants and other socially vulnerable individuals. Though the new legislation increases Medicaid eligibility, many individuals will still not qualify and may not be able, even with newly-available subsidies, to afford a policy that provides adequately for their needs as they face a complicated chronic illness. Health insurance companies have wide discretion in service provision and may not necessarily elect to pay for the early treatment of hepatitis B without estimates of the costs and benefits of such care.
Our model assumes knowledge of hepatitis B infection at its outset, yet it is estimated that less than one-third of persons infected are aware of their infection and many who are diagnosed are not managed appropriately(22). Even with improved access to health services, the thousands of U.S. residents who do not know of their infection would continue essentially to live out a Late Care scenario. In order to achieve the benefits of Early Care, it is thus absolutely critical that persons be screened for hepatitis B infection. Though virtually all pregnant women in the U.S. are screened, there is no comprehensive source of screening for other at-risk groups, though scattered community-based screening programs exist (30,45). A recent study indicated that universal screening of at-risk populations, along with targeted vaccination and availability of treatment, could be a highly cost-effective public health measure(46). The CDC has recently issued broader and more forceful recommendations for routine hepatitis B screening to include a wider range of at-risk groups and referral to care for those who are infected(8).
We anticipate that greater attention to hepatitis B screening will help highlight the need for improvement in treatment access. Given that screening is likely to become more widespread, we anticipate a parallel need for better access to treatment, if only to prevent an ethically untenable scenario where individuals are informed of their hepatitis B infection status but have no way to obtain care. The federal government has recognized this issue in the past: a CDC screening program for breast and cervical cancer created a similar scenario, and in response, the Breast and Cervical Cancer Prevention and Treatment Act of 2000 allowed states to expand Medicaid coverage for women screened through this program regardless of additional costs, an option that was being implemented by all 50 states within three years of the law's passage(47). The results from our model demonstrate the critical importance of linking hepatitis B screening to access to care and treatment in order to achieve maximal health gains.
Because of our improved understanding of the natural history of chronic hepatitis B infection and the availability of newer, more potent and effective treatments, we have the opportunity to reduce long-term morbidity and mortality from this infection. Obtaining the greatest benefits and cost savings from a “societal” perspective can only come about by expanding access to care and coverage for chronic hepatitis B, improving the possibility of adequate disease management through early-stage treatment. Our model predicts that this improvement in access would not only decrease morbidity and save lives but would also be highly cost-effective in the long run. The model provides a potential tool for evaluating the impact, costs and benefits of strategies to achieve these goals, and it could be used to optimize approaches aimed at correcting this longstanding health disparity.
Supplementary Material
Acknowledgments
Preliminary results of this model were presented at the 135th Annual Meeting and Expo of the American Public Health Association (APHA) on November 5, 2007, as Sodhi NK, Peng C, Wan K, Baker P, Young P, Pollack HJ, “Rationale for extending Medicaid eligibility to uninsured persons with chronic hepatitis B infection,” and at the 137th Annual Meeting and Expo of APHA on November 9, 2008, as Post S, Sodhi N, Peng C, Wan K, Pollack H, “Evaluating costs and benefits of expanding access to comprehensive care for chronic hepatitis B infection.” This study was funded by grants from the Centers for Disease Control and Prevention (DP07-707); Racial and Ethnic Approaches to Community Health across the US; the National Center on Minority Health and Health Disparities (P60 MD000538); the New York University Center for the Study of Asian American Health; and Gilead Sciences. The funders had no role in the design of the model, analysis, or decision to publish; or in the preparation, review, or approval of the manuscript. The authors thank Jeffrey Levi of the Trust for America's Health, Andrew Hindman and Carol Brosgart of Gilead Sciences, and Gaylee Morgan and Jack Meyer of Health Management Associates for valuable discussions about strategies for improving access to care and estimating hepatitis B costs.
References
- 1.El-Serag HB, Mason AC. Risk factors for the rising rates of primary liver cancer in the United States. Arch Intern Med. 2000 Nov 27;160(21):3227–3230. doi: 10.1001/archinte.160.21.3227. [DOI] [PubMed] [Google Scholar]
- 2.Kao JH, Chen DS. Changing disease burden of hepatocellular carcinoma in the Far East and Southeast Asia. Liver Int. 2005 Aug;25(4):696–703. doi: 10.1111/j.1478-3231.2005.01139.x. [DOI] [PubMed] [Google Scholar]
- 3.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004 Mar;11(2):97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 4.Cohen C, Evans AA, London WT, Block J, Conti M, Block J. Underestimation of chronic hepatitis B virus infection in the United States of America. J Viral Hepat. 2008 Jan;15(1):12–13. doi: 10.1111/j.1365-2893.2007.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McQuillan GM, Coleman PJ, Kruszon-Moran D, Moyer LA, Lambert SB, Margolis HS. Prevalence of hepatitis B virus infection in the United States: the National Health and Nutrition Examination Surveys, 1976 through 1994. Am J Public Health. 1999;89(1):14–18. doi: 10.2105/ajph.89.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lok A, McMahon BJ. Chronic hepatitis B. Hepatology. 2001;34(6):1225–1241. doi: 10.1053/jhep.2001.29401. [DOI] [PubMed] [Google Scholar]
- 7.Gish RG, Gadan AC. Chronic Hepatitis B: current epidemiology in the Americas and implications for management. J Viral Hepat. 2006 Dec;13(12):787–798. doi: 10.1111/j.1365-2893.2006.00787.x. [DOI] [PubMed] [Google Scholar]
- 8.Weinbaum CM, Williams I, Mast EE, Wang SA, Finelli L, Wasley A, et al. Recommendations for Identification and Public Health Management of Persons with Chronic Hepatitis B Virus Infection. Morbidity and Mortality Weekly Report Recommendations and Reports. 2008 Sep 19;57(RR-8):1–20. [PubMed] [Google Scholar]
- 9.Dienstag JL, Goldin RD, Heathcote EJ, Hann HWL, Woessner M, Stephenson SL, et al. Histological outcome during long-term lamivudine therapy. Gastroenterology. 2003;124(1):105–117. doi: 10.1053/gast.2003.50013. [DOI] [PubMed] [Google Scholar]
- 10.Lai CL, Rosmawati M, Lao J, Van Vlierberghe H, Anderson FH, Thomas N, et al. Entecavir is superior to lamivudine in reducing hepatitis B virus DNA in patients with chronic hepatitis B infection. Gastroenterology. 2002 Dec;123(6):1831–1838. doi: 10.1053/gast.2002.37058. [DOI] [PubMed] [Google Scholar]
- 11.Liaw YF, Sung JJY, Chow WC, Farrell G, Lee CZ, Yuen H, et al. Lamivudine for Patients with Chronic Hepatitis B and Advanced Liver Disease. New England Journal of Medicine. 2004;351(15):1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 12.Lin SM, Sheen IS, Chien RN, Chu CM, Liaw YF. Long-term beneficial effect of interferon therapy in patients with chronic hepatitis B virus infection. Hepatology. 1999 Mar;29(3):971–975. doi: 10.1002/hep.510290312. [DOI] [PubMed] [Google Scholar]
- 13.Marcellin P, Chang TT, Lim SG, Tong MJ, Sievert W, Shiffman ML, et al. Adefovir Dipivoxil for the Treatment of Hepatitis B e Antigen-Positive Chronic Hepatitis B. New England Journal of Medicine. 2003;348(9):808–816. doi: 10.1056/NEJMoa020681. [DOI] [PubMed] [Google Scholar]
- 14.Rizzetto M, Tassopoulos NC, Goldin RD, Esteban R, Santantonio T, Heathcote EJ, et al. Extended lamivudine treatment in patients with HBeAg-negative chronic hepatitis B. J Hepatol. 2005 Feb;42(2):173–179. doi: 10.1016/j.jhep.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006 Jan 4;295(1):65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 16.Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006 Mar;130(3):678–686. doi: 10.1053/j.gastro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Tang B, Kruger WD, Chen G, Shen F, Lin WY, Mboup S, et al. Hepatitis B Viremia is Associated with Increased Risk of Hepatocellular Carcinoma in Chronic Carriers. Journal of Medical Virology. 2004 Jan;72(1):35–40. doi: 10.1002/jmv.10559. [DOI] [PubMed] [Google Scholar]
- 18.Keeffe EB, Dieterich DT, Han SH, Jacobson IM, Martin P, Schiff ER, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: an update. Clin Gastroenterol Hepatol. 2006 Aug;4(8):936–962. doi: 10.1016/j.cgh.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 19.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009 Sep;50(3):661–2. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 20.Barazani Y, Hiatt JR, Tong MJ, Busuttil RW. Chronic viral hepatitis and hepatocellular carcinoma. World J Surg. 2007 Jun;31(6):1243–1248. doi: 10.1007/s00268-007-9041-3. [DOI] [PubMed] [Google Scholar]
- 21.Bennett WG, Inoue Y, Beck JR, Wong JB, Pauker SG, Davis GL. Estimates of the cost-effectiveness of a single course of interferon-alpha 2b in patients with histologically mild chronic hepatitis C. Ann Intern Med. 1997 Nov 15;127(10):855–865. doi: 10.7326/0003-4819-127-10-199711150-00001. [DOI] [PubMed] [Google Scholar]
- 22.Colvin HM, Mitchell AE, editors. Hepatitis and liver cancer: a national strategy for prevention and control of hepatitis B and C. Washington, DC: National Academies Press; 2009. [cited 2010 June 3]. Available from: http://www.nap.edu/catalog/12793.html. [PubMed] [Google Scholar]
- 23.Chu CM, Liaw YF. Chronic hepatitis B virus infection acquired in childhood: special emphasis on prognostic and therapeutic implication of delayed HBeAg seroconversion. J Viral Hepat. 2007 Mar;14(3):147–152. doi: 10.1111/j.1365-2893.2006.00810.x. [DOI] [PubMed] [Google Scholar]
- 24.Kanwal F, Farid M, Martin P, Chen G, Gralnek IM, Dulai GS, et al. Treatment alternatives for hepatitis B cirrhosis: a cost-effectiveness analysis. Am J Gastroenterol. 2006 Sep;101(9):2076–2089. doi: 10.1111/j.1572-0241.2006.00769.x. [DOI] [PubMed] [Google Scholar]
- 25.Liaw YF, Chu CM, Su IJ, Huang MJ, Lin DY, Chang-Chien CS. Clinical and histological events preceding hepatitis B e antigen seroconversion in chronic type B hepatitis. Gastroenterology. 1983 Feb;84(2):216–219. [PubMed] [Google Scholar]
- 26.Lok AS, Lai CL, Wu PC, Leung EK, Lam TS. Spontaneous hepatitis B e antigen to antibody seroconversion and reversion in Chinese patients with chronic hepatitis B virus infection. Gastroenterology. 1987 Jun;92(6):1839–1843. doi: 10.1016/0016-5085(87)90613-5. [DOI] [PubMed] [Google Scholar]
- 27.Martins A, Cortez-Pinto H, Marques-Vidal P, Mendes N, Silva S, Fatela N, et al. Treatment and prognostic factors in patients with hepatocellular carcinoma. Liver Int. 2006 Aug;26(6):680–687. doi: 10.1111/j.1478-3231.2006.001285.x. [DOI] [PubMed] [Google Scholar]
- 28.Shepherd J, Jones J, Takeda A, Davidson P, Price A. Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation. Health Technol Assess. 2006 Aug;10(28):iii–iv. xi–xiv, 1–183. doi: 10.3310/hta10280. [DOI] [PubMed] [Google Scholar]
- 29.Pollack H, Sherman A, Tsang T, Wan K, Lupatkin H, Villanueva G, et al. Clinical characteristics of Asian Americans infected with hepatitis B diagnosed by community-based screenings in New York City. Hepatol. 2006;44:568A. Abstract. [Google Scholar]
- 30.Pollack H, Wan K, Ramos R. Screening for chronic hepatitis B among Asian/Pacific Islander populations—New York City, 2005. MMWR Morbid Mortal Wkly Rep. 2006;55:505–509. [PubMed] [Google Scholar]
- 31.Arias E. United States Life Tables, 2004. National Vital Statistics Reports 2007. 2007 Dec 28;56(9):1–40. [PubMed] [Google Scholar]
- 32.Keeffe EB, Dieterich DT, Han SB, Jacobson IM, Martin P, Schiff ER, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States. Clinical Gastroenterology and Hepatology. 2004;2(2):87–106. doi: 10.1016/s1542-3565(03)00312-4. [DOI] [PubMed] [Google Scholar]
- 33.Berg T, Moller B, Trinh H, Chan S, Marcellin P, Suarez E, et al. Tenofovir disoproxil fumarate (TDF) versus emtricitabine plus TDF for treatment of chronic hepatitis B (CHB) in subjects with persistent viral replication in receiving adefovir dipivoxil (ADV); European Association for the Study of the Liver 44th Annual Meeting; 2009 April 22-26; Copenhagen, Denmark. [cited 2010 May 30]. Available from: http://www.natap.org/2009/EASL/EASL_58.htm. [Google Scholar]
- 34.Heathcote J, Gane E, DeMan R, Lee S, Flisiak R, Manns M, et al. A Randomized Double-Blind Comparison of Tenofovir DF (TDF) versus Adefovir Dipivoxil (ADV) for the Treatment of HBeAg Positive Chronic Hepatitis B (CHB): Study GS-US-174-0103; 58th Annual Meeting of the American Association for the Study of Liver Diseases; November 2-6, 2007. [Google Scholar]
- 35.Marcellin P, Buti M, Krastev Z, Germanidis G, Kaita K, Kotsev I, et al. A Randomized Double-Blind Comparison of Tenofovir DF (TDF) versus Adefovir Dipivoxil (ADV) for the Treatment of HBeAg-Negative Chronic Hepatitis B (CHB): Study GS-US-174-0102; 58th Annual Meeting of the American Association for the Study of Liver Diseases; November 2-6, 2007. [Google Scholar]
- 36.Marcellin P, Chang TT, Lim SG, Tong MJ, Sievert W, Shiffman ML, et al. Adefovir Dipivoxil for the Treatment of Hepatitis Be Antigen-Positive Chronic Hepatitis B. N Engl J Med. 2003;348(9):808. doi: 10.1056/NEJMoa020681. [DOI] [PubMed] [Google Scholar]
- 37.De Simone P, Vignali C, Petruccelli S, Carrai P, Coletti L, Montin U, et al. Cost analysis of tumor downsizing for hepatocellular carcinoma liver transplant candidates. Transplant Proc. 2006 Dec;38(10):3561–3563. doi: 10.1016/j.transproceed.2006.10.069. [DOI] [PubMed] [Google Scholar]
- 38.Sarasin FP, Giostra E, Mentha G, Hadengue A. Partial hepatectomy or orthotopic liver transplantation for the treatment of resectable hepatocellular carcinoma? A cost-effectiveness perspective. Hepatology. 1998 Aug;28(2):436–442. doi: 10.1002/hep.510280222. [DOI] [PubMed] [Google Scholar]
- 39.Canada Patented Medicine Prices Review Board. Report on New Patented Drugs -- Viread. 2004 [Google Scholar]
- 40.Centers for Medicare and Medicaid Services. State Drug Utilization Data. 2006 [Google Scholar]
- 41.Jing Y, Klein P, Kelton CM, Li X, Guo JJ. Utilization and Spending Trends for Antiretroviral Medications in the U.S. Medicaid Program from 1991 to 2005. AIDS Research and Therapy. 2007 Oct 16;4:22. doi: 10.1186/1742-6405-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong JB, Koff RS, Tine F, Pauker SG. Cost-effectiveness of interferon-alpha 2b treatment for hepatitis B e antigen-positive chronic hepatitis B. Ann Intern Med. 1995 May 1;122(9):664–675. doi: 10.7326/0003-4819-122-9-199505010-00004. [DOI] [PubMed] [Google Scholar]
- 43.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. Journal of the American Medical Association. 1996 Oct 16;276(15):1253–8. [PubMed] [Google Scholar]
- 44.Chong CA, Gulamhussein A, Heathcote EJ, Lilly L, Sherman M, Naglie G, et al. Health-state Utilities and Quality of Life in Hepatitis C Patients. American Journal of Gastroenterology. 2003 Mar;98(3):630–8. doi: 10.1111/j.1572-0241.2003.07332.x. [DOI] [PubMed] [Google Scholar]
- 45.San Francisco Hep B Free Campaign. San Francisco, CA: Community Initiatives Inc.; 2007-2010. [cited 2009 September 21]. homepage on the Internet. Available from: http://www.sfhepbfree.org/ [Google Scholar]
- 46.Hutton DW, Tan D, So SK, Brandeau ML. Cost-effectiveness of screening and vaccinating Asian and Pacific Islander adults for hepatitis B. Ann Intern Med. 2007 Oct 2;147(7):460–469. doi: 10.7326/0003-4819-147-7-200710020-00004. [DOI] [PubMed] [Google Scholar]
- 47.Lantz PM, Weisman CS, Itani Z. A disease-specific Medicaid expansion for women. The Breast and Cervical Cancer Prevention and Treatment Act of 2000. Women's Health Issues. 2003;13(3):79–92. doi: 10.1016/s1049-3867(03)00032-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.