Abstract
Objective
Multiple sclerosis (MS) lesions demonstrate immunopathological heterogeneity in patterns of demyelination. Previous cross-sectional studies reported immunopatterns of demyelination were identical among multiple active demyelinating lesions from the same individual, but differed between individuals, leading to the hypothesis of intraindividual pathological homogeneity and interindividual heterogeneity. Other groups suggested a time-dependent heterogeneity of lesions. The objective of our present study was to analyze tissue samples collected longitudinally to determine whether patterns of demyelination persist over time within a given patient.
Methods
Archival tissue samples derived from patients with pathologically confirmed CNS inflammatory demyelinating disease who had undergone either diagnostic serial biopsy or biopsy followed by autopsy, were analyzed immunohistochemically. Inclusion criteria was the presence of early active demyelinating lesions - required for immunopattern classification - obtained from the same patient at two or more time points.
Results
Among 1321 surgical biopsies consistent with MS, 22 cases met study inclusion criteria. Twenty-one patients (95%) showed a persistence of immunopathological patterns in tissue sampled from different time points. This persistence was demonstrated for all major patterns of demyelination. A single patient showed features suggestive of both pattern II and pattern III on biopsy, but only pattern II among all active lesions examined at autopsy.
Interpretation
These findings continue to support the concept of patient-dependent immunopathological heterogeneity in early MS and suggest that the mechanisms and targets of tissue injury may differ among patient subgroups. These observations have potentially significant implications for individualized therapeutic approaches.
Keywords: Multiple sclerosis, histopathology, intra-individual, homogeneity, heterogeneity, active demyelination, persistence over time
Introduction
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system (CNS), and the most common cause of non-traumatic disability in young adults.1 MS is heterogeneous with respect to clinical, genetic, radiographic, and pathological features. Pathological hallmarks include multifocal demyelination, inflammation, gliosis and axonal damage. MS lesions evolve differently during early versus chronic disease phases, and within each phase, different stages and types of demyelinating activity are evident. Demyelinating activity is defined based on the sequential degradation of myelin protein products within macrophages.2 Early active lesions contain both minor and major myelin protein degradation products within macrophages, and represent an early stage in lesion formation. MS brains may contain multiple lesions, any of which may consist of several areas in different demyelinating stages.
Studies of early active MS lesions described heterogeneity in immunopatterns of demyelination.3 Active lesions were classified into four categories based on loss of specific myelin proteins, plaque topography, oligodendrocyte destruction and evidence for complement and immunoglobulin deposition. We refer to these as “patterns of demyelination.” Patterns I and II showed T cell/macrophage-associated demyelination with parallel loss of all myelin proteins. Pattern II was selectively associated with immunoglobulin and complement deposited along myelin sheaths and present within macrophages, suggesting a pathogenic role for humoral mechanisms. Pattern III lesions were characterized by the presence of apoptotic oligodendrocytes and a preferential loss of myelin associated glycoprotein (MAG). MAG is located in distal oligodendrocyte processes, and its selective loss is considered a marker of metabolically stressed oligodendrocytes, leading to a dying back oligodendrogliopathy. MAG loss is also found in progressive multifocal leukoencephalopathy, and in hypoxic and ischemic conditions.4,5 Pattern IV lesions were rare and associated with non-apoptotic oligodendrocyte death in periplaque non-demyelinated white matter. Pattern II was most frequent, followed by patterns III, I and IV. Immunopatterns were identical among multiple active lesions analyzed within a given patient, but differed between individuals. Based on these results we proposed that early active demyelinating MS lesions showed intraindividual immunopathologic homogeneity and interindividual heterogeneity.
This patient dependent heterogeneity hypothesis has been debated. Pattern III-like oligodendrocyte apoptosis in the absence of inflammation in some plaque regions, and pattern II-like complement activation in other regions within the same case was interpreted as immunopattern overlap, suggesting demyelination patterns were stage rather than patient specific.6 Another study suggested antibody and complement-mediated myelin phagocytosis was the dominant mechanism in all lesions among chronic MS patients.7
This study’s aim was to determine, within a cohort of patients with pathologically confirmed CNS inflammatory demyelination who had either serial biopsies or biopsy followed by autopsy, whether immunopatterns of active demyelination persist over time within a given patient.
Methods
This study was approved by ethical review committees of the University Medical Center Göttingen (# 19/09/10) and the Mayo Clinic (IRB # 2067-99) and involved analysis of formalin-fixed paraffin-embedded (FFPE) archival tissues from patients with biopsy or autopsy proven CNS inflammatory demyelination diagnosed by at least two board-certified neuropathologists (JEP, WB, IM). Brain biopsies were obtained in the context of routine clinical care and consent obtained by treating physicians. Repeated biopsies were performed due to persisting diagnostic uncertainty or atypical course. No individuals underwent surgery for research purposes.
Study Cohort
Inclusion criteria were: (1) histopathological diagnosis of MS-compatible inflammatory demyelinating lesions; (2) biopsy material available from ≥2 biopsies occurring at different times or tissue available from one biopsy followed by an autopsy; (3) presence of early active demyelinating brain lesions available from at least two time points from the same patient; (4) reliable tissue staining; and (5) sufficient tissue area for analyses (≥1mm2 or ≥10 morphometric grids at 100×). Exclusion criteria were (1) acute disseminated encephalomyelitis, and neuromyelitis optica (NMO) defined based on published criteria;8–11 or (2) evidence of other disease (e.g., neoplasm, infection).
Histopathology
Formalin-fixed, paraffin-embedded (FFPE) 4 µm thick sections were stained with haematoxylin and eosin, Luxol-fast blue/periodic acid–Schiff and Bielschowsky’s silver impregnation. Immunohistochemical staining was performed (avidin–biotin-based) using the following antibodies: anti-MAG, anti-MOG, anti-CNPase (2´3´-cyclic nucleotide 3´phosphodiesterase), anti-PLP (proteolipid protein), anti-MBP (myelin basic protein) and 3 different antibodies directed against the TCC and C9neo (terminal complement complex, complement C9neo antigen). Anti-AQP4 (aquaporin-4) was stained in selected cases. Detailed antibody information is given in the Web extra table 1. Positive controls and negative controls (omitting the primary antibody) were used for all antibodies.
Classification of lesions
Lesions were first staged according to demyelinating activity based on published criteria.2 Early active lesions contained myelin–laden macrophages immunoreactive for minor (CNPase, MOG, MAG) and major myelin proteins (MBP, PLP), whereas late active lesions contained macrophages immunoreactive for major myelin proteins only. Inactive areas lacked myelin-laden macrophages. As early active demyelination represents the earliest demyelination stage (Fig 1), it is an absolute prerequisite for immunopattern classification.
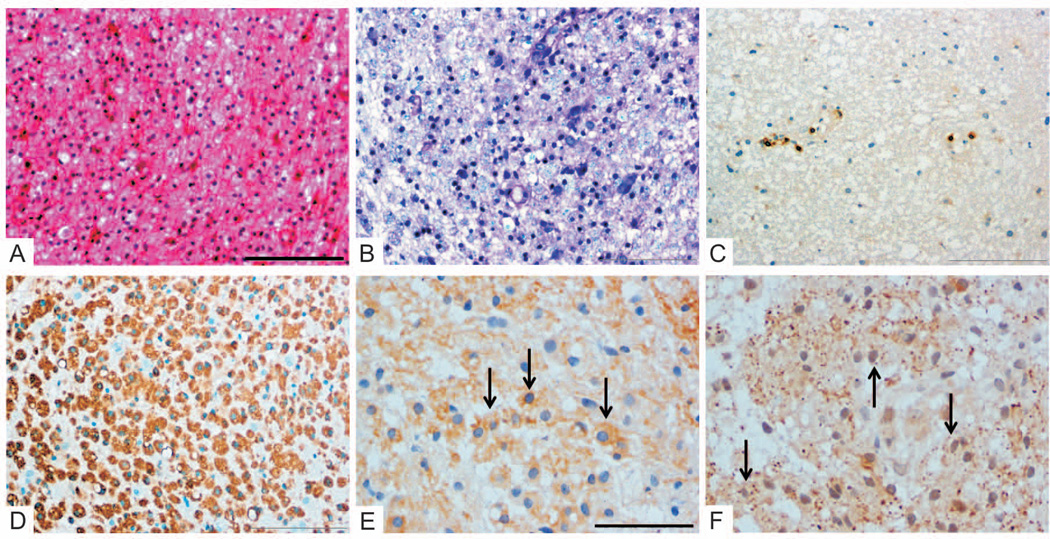
Figure 1. Early active demyelinating MS lesion.
Early MS lesions are characterized by cellular lesions infiltrated by numerous macrophages and the presence of reactive gliosis (A: HE). Lesions are demyelinated and display LFB-positive degradation products within macrophages, indicating recent myelin breakdown (B: LFB/PAS). T cell inflammation is variable and may be low (C: anti-CD3), whereas macrophages are abundant (D: KiM1P staining). Early active demyelinating lesions, which represent the earliest lesion stage, contain macrophages immunoreactive for minor myelin proteins (E: anti-MOG, F: anti-MAG, single of the numerous myelin laden macrophages are indicated by arrows).
Original magnifications: A–D: ×20, E/F: ×40; Scale bars: A: 200µm, E: 100µm.
Among 1321 surgical biopsies pathologically diagnosed with MS-compatible inflammatory demyelination, 79 were biopsied ≥2 times or an autopsy was performed at some point following initial biopsy. Twenty-two cases met study inclusion criteria (17 two biopsies (bx/bx), 4 with a biopsy followed by an autopsy (bx/ax), and 1 with serial biopsies followed by an autopsy (bx/bx/ax)). Among the 17 serially biopsied patients, one had three biopsies, but only the first and third biopsy contained active demyelinating lesions.
Early active lesions in this cohort were next classified into immunopathological patterns I–IV according to published criteria,3 by a minimum of 3 study investigators (IM, WB, HL, and/or CFL), each blinded to immunopattern classification rendered on previous tissue samples from the same patient. There was agreement among the investigators regarding immunopattern classification on all included cases.
Tissue sections were analyzed using an Olympus BX41 microscopy equipped with a DP20 camera (Olympus Optical Co, Ltd., Hamburg, Germany). Images were prepared in Corel PHOTO-PAINT®, version 13 (including adjustment of contrast and brightness).
Clinical and Radiographic Follow-up
Clinical information was obtained via multiple sources: medical record review (n=22); face-to-face encounter and examination (n=8); patient letter or telephone contact (n=5); and family or physician contact (n=3). Clinical course was categorized based on established criteria.12 Patients were diagnosed as having definite or probable MS by McDonald or Poser criteria.13,14 Patients with a single neurological episode at last follow-up were classified as a clinically-isolated syndrome (CIS).
Limited retrospective MRI information was available on a subset of cases that met study inclusion. Ten patients had at least one MRI scan prior to each biopsy, as well as at least one post-biopsy MRI scan available for review. An additional 2 cases had only post-biopsy MRI scans available. Among these 10 cases, 5 had two biopsies performed at different lesion locations, with an MRI obtained ≤ 1 month prior to each biopsy. Biopsied lesions were analyzed with respect to the presence of well-defined or ill-defined lesion margins, evidence for T2-weighted hypointense rims, and enhancement pattern [i.e. homogenous, heterogenous, ring or arc-like, punctuate, nodular, other]. Pre- as well as post-biopsy MRI scans were further assessed for the presence of uni- versus multifocal lesions, and whether Barkhof criteria was met.15
Results
Clinical features stratified by immunopattern classification are summarized in Table 1, with index attack symptoms leading to initial biopsy summarized in Figure 2. All three immunopatterns had a similar duration from first attack/symptom onset to first biopsy.
Table 1.
Clinical characteristics of study subjects. Values shown are number (%) or median (minimum, maximum).
| Characteristic | All subjects | Pattern I | Pattern II | Pattern III | Overlap case |
|---|---|---|---|---|---|
| Number of patients | 22 | 5 | 14 | 2 | 1 |
| Men, n (%) | 13 (59) | 2 (40) | 8 (57) | 2 (100) | 1 (100) |
| Age at symptom onset, y | 40 (10, 66) | 22 (10, 40) | 42 (32, 66) | 51 (43, 58) | 23 |
| Duration symptom onset to first biopsy, days | 20 (3, 9·1 y) | 26 (5, 81) | 20 (3, 9·1 y) | 26 (11, 40) | 3 |
| Duration between biopsies, days | 50 (14, 7·6 y) | 27 (23, 352) | 114 (14, 7·6 y) | 20 | n/a |
| Duration first biopsy to autopsy, days | 51 (1, 9·9 y) | n/a | 51 (1, 9·9 y) | 17 | 315 |
| Duration symptom onset to last follow-up, y | 10·0 (0·2, 17·5) | 11·5 (5·3, 17·5) | 10·0 (0·3, 16·1) | 4·6 (0·2, 9·1) | 0·9 |
| Clinical course prior to biopsy, n (%) | |||||
| First neurological event | 15 (79) | 4 (80) | 8 (73) | 2 (100) | 1 (100) |
| Relapsing-remitting | 4 (21) | 1 (20) | 3 (27) | 0 | 0 |
| Clinical course at most recent follow-up, n (%) | |||||
| Monophasic | 2 (11) | 1 (20) | 1 (9) | 0 | 0 |
| Relapsing-remitting | 10 (53) | 3 (60) | 6 (55) | 0 | 1 (100) |
| Secondary progressive | 6 (32) | 0 | 4 (36) | 2 (100) | 0 |
| Progressive relapsing | 1 (5) | 1 (20) | 0 | 0 | 0 |
| Diagnosis at most recent follow-up, n (%) | |||||
| Multiple sclerosis | 17 (89) | 4 (80) | 10 (91) | 2 (100) | 1 (100) |
| Clinically isolated syndrome | 2 (11) | 1 (20) | 1 (9) | 0 | 0 |
| Estimated EDSS at index attack | 4 (1, 10) | 4 (1, 8) | 3 (2, 10) | 7 (4, 10) | 4 |
| Estimated EDSS at last follow-up | 6 (1, 10) | 2 (1, 10) | 6 (1, 10) | 8 (6, 10) | 5 |
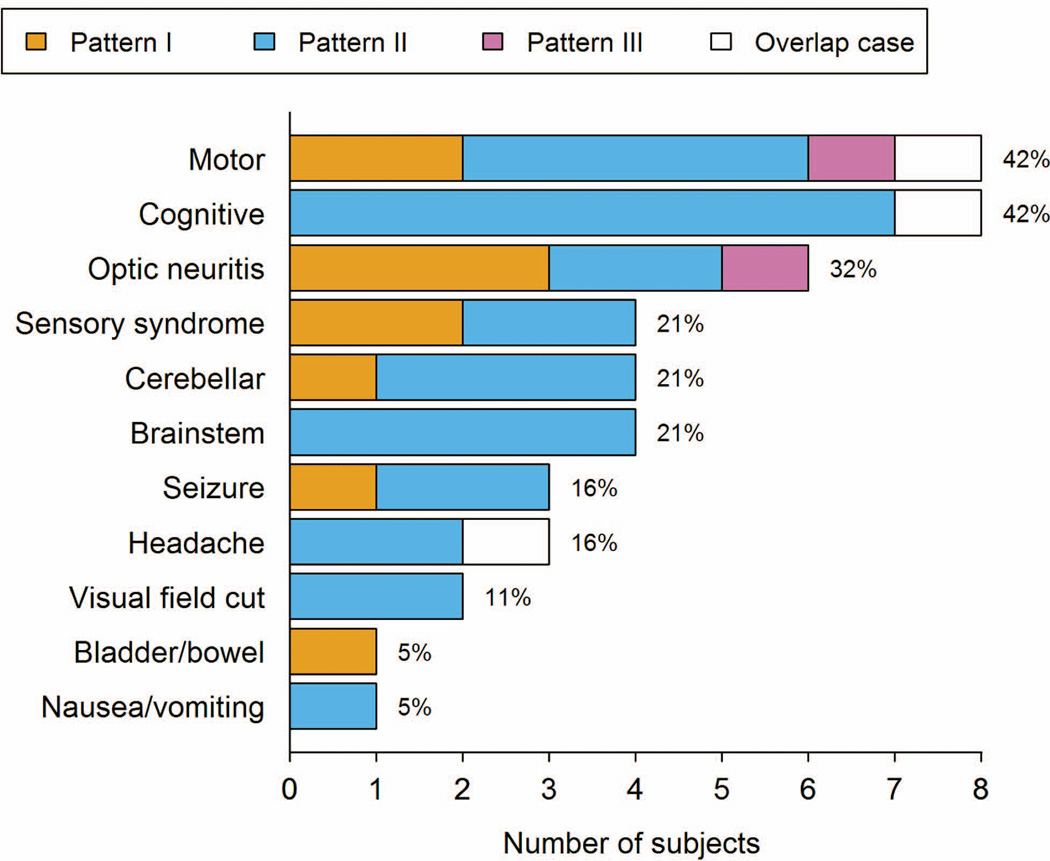
Figure 2.
Bar plot of symptoms leading to initial biopsy overall and relative to immunopattern. Percentages shown are based on 19 subjects with documented symptoms. (We could not ascertain symptoms for three subjects.) Subjects can have multiple symptoms.
Nineteen cases had detailed clinical information, with 89% having clinical definite MS and 11% CIS at last follow-up. Fifteen patients had acute attack treatment data available. Eight received steroids (prednisone, dexamethasone, or intravenous methylprednisolone) a median of 2 weeks prior to the initial biopsy (range 3 days-4 months), whereas 7 received steroids shortly after the first biopsy (median 2 weeks, range 1 day-2 months). Three patients received plasma exchange (2 pre-biopsy, 1 post-biopsy), and 11 patients long-term immunomodulatory treatments. Nine were treated following the first biopsy (2 mitoxantrone; 2 intravenous immunoglobulins; 2 cyclophosphamide; 1 natalizumab; 1 interferon beta-1a; 1 azathioprine) and 2 after the second biopsy (1 interferon beta-1b; 1 rituximab). No correlation between immunopattern and therapy administered was found. However, small numbers preclude definitive conclusions.
Persistence of immunopathological patterns
An identical immunopathological pattern was observed across serially sampled lesions from two distinct time points in 21/22 patients (95%). Immunopathological distribution in the cohort included 5 pattern I (all bx/bx); 14 pattern II (11 bx/bx; 2 bx/ax, and 1 bx/bx/ax); and two pattern III (1 bx/bx; 1 bx/ax) cases. We describe one case separately below. Pattern IV was not found, consistent with its rarity. To provide a numeric measure of agreement between baseline and follow-up immunopattern we calculated an intraclass kappa statistic based on a two-ratings, three-category model.16 The observed value of 0.91 (approximate 95% CI, 0.74 to 1.0) can be interpreted as percentage agreement corrected for chance. This is generally considered very high,17 although we interpret it cautiously as kappa can be affected by baseline prevalences and is sensitive to different patterns of agreement.
Figures 3 and 4 show representative cases with serially sampled lesions from one pattern I and one pattern II patient, respectively. Pattern I and II active lesions demonstrated similar loss of MOG (Fig 3A/E; Fig 4A/D) and MAG (Fig 3B/F; Fig 4B/E) in both the first biopsy and the subsequent biopsy/autopsy. In addition, the pattern II case showed complement activation products within macrophages in both biopsies (Fig 4C/F). Figure 5 demonstrates pattern III persistence among active lesions in both biopsies from the same patient. The pattern III lesions were characterized by a preferential loss of MAG (Fig 5B/F) relative to MOG (Fig 5A/E), and apoptotic oligodendrocytes (Fig 5C/G).
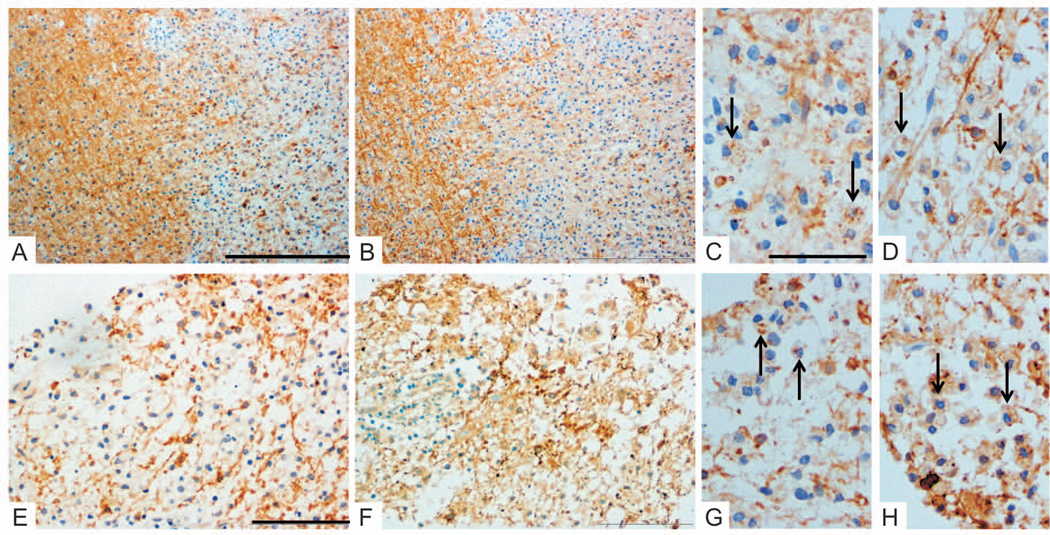
Figure 3. Persistence of pattern I pathology.
Pattern I immunopathology was observed in both biopsies from the same MS patient performed at different time points (interval 3mo 28days, patient #2) and from different lesion locations (left occipital and right parieto-occipital). Early active lesions in both specimens revealed demyelination with similar loss of myelin proteins (A/C/E/G: anti-MOG, B/D/F/H: anti-MAG). Minor myelin proteins within macrophages indicate early active demyelination (arrows in C,D,G,H). Complement activation products within macrophages were absent (not shown).
Original magnifications: A/B: ×10, C/D/G/H: ×40, E/F: ×20; Scale bars: A: 500µm, C: 100µm, E: 200µm.
Figure 4. Persistence of pattern II pathology.
Pattern II immunopathology was observed in both biopsies from the same MS patient performed at different time points and same lesion location (interval 1mo 8days, patient #18). Early active lesions in both specimens revealed demyelination with similar loss of myelin proteins (A/D: anti-MOG, B/E: anti-MAG, inserts in higher magnification to show minor myelin proteins within macrophages), as well as complement activation products within macrophages (C/F: anti-TCC).
Original magnifications: A/B: ×20, C/F: ×40, D/E: ×10; Scale bars: A: 200µm, C: 100µm, D: 500µm.
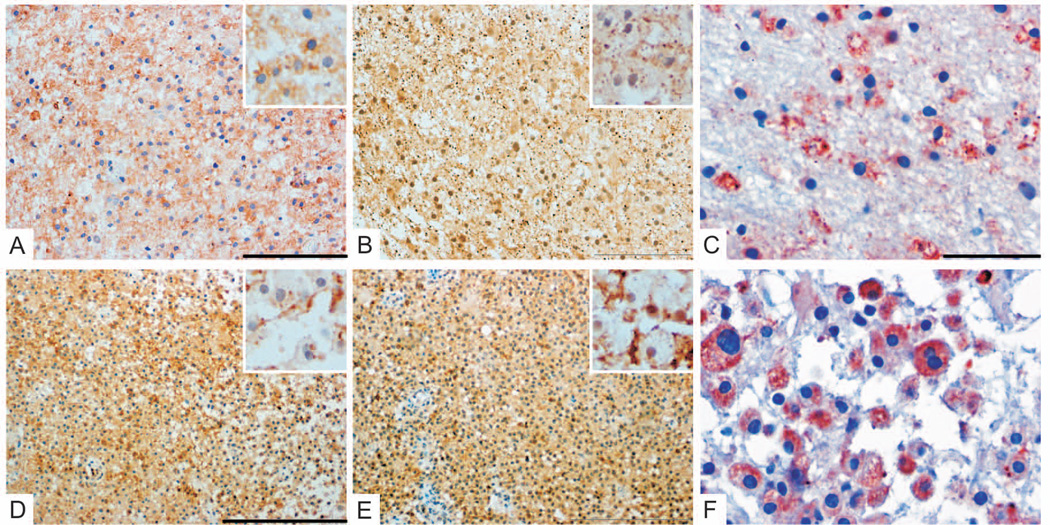
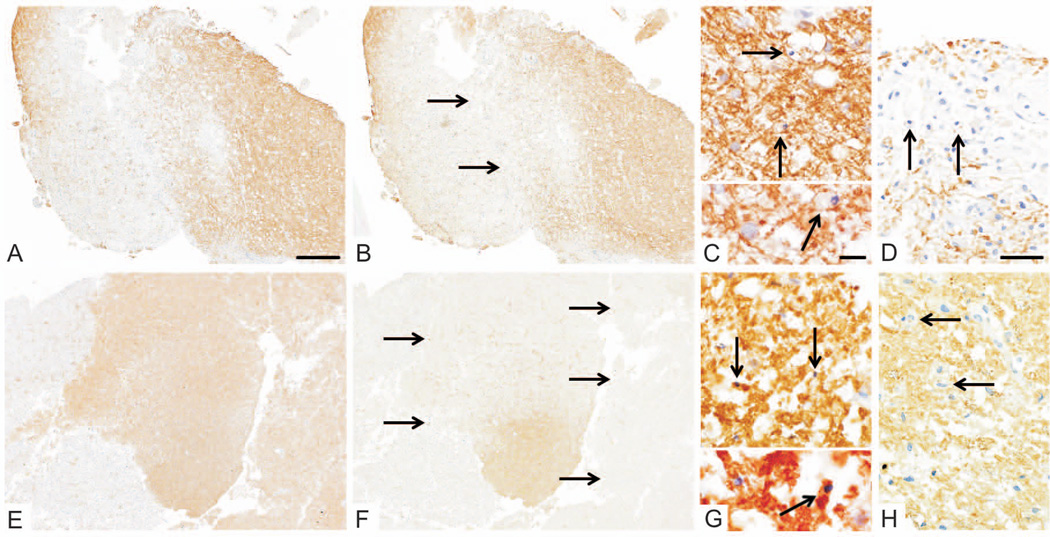
Figure 5. Persistence of pattern III pathology.
Pattern III pathology is observed in subsequent biopsies from the same patient (interval 20days, patient #21) with the same lesion biopsied twice. Both specimens were characterized by a preferential loss of MAG (B/F, MAG loss indicated by arrows) relative to MOG (A/E). Numerous apoptotic oligodendrocytes were present in areas of MAG loss (arrows, C: anti-CNPase, G: anti-PLP). Minor myelin proteins within macrophages indicate early active demyelinating activity (arrows; D/H: anti-MOG).
Original magnifications: A/B/E/F: ×4, C/G: upper regions ×60, lower regions ×100, D/H: ×40; Scale bars: A: 500µm, C: 50µm for upper region and 10µm for lower region, D: 20µm.
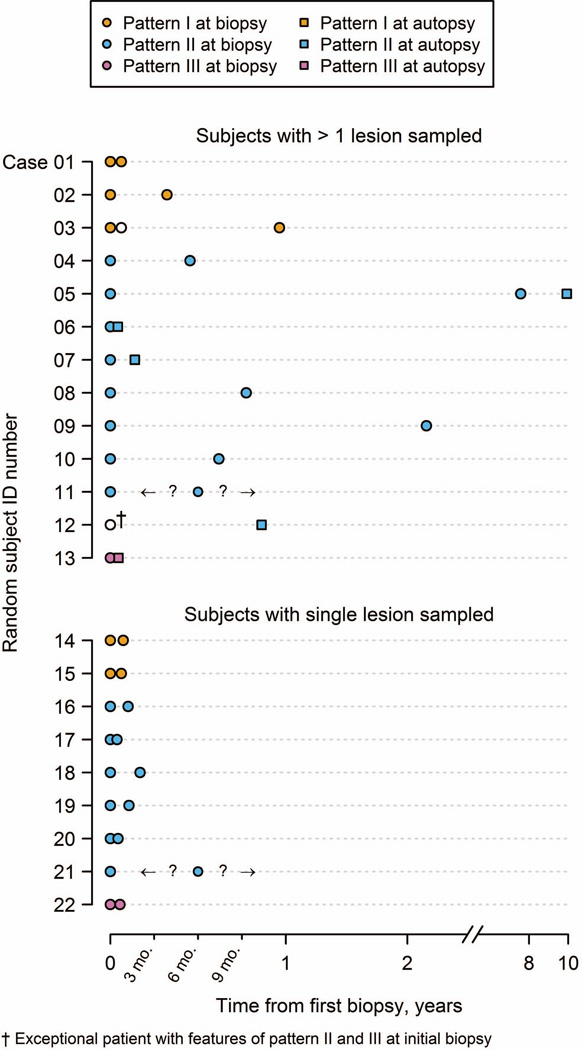
Among the 21 patients, active lesions biopsied from different anatomical regions within the same patient were available for immunopattern classification in 12 cases (8 bx/bx; 3 bx/ax; 1 bx/bx/ax), whereas 9 (all bx/bx) had the same lesion biopsied on two separate occasions. Figure 6 summarizes the immunopattern classification relative to both timing of biopsy or autopsy, and distinguishes between cases with a single lesion biopsied twice, versus more than one lesion sampled from two different regions. Autopsied subjects containing multiple early active demyelinating lesions (n=4 cases) uniformly showed intraindividual homogeneity in immunopathological pattern across their active lesions.
Figure 6.
Relationship of immunopatterns relative to time of biopsy and/or autopsy. To better show the data, the x-axis is linear for the first twelve months and logarithm thereafter. Question marks indicate unknown second biopsy date.
Exceptional patient with histological features associated with pattern II and pattern III
A single bx/ax case suggested the presence of different immunopattern classifications between the initial biopsy and subsequent autopsy. Brain biopsy revealed complement activation products within macrophages suggestive of pattern II, and a small pattern III-like discrete area containing scattered apoptotic oligodendrocytes and MAG-loss. The autopsy performed 10 months later contained several small active demyelinating lesions, all demonstrating pattern II, but no pattern III pathology. This patient was diagnosed with relapsing-remitting MS at last follow-up, and died due to valproate-induced hepatotoxicity.
Identical MRI features in consecutively biopsied lesions
Among the twelve patients with pre and/or post-biopsy scans available for review, multifocal lesions were present on 6 pre-biopsy (1 pattern I, 4 pattern II, and 1 pattern III) and 9 post-biopsy (1 pattern 1, 5 pattern II, 2 pattern III, and the pattern overlap case) studies. Four cases met Barkhof criteria on a pre-biopsy scan (1 pattern I, and 3 pattern II), and 7 on a post-biopsy scan (1 pattern I, 5 pattern II, 1 pattern III). Radiographic features of lesions biopsied in two different locations were similar among 5 patients. Both biopsied lesions from two pattern II cases demonstrated well defined margins, as well as colocalization of a T2W hypointense rim, and gadolinium ring enhancement. The biopsied lesion characteristics were also similar in three additional cases (two pattern I and one pattern II) and were characterized on both pre-biopsy scans by a more diffuse lesion margin edge and heterogenous gadolinium enhancement without an associated T2W hypointense rim.
Discussion
The pathological evolution of MS lesions has not been thoroughly investigated due to the rarity of having multiple archival brain tissue samples available for analysis from the same patient at different disease time points. This study provided a unique opportunity to examine active demyelinating lesions longitudinally to determine whether active lesions from the same patient show a consistent immunopathological pattern over time. We previously reported that multiple early active lesions in the same MS patient are homogenous with respect to immunopatterns.3,18 We now describe in a larger cohort that, within an individual, distinct immunopatterns persist in active lesions, i.e., display temporal homogeneity. All three demyelination patterns (I–III) persisted over time, with pattern II being most frequent.3 We also demonstrate that pathological homogeneity is chronologically preserved both when serial biopsies target the same active lesion at different time points, and when active lesions located in different regions are examined via a subsequent biopsy or at autopsy. Furthermore, detailed clinical follow-up revealed most patients had MS at last follow-up (n=17). Taken together, our findings reinforce the concept of intraindividual immunopathological homogeneity and interindividual immunopathological heterogeneity in early MS. Pathological heterogeneity may reflect either distinct triggers or different host genetic factors influencing the character of immune-mediated inflammation, and the susceptibility of the tissue with respect to glial, axonal and neuronal injury.
This hypothesis of patient-dependent heterogeneity has not been universally accepted. According to Barnett and Prineas (2004), all MS lesions begin with a pattern III-like lesion with oligodendrocyte apoptosis, followed by superimposed pattern II-like demyelination and complement activation.6 The authors concluded that an overlap of immunopathologic patterns was found within a patient, and proposed heterogeneity is stage, rather than patient-dependent. While this could plausibly explain our single case with an ambiguous overlap of immunopathological patterns, if in fact immunopatterns evolved over time, we would have expected additional serial biopsy or autopsy cases to demonstrate pattern overlap in both the current and previously published studies. Since MAG loss is a marker of metabolically stressed oligodendrocytes, we cannot exclude that superimposed hypoxic or ischemic confounding factors possibly related to the biopsy itself may have contributed to the MAG loss seen in this single pattern overlap biopsy. The subsequent autopsy in this patient permitted more extensive sampling of additional active lesions. All demonstrated pattern II, with no evidence of pattern III.
If all lesions began as pattern III, as suggested by Barnett and Prineas, the time interval between symptom onset and biopsy or autopsy should be shorter among pattern III versus pattern II. No such correlation has been found in either the current series or in published series which included a larger number of immunopattern classified cases.19 Furthermore, most evidence suggesting MS lesion formation is stage dependent is based on a pediatric autopsy case of presumed fulminant MS, with a clinical presentation and clinical course possibly more compatible with NMO.6 We recently reported that a subset of active demyelinating NMO supraspinal lesions simultaneously show pathological features resembling an overlap of MS pattern II and III. This strikingly resembles the stage-dependent characteristics described by Barnett and Prineas in a brain stem lesion, raising the possibility of a misclassified case of NMO.20
Another study suggested antibody and complement-mediated myelin phagocytosis was the dominant mechanism of demyelination in all lesions examined among patients with established MS.7 However this study analyzed MS lesions from late disease stages (median 22.2 years) and did not use the same classification of lesion activity used by Lucchinetti et al. which examined an early MS cohort (median 3.8 years).3 Therefore given their rare occurrence in chronic MS, it is unlikely early active lesions (a prerequisite for immunopattern classification) were available for analysis in this published series.
It must be emphasized that all cases enrolled in our current study required the presence of early active lesions, implying they had “active” disease. Furthermore, the median duration between serial biopsies or biopsy and autopsy in our cohort was short (median 50 days and 51 days, respectively). A single pattern bx/bx/ax pattern II case had a considerably longer interval (case 5) whose second biopsy was 7.6 years and autopsy 9.9 years after the first biopsy. It remains to be determined to what extent immunopathological heterogeneity persists into chronic disease phases, when active demyelination is less common.21
Several independent laboratories have published studies supporting the concept of pathogenic heterogeneity in early MS. Distinct cellular expression patterns of chemokine receptors were observed in pattern II and III MS lesions;22 antigen microarrays identified unique serum autoantibody signatures in pattern II versus I;23 mitochondrial defects have been described only in pattern III;24 and pattern II patients were more likely to respond favorably to plasma exchange than patterns I or III.25 Taken together, we conclude that the preponderance of published pathological evidence continues to support our hypothesis of interindividual heterogeneity and intraindividual homogeneity in MS.
Although our conclusions are based on a cohort of patients with biopsy-proven inflammatory demyelinating disease which, by definition, carry potential biases, published evidence suggests that these findings can be extrapolated to prototypic MS. Despite atypical clinical presentations (i,e, tumefactive lesions on MRI; seizures, etc), we found most patients (89%) developed prototypic MS at last follow-up. We previously reported that a series of biopsy cases (n=91) developed clinically definite or probable MS during a median follow up of 4.4 years. Subsequent clinical course and disability in this biopsy patient group was indistinguishable from the non-biopsied Olmsted County prevalence cohort matched for disease duration, age, and sex (n>200).19 A subsequent clinical-radiographic study of 168 patients with tumefactive biopsied inflammatory demyelinating lesions reported 70% developed definite MS at last follow-up and another 9% developed probable MS.26 In these cases, 83% presented with multiple lesions and 55% fulfilled Barkhof radiographic criteria for MS at the time of last MRI.26 Furthermore, among biopsy patients with inflammatory cortical demyelination and long-term clinical follow-up, 75% were diagnosed with definite MS, comparable to our published cohorts.27 The extent to which serially biopsied cases are less representative than the broader cross-sectional biopsy cohort is difficult to evaluate. Age at symptom onset was quite similar between the two cohorts. There was a lower proportion of women in the current study but this difference was not statistically significant. Those in the current study did tend to have a shorter time from symptom onset to biopsy on average, although nearly a third of the larger cohort was biopsied within one month of symptom onset. Of the 15 cases with serial biopsy (rather than biopsy followed by autopsy) and available clinical data, 13/15 received a diagnosis of MS by last follow-up. We suspect that a second biopsy mostly reflects chance occurrences, such as having a misread or inconclusive initial biopsy, or a progressive clinical or radiographic course despite steroid therapy, rather than a systematic difference in underlying pathology. These findings lead us to conclude that patients whose demyelinating disease was diagnosed atypically by biopsy nevertheless comprise a representative and informative cohort of MS patients.
Limitations of our study include variable tissue preservation and fixation, and biopsy-associated bleeding, which have the potential to limit reliable immunohistopathological characterization. However we restricted analysis to serial cases with sufficient tissue and reliable staining at two or more time points in order to interpret immunopattern classification. Pattern II lesions may also be over-represented in our biopsy cohort. Ring-enhancement on MRI correlates with the dense accumulation of macrophages at the active plaque border, a histopathological feature typical of pattern I and II lesions.28 It is therefore possible that ring-enhancing lesions were more likely to be biopsied to exclude tumor. Available radiographic data on the cohort was also limited. In addition, given the low number of pattern III lesions with a relatively short interval between the two biopsies, one cannot completely exclude the possibility that pattern III could be a precursor of other patterns. However if this was the case, we would have expected to more commonly see mixed patterns within a person. It is also important to interpret our findings in this serial biopsy/autopsy cohort in the context of existing background knowledge about cross-sectional intra-patient pattern homogeneity.
In our present cohort, pattern II lesions, indicative for involvement of pathogenic auto-antibodies, were dominant. However, in contrast to NMO, it has been notoriously difficult to identify potentially pathogenic auto-antibody reactions in MS patients. Antibodies against MOG epitopes and neurofascin have been described in subsets of patients.29–33 Proof of principle for distinguishing different patterns of demyelination in MS was demonstrated by antigen microarray profiles that analysed patterns of antibody reactivity in MS serum against CNS proteins, lipids and heat shock proteins.23 However, this study did not address whether these antibodies were directed against epitopes expressed on the surface of myelin or oligodendrocytes.
Future research needs to focus on the therapeutic relevance of immunopathological patterns. Proof of principle for a MS pattern-specific therapeutic response was reported by Keegan et al. 2005, who reported that plasma exchange for steroid-refractory MS attacks was selectively effective in pattern II patients.25 Variable responses of MS patients to current immunomodulatory therapies may relate in part to differences in the underlying effector mechanisms of tissue damage. Pattern I MS lesions, in which T cells and macrophages predominate might be more responsive to either interferons or glatiramer acetate which act on macrophages/microglia,34 whereas B cell-targeted therapies, such as rituximab and ocrelizumab, may plausibly be more effective in patients with pattern II pathology.35 In patients with pattern III, mitochondrial defects have been described, therefore targeting pathways limiting oxidative damage may be particularly effective.
In conclusion, this study demonstrates that the same immunopattern persists within a patient during active disease and brings additional evidence for patient dependent heterogeneity in immunopathological patterns, suggesting the mechanisms and targets of tissue injury in early active MS lesions may differ among patient subgroups. While histological analysis is presently the gold standard to determine those patterns, there remains a critical need to identify paraclinical or radiographic markers which reliably differentiate immunopatterns in order to eliminate biopsy-related risks, and to facilitate prospective identification and longitudinal follow-up of immunopattern-classified non-biopsied MS patients. Although we observed among 5 cases serially biopsied from different locations that the pre-biopsy radiographic features of both biopsied lesions were similar, ongoing studies on a larger cohort of biopsied MS cases will determine whether specific MRI features correlate with specific immunopatterns. Our observations have potentially significant therapeutic implications, as individualized therapeutic approaches to treat early relapsing disease may need to be considered if different patterns of active demyelination persist within a given patient, rather than show a stage-dependent change.
Supplementary Material
Acknowledgement
This work was supported by grants from the German Ministry for Education and Research (BMBF, “German Competence Network Multiple Sclerosis” (KKNMS), Pattern MS/NMO) (to IM, WB), the Saskatchewan Health Research Foundation (to BFP), the Canada Research Chairs program (to BFP), the National Multiple Sclerosis Society (NMSS RG3185-B-3 to CFL)), and the National Institutes of Health (1R01NS049577, to CFL). We acknowledge Sven Müller for outstanding administrational help and Doris Bode and Patricia Ziemer for excellent technical assistance. We thank Prof. Paul Morgan who provided antibodies.
IM received grants from the German Ministry for Education and Research (BMBF, “German Competence Network Multiple Sclerosis” (KKNMS), Pattern MS/NMO) and speaking honoraria and travel expenses from BiogenIdec, BayerHealthCare and Serono. BFP has served as a speaker for Teva Canada Innovation and receives research support from the Saskatchewan Health Research Foundation and the Canada Research Chairs program. WB received grants from the German Ministry for Education and Research (BMBF, “German Competence Network Multiple Sclerosis” (KKNMS), Pattern MS/NMO) and honoraria for board membership and consultancy from Teva Pharma, BiogenIdec, Genzyme and Novartis as well as speaking honoraria from Teva Pharma, BiogenIdec, Merck-Serono, Novartis, Bayer Vital, Sanofi and Genzyme; grants to his institution were paid from Teva Pharma, BiogenIdec and Novartis. CFL receives research support from the NIH (NS49577-R01), the Guthy-Jackson Charitable Foundation, and the National Multiple Sclerosis Society (RG 3185 B 3). CFL shares in royalties from marketing of kits for detecting AQP4 autoantibody (#7101679 issued 2006) and from the sale of Blue Books of Neurology: Multiple Sclerosis 3 (Saunders Elsevier, 2010);
Role of the funding source
The funding sources did not play any role in writing of the manuscript and the decision to submit the manuscript for publication. We have not been paid to write this article by a pharmaceutical company or other agency. CFL and IM had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Conflicts of interest
SDW, JMF, JEP, GY and HL have nothing to disclose.
Authors´ contributions
WB, CFL and IM designed the study. Data collection was performed by BFP, JMF, JEP, HL, WB, YG, JF, CFL and IM. Data were analyzed by SDW, CFL, HL, WB, JF, and IM and interpreted by SDW, CL, WB, HL and IM. Figures were created by SDW, GY, CFL and IM. All authors were involved in manuscript preparation / writing the manuscript.
References
- 1.Anderson DW, Ellenberg JH, Leventhal CM, et al. Revised estimate of the prevalence of multiple sclerosis in the United States. Ann Neurol. 1992;31:333–336. doi: 10.1002/ana.410310317. [DOI] [PubMed] [Google Scholar]
- 2.Brück W, Porada P, Poser S, et al. Monocyte/macrophage differentiation in early multiple sclerosis lesions. Ann Neurol. 1995;38:788–796. doi: 10.1002/ana.410380514. [DOI] [PubMed] [Google Scholar]
- 3.Lucchinetti C, Brück W, Parisi J, et al. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 4.Aboul-Enein F, Rauschka H, Kornek B, et al. Preferential loss of myelin-associated glycoprotein reflects hypoxia-like white matter damage in stroke and inflammatory brain diseases. J Neuropathol Exp Neurol. 2003;62:25–33. doi: 10.1093/jnen/62.1.25. [DOI] [PubMed] [Google Scholar]
- 5.Itoyama Y, Webster HD, Sternberger NH, et al. Distribution of papovavirus, myelin-associated glycoprotein, and myelin basic protein in progressive multifocal leukoencephalopathy lesions. Ann Neurol. 1982;11:396–407. doi: 10.1002/ana.410110414. [DOI] [PubMed] [Google Scholar]
- 6.Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: Pathology of the newly forming lesion. Ann Neurol. 2004;55:458–468. doi: 10.1002/ana.20016. [DOI] [PubMed] [Google Scholar]
- 7.Breij EC, Brink BP, Veerhuis R, et al. Homogeneity of active demyelinating lesions in established multiple sclerosis. Ann Neurol. 2008;63:16–25. doi: 10.1002/ana.21311. [DOI] [PubMed] [Google Scholar]
- 8.van Bogaert L. Post-infectious encephalomyelitis and multiple sclerosis. The significance of perivenous emcephalomyelitis. J Neuropathol Exp Neurol. 1950;9:219–249. doi: 10.1097/00005072-195007000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Young NP, Weinshenker BG, Parisi JE, et al. Perivenous demyelination: association with clinically defined acute disseminated encephalomyelitis and comparison with pathologically confirmed multiple sclerosis. Brain. 2010;133:333–348. doi: 10.1093/brain/awp321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hart MN, Earle KM. Haemorrhagic and perivenous encephalitis: a clinical-pathological review of 38 cases. J Neurol Neurosurg Psychiatry. 1975;38:585–591. doi: 10.1136/jnnp.38.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wingerchuk DM, Lennon VA, Pittock SJ, et al. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66:1485–1489. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- 12.Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. Neurology. 1996;46:907–911. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]
- 13.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 14.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 15.Barkhof F, Filippi M, Miller DH, et al. Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain. 1997;120:2059–2069. doi: 10.1093/brain/120.11.2059. [DOI] [PubMed] [Google Scholar]
- 16.Chmura Kraemer H, Periyakoil VS, Noda A. Kappa coefficients in medical research. Stat Med. 2002;21:2109–2129. doi: 10.1002/sim.1180. [DOI] [PubMed] [Google Scholar]
- 17.Rosner B. Fundamentals of biostatistics. 7th ed. Boston: Brooks/Cole, Cengage Learning; 2011. [Google Scholar]
- 18.König FB, Wildemann B, Nessler S, et al. Persistence of immunopathological and radiological traits in multiple sclerosis. Arch Neurol. 2008;65:1527–1532. doi: 10.1001/archneur.65.11.1527. [DOI] [PubMed] [Google Scholar]
- 19.Pittock SJ, McClelland RL, Achenbach SJ, et al. Clinical course, pathological correlations, and outcome of biopsy proved inflammatory demyelinating disease. J Neurol Neurosurg Psychiatry. 2005;76:1693–1697. doi: 10.1136/jnnp.2004.060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruck W, Popescu B, Lucchinetti CF, et al. Neuromyelitis optica lesions may inform multiple sclerosis heterogeneity debate. Ann Neurol. 2012;72:385–394. doi: 10.1002/ana.23621. [DOI] [PubMed] [Google Scholar]
- 21.Prineas JW, Kwon EE, Cho E-S, et al. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. 2001;50:646–657. doi: 10.1002/ana.1255. [DOI] [PubMed] [Google Scholar]
- 22.Mahad DJ, Trebst C, Kivisakk P, et al. Expression of chemokine receptors CCR1 and CCR5 reflects differential activation of mononuclear phagocytes in pattern II and pattern III multiple sclerosis lesions. J Neuropathol Exp Neurol. 2004;63:262–273. doi: 10.1093/jnen/63.3.262. [DOI] [PubMed] [Google Scholar]
- 23.Quintana FJ, Farez MF, Viglietta V, et al. Antigen microarrays identify unique serum autoantibody signatures in clinical and pathologic subtypes of multiple sclerosis. Proc Natl Acad Sci U S A. 2008;105:18889–18894. doi: 10.1073/pnas.0806310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahad D, Ziabreva I, Lassmann H, et al. Mitochondrial defects in acute multiple sclerosis lesions. Brain. 2008;131(Pt7):1722–1735. doi: 10.1093/brain/awn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keegan M, König F, McClelland R, et al. Relation between humoral pathological changes in multiple sclerosis and response to therapeutic plasma exchange. Lancet. 2005;366:579–582. doi: 10.1016/S0140-6736(05)67102-4. [DOI] [PubMed] [Google Scholar]
- 26.Lucchinetti CF, Gavrilova RH, Metz I, et al. Clinical and radiographic spectrum of pathologically confirmed tumefactive multiple sclerosis. Brain. 2008;131(Pt 7):1759–1775. doi: 10.1093/brain/awn098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucchinetti CF, Popescu BF, Bunyan RF, et al. Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med. 2011;365:2188–2197. doi: 10.1056/NEJMoa1100648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brück W, Neubert K, Berger T, et al. Clinical, radiological, immunological and pathological findings in inflammatory CNS demyelination - possible markers for an antibody-mediated process. Mult.Scler. 2001;7:173–177. doi: 10.1177/135245850100700307. [DOI] [PubMed] [Google Scholar]
- 29.O'connor KC, McLaughlin KA, De Jager PL, et al. Self-antigen tetramers discriminate between myelin autoantibodies to native or denatured protein. Nat Med. 2007;13:211–217. doi: 10.1038/nm1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathey EK, Derfuss T, Storch MK, et al. Neurofascin as a novel target for autoantibody-mediated axonal injury. J Exp Med. 2007;204:2363–2372. doi: 10.1084/jem.20071053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitley J, Woodhall M, Waters P, et al. Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology. 2012 doi: 10.1212/WNL.0b013e31826aac4e. [DOI] [PubMed] [Google Scholar]
- 32.Di PF, Mader S, Rostasy K, et al. Temporal dynamics of anti-MOG antibodies in CNS demyelinating diseases. Clin Immunol. 2011;138:247–254. doi: 10.1016/j.clim.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 33.Rostasy K, Mader S, Schanda K, et al. Anti-myelin oligodendrocyte glycoprotein antibodies in pediatric patients with optic neuritis. Arch Neurol. 2012;69:752–756. doi: 10.1001/archneurol.2011.2956. [DOI] [PubMed] [Google Scholar]
- 34.Weber MS, Prod'homme T, Youssef S, et al. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat Med. 2007;13:935–943. doi: 10.1038/nm1620. [DOI] [PubMed] [Google Scholar]
- 35.Aktas O, Kieseier B, Hartung HP. Neuroprotection, regeneration and immunomodulation: broadening the therapeutic repertoire in multiple sclerosis. Trends Neurosci. 2010;33:140–152. doi: 10.1016/j.tins.2009.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.