Summary
Objectives
The prevalence of vitamin D insufficiency in Africans with AIDS-associated Kaposi sarcoma (AIDS-KS) and the role of vitamin D in AIDS-KS progression are unknown. We hypothesized that a high prevalence of vitamin D deficiency would be found in Zimbabweans with AIDS-KS and that low baseline vitamin D would correlate with progression of AIDS-KS.
Methods
Ninety subjects were enrolled in a prospective pilot study investigation of the effect of antiretroviral therapy in the treatment of AIDS-KS in Harare, Zimbabwe. Co-formulated abacavir, lamivudine, and zidovudine was initiated; chemotherapy was provided at the discretion of the provider. Participants were followed for 96 weeks. 25-Hydroxyvitamin D was measured in stored specimens collected at study entry. The relationship between vitamin D and clinical response was described by odds ratio and 95% confidence interval.
Results
Samples were available for 85 participants; 45 (53%) subjects had inadequate (<75 nmol/l) 25-hydroxyvitamin D. HIV-1 RNA was significantly higher among those with insufficient vitamin D (4.7 vs. 4.5 log, p = 0.04). Tumor response, survival, and KS-IRIS were not associated with vitamin D (p ≥0.3).
Conclusions
Vitamin D insufficiency was common among Zimbabweans with AIDS-KS but not associated with outcomes after initiation of antiretroviral therapy.
Keywords: AIDS-KS, Vitamin D insufficiency, HIV
Introduction
AIDS-associated Kaposi’s sarcoma (AIDS-KS) is the most common tumor in Zimbabwe.1 Without antiretroviral therapy (ART), AIDS-KS is associated with more than 85% mortality, due to progression of KS or complications associated with advanced AIDS.2 With effective ART, the mortality associated with AIDS-KS is improved, but the response of clinical AIDS-KS lesions is discouraging, at less than 20%.3
Moderate associations of low vitamin D levels with cancer progression have been observed in stomach, esophageal, gallbladder, colon, breast, prostate, and pancreatic cancer.4–7 Cancers with the most sensitive vitamin D effect appear to be those of rapidly proliferating tissue, specifically the oral–gastrointestinal tract and the bone marrow.8 Forms of vitamin D have been shown to inhibit tumor angiogenesis, and calcitriol both inhibits9–11 and upregulates vascular endothelial growth factor and angiogenesis in non-KS tumors.12,13 Little is known about the effect of vitamin D or the vitamin D receptor on vascular tumors such as KS. In vitro, both the activated form of vitamin D, 1,25-dihydroxycholecalciferol (1,25(OH)2D, calcitriol), and a vitamin D analogue have demonstrated inhibition of KS transformed cell lines.14–18 Mice with implanted KS tumors treated with calcitriol or a vitamin D analogue showed significant slowing of tumor growth.14,17 Of eight male patients with KS treated with the topical synthetic vitamin D receptor agonist calcipotriene, 50% had improvement in cutaneous lesions.14
A high prevalence of vitamin D insufficiency occurs in persons with HIV-1 infection in the USA and in European countries.19–22 Persons recently emigrated from Africa to the USA, Europe, or Australia often have vitamin D insufficiency.23–26 Existing data on vitamin D among persons with or without HIV-1 residing in Sub-Saharan Africa also suggest a high prevalence of vitamin D insufficiency or deficiency.27–32 Based on the existing epidemiological and in vitro and in vivo data described above, we hypothesized that a high prevalence of vitamin D deficiency would be found in Zimbabweans with AIDS-KS and that low baseline vitamin D would be associated with the progression of AIDS-KS.
Methods
Study population
The current study was a retrospective analysis of existing data and stored specimens from 90 subjects enrolled in a prospective investigation of the effect of ART in the treatment of AIDS-KS (Clinicaltrials.gov number NCT00834457) at the Parirenyatwa Hospital Kaposi Sarcoma Clinic in Harare, Zimbabwe between June 2003 and May 2005.3 The study was reviewed and approved by the Medical Research Council of Zimbabwe and the Colorado Multiple Institutional Review Board, and informed consent was obtained from all participants. All participants had KS confirmed by histopathological examination. Other entry criteria included age >18 years, confirmed presence of HIV-1 antibody, hemoglobin >7.5 g/dl, absolute neutrophil count >750 cells/μl, agreement not to participate in a conception process during study participation, ART naïve, no receipt of chemotherapy or radiation therapy ≤45 days before study entry, and no intention to relocate out of the Harare area for the duration of study participation. Co-formulated abacavir, lamivudine, and zidovudine was initiated in all participants on day 0. Participants were followed for 96 weeks.3
Clinical and immunological/virological outcomes
The stage of KS was assessed using the criteria of both Krigel et al. and the AIDS Clinical Trials Group, as described previously.3,33,34 Clinical response was defined as either complete or partial resolution of clinically apparent KS disease at week 96. Complete resolution was defined as the absence of KS lesions and tumor-related edema for a minimum of 4 weeks, lasting until the end of the follow-up period. Partial resolution was defined as ≥50% improvement in KS lesions without complete resolution. KS-associated immune reconstitution inflammatory syndrome (KS-IRIS) was defined as any progression of KS occurring ≤12 weeks after initiation of ART and was associated with an increased CD4+ lymphocyte count of at least 50 cells/μl above the baseline value, before or at the time of documented KS progression. Virological failure was defined as failure to suppress or failure to maintain suppression of the plasma HIV-1 RNA level to <400 copies/ml after week 24.
CD4 count, plasma HIV-1 RNA, and HHV-8 DNA in peripheral blood mononuclear cells (PBMC) and plasma were measured as described elsewhere.3,35 Plasma samples obtained at study entry were assayed for 25-hydroxyvitamin D (25(OH)D) with the Liaison immunoluminometric direct assay (DiaSorin, Stillwater, MN, USA). Vitamin D deficiency was defined as 25(OH)D ≤50 nmol/l and insufficiency as >50 nmol/l but <75 nmol/l. Inadequate and adequate 25(OH)D were defined as <75 nmol/l and ≥75 nmol/l, respectively.20,36,37 The rainy season was defined as November through April and the dry season as May through October.
Data analyses
Continuous variables are reported as the median and interquartile range (IQR) and categorical variables as the frequency and percentage. Continuous variables were tested with the Mann–Whitney test and categorical variables with the Chi-square test or Fisher’s exact test. The relationship between vitamin D and clinical response was described by odds ratio (OR) and 95% confidence interval (95% CI) and tested with a Chi-square or Fisher’s exact test. Logistic regression models were used to adjust for possible confounding of HIV-1 viral load and CD4 count in predicting clinical outcomes. Other analyses were considered exploratory and were not corrected for multiple comparisons. Statistical analyses were conducted in SAS v. 9.2 (SAS Institute) and GraphPad Prism (GraphPad Software Inc.), and assumed a two-sided significance level of 0.05.
Results
Prevalence of vitamin D insufficiency and study participant characteristics
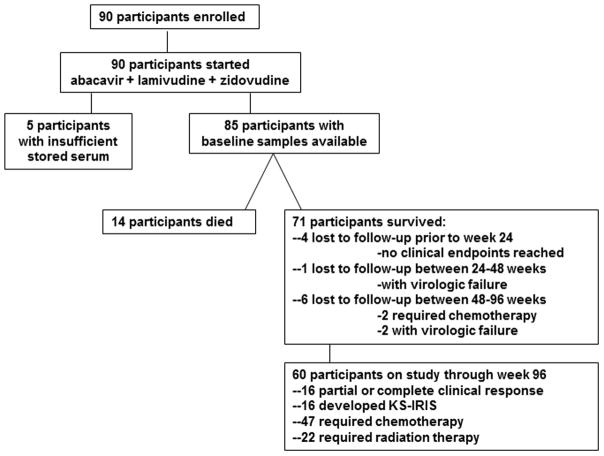
Baseline serum samples were available for 85 of 90 subjects. Of those with available samples for analysis, the median 25(OH)D was 72.5 nmol/l (IQR 56.3–88.8 nmol/l). Forty-five (53%) subjects had inadequate 25(OH)D and 17 (20%) had 25(OH)D insufficiency. Fourteen subjects died, 11 subjects were lost to follow-up prior to week 96, and 60 subjects completed 96 weeks of observation (Figure 1). Further characteristics of the study population are provided in Table 1. Those enrolling during the rainy season had higher median 25(OH)D levels (78.75 vs. 62.5 nmol/l, p = 0.01).
Figure 1.
Flowchart of study participants.
Table 1.
Comparison of demographic and clinical characteristics between persons with insufficient and adequate 25(OH)D.
| Characteristic | Inadequate 25(OH)D N=45 | Adequate 25(OH)D N=40 | P value |
|---|---|---|---|
| Demographics | |||
| Age | 38 (33–45) | 36 (31–43) | 0.4 |
| Gender (female) | 14 (31) | 12 (30) | 0.9 |
| BMI | 21.5 (20.0–22.8) | 21.5 (19.0–24.3) | 1.0 |
| Disease stage | |||
| Stage 4 KS | 23 (51) | 17 (43) | 0.4 |
| ACTG T1 | 38 (84) | 33 (83) | 0.8 |
| Baseline laboratory | |||
| Hemoglobin | 11 (9.4–11.9) | 11.3 (10.3–12.6) | 0.2 |
| CD4+ lymphocyte count | 117 (76–208) | 141 (70–222) | 0.5 |
| HIV-1 VL (log) | 4.7 (4.3–5.0) | 4.5 (3.9–4.9) | 0.04 |
| HHV-8 plasma VL (log) | 2.9 (2.1–3.5) | 2.8 (1.8–3.4) | 0.4 |
| HHV-8 PBMC VL (log) | 3.2 (2.4–4.4) | 3.5 (2.5–4.1) | 0.8 |
| 96 week changea | |||
| Increase in CD4+ lymphocyte count | 85 (21–291) | 105 (30–202) | 0.8 |
| Decrease in HIV-1 VL (log) | 1.9 (1.3–2.4) | 1.3 (0.8–1.9) | 0.04 |
| Decrease in HHV-8 plasma VL (log) | 0.5 (0–1.5) | 0.4 (0–1.5) | 0.8 |
| Decrease in HHV-8 PBMC VL (log) | 0.9 (−0.4–2.3) | 1.0 (0–2.0) | 0.9 |
Median and intraquartile range or number and percentage are reported for each variable; BMI: body mass index; KS: Kaposi Sarcoma; ACTG T1: AIDS Clinical Trials Group Tumor 1 Staging; VL: viral load; HHV-8: Human Herpes Virus 8; PBMC: peripheral blood mononuclear cells.
week 96 or last available results
Relationship of vitamin D status and clinical outcomes
Twenty-nine subjects were diagnosed with stage 4 KS at study entry and 71 with T1 disease; the median vitamin D in these subjects was 66.5 nmol/l (IQR 55.8–88.8) and 73 nmol/l (IQR 58–90), respectively. The proportion of stage 4 or T1 disease was similar between the 25(OH)D groups, and 25(OH)D levels were similar between persons with earlier and later stages of AIDS-KS, as shown in Table 1 and Table 2, respectively. Sixteen subjects (19%) had a partial or complete clinical response, 16 (19%) developed KS-IRIS, 49 (58%) required chemotherapy, and 22 (26%) required radiation therapy by 96 weeks. Clinical response (OR 0.6, 95% CI 0.2, 1.9; p = 0.4), development of KS-IRIS (OR 0.4, 95% CI 0.2, 1.9; p = 0.4), need for chemotherapy (OR 1.2, 95% CI 0.5, 2.8; p = 0.7), and need for radiation therapy (OR 0.6, 95% CI 0.2, 1.5; p = 0.2) were similar between those with inadequate and adequate 25(OH)D. ORs and significance remained unchanged after adjustment for HIV-1 viral load and CD4 count. The median 25(OH)D was not significantly different between those with or without a clinical response, KS-IRIS, chemotherapy, or radiation therapy (Table 2).
Table 2.
Median 25(OH)D levels by baseline disease stage and clinical outcomes
| Clinical characteristic | Median 25(OH)D | Interquartile range | P value |
|---|---|---|---|
| KS Clinical Stage | 0.8 | ||
| 2 or 3 | 75 | 57–91 | |
| 4 | 72 | 56–89 | |
| ACTG Stage | 0.4 | ||
| 0 | 73 | 40–85 | |
| 1 | 73 | 58–90 | |
| Survival Status | 0.3 | ||
| Alive at 96 weeks | 69 | 55–85 | |
| Died by 96 weeks | 63 | 46–79 | |
| Clinical Response | 0.7 | ||
| Partial or complete | 64 | 56–88 | |
| No response | 73 | 58–92 | |
| Immune Response | |||
| No KS-IRIS | 73 | 57–89 | 0.7 |
| Developed KS-IRIS | 64 | 56–88 | |
| HIV-1 virological response | 66 | 55–86 | 0.3 |
| HIV-1 virological failure | 83 | 58–93 | |
| Adjunctive therapy | |||
| No chemotherapy | 66 | 52–93 | 0.8 |
| Chemotherapy | 73 | 57–88 | |
| No radiation | 75 | 58–90 | 0.5 |
| Radiation therapy | 67 | 55–86 | |
KS: Kaposi Sarcoma; ACTG: AIDS Clinical Trials Group; KS-IRIS: Kaposi Sarcoma- Immune reconstitution inflammatory syndrome.
Baseline 25(OH)D measurements were available for 12 of 14 participants who died during the study. Those who died during the study had similar 25(OH)D (63 nmol/l, IQR 46–79) as the 60 subjects known to have survived to 96 weeks (69 nmol/l, IQR 55–85; p = 0.27). The odds of death by 96 weeks was not significantly associated with 25(OH)D (OR 0.5, 95% CI 0.1, 1.9; p = 0.3) and remained unchanged when adjusting for plasma HIV-1 RNA and CD4+ lymphocyte count.
Vitamin D and immunological/virological parameters
25(OH)D insufficiency was associated with significantly higher baseline plasma HIV- 1 RNA and greater decrease in HIV-1 viral load with ART initiation (p = 0.04, Table 1). Baseline CD4+ count and CD4+ cell increase with ART were slightly lower in persons with vitamin D insufficiency, however this did not reach statistical significance (p ≥0.5). HHV-8 viral load in plasma and PBMC were similar between the 25(OH)D groups (Table 1).
Discussion
Few studies have evaluated the prevalence of vitamin D insufficiency in HIV-infected adults living in Sub-Saharan Africa and no prior published studies have evaluated 25(OH)D insufficiency among Zimbabweans or in persons with KS-AIDS. The limited in vitro data on vitamin D or vitamin D agonists in KS and in other vascular tumors have been conflicting. Our study provides the first analyses of vitamin D insufficiency among HIV-infected adults in Zimbabwe and is the first to investigate relationships between 25(OH)D insufficiency and clinical and immunological/virological outcomes of persons with KS-AIDS.
The higher baseline HIV-1 RNA observed among subjects with 25(OH)D insufficiency is reported in the literature and may reflect the integral role of vitamin D in innate immunity.19,38,39 Unexpectedly, we observed a greater decrease in HIV-1 RNA in persons with inadequate 25(OH)D and slightly higher baseline 25(OH)D among persons with virological failure. Early in vitro studies of HIV and vitamin D suggested an increase in HIV-1 replication with 1,25(OH)2D partially mediated by inflammatory cytokines,40–43 thus, theoretically, higher 25(OH)D may have led to increased HIV-1 expression. Subsequent studies have largely suggested a suppressive effect of 25(OH)D and 1,25(OH)2D.39,44 Alternatively, higher levels of immune activation, inflammatory cytokines, or HIV-1 RNA itself may interfere with conversion of 25(OH)D,45,46 thus our findings may not accurately represent the levels of biologically active vitamin D, 1,25(OH)2D. The short half-life and transient changes in 1,25(OH)2D make this assay much less useful in determining vitamin D status.37
A potential limitation of our study is that we studied only the relationship between baseline 25(OH)D and clinical and immunological/virological outcomes. Our analysis would not have captured the impact of changes in 25(OH)D associated with ART initiation or nutritional status that may have occurred during the course of the study.21 The small sample population and event number may have limited our power to detect significant differences in outcomes (i.e., survival, clinical response, receipt of adjunctive therapy, development of KS-IRIS). Receipt of chemotherapy or radiation therapy was affected by availability. Regardless of these limitations, we found that more than one half of Zimbabweans with KS-AIDS enrolling in our study had insufficient 25(OH)D. While persons with KS-AIDS may not be representative of the majority of HIV-infected Zimbabweans, the mean 25(OH)D level (75.5 nmol/l) of our population is similar to the weighted mean (84.7 nmol/l) of other African populations with and without HIV infection.23,24,28–31,47,48
In summary, despite associations between vitamin D deficiency and severe disease, AIDS, and death,22,49 we were unable to demonstrate improved KS-AIDS outcomes in clinical response, need for chemotherapy or radiation, development of KS-IRIS, survival rates, or more favorable immunological response50 to ART in association with higher levels of vitamin D. Larger studies should investigate longitudinal associations with vitamin D state in persons with AIDS-KS and other malignancies prior to routinely recommending vitamin D replacement.
Acknowledgments
This work was supported by GlaxoSmithKline, NIH T32 A1007447-1, and the Hartford Foundation Center of Excellence.
Footnotes
Conflict of interest: The sponsors were not involved in the study design, collection, analysis, or interpretation of the data, or in the writing of this manuscript. The contents are the authors’ sole responsibility and do not necessarily represent official NIH views. The authors report no other potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chokunonga E, Levy LM, Bassett MT, Mauchaza BG, Thomas DB, Parkin DM. Cancer incidence in the African population of Harare, Zimbabwe: second results from the cancer registry 1993–1995. Int J Cancer. 2000;85:54–9. doi: 10.1002/(sici)1097-0215(20000101)85:1<54::aid-ijc10>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 2.Olweny CL, Borok M, Gudza I, Clinch J, Cheang M, Kiire CF, et al. Treatment of AIDS-associated Kaposi’s sarcoma in Zimbabwe: results of a randomized quality of life focused clinical trial. Int J Cancer. 2005;113:632–9. doi: 10.1002/ijc.20606. [DOI] [PubMed] [Google Scholar]
- 3.Borok M, Fiorillo S, Gudza I, Putnam B, Ndemera B, White IE, et al. Evaluation of plasma human herpesvirus 8 DNA as a marker of clinical outcomes during antiretroviral therapy for AIDS-related Kaposi sarcoma in Zimbabwe. Clin Infect Dis. 2010;51:342–9. doi: 10.1086/654800. [DOI] [PubMed] [Google Scholar]
- 4.Giovannucci E. Vitamin D and cancer incidence in the Harvard cohorts. Ann Epidemiol. 2009;19:84–8. doi: 10.1016/j.annepidem.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Mizoue T, Kimura Y, Toyomura K, Nagano J, Kono S, Mibu R, et al. Calcium, dairy foods, vitamin D, and colorectal cancer risk: the Fukuoka Colorectal Cancer Study. Cancer Epidemiol Biomarkers Prev. 2008;17:2800–7. doi: 10.1158/1055-9965.EPI-08-0369. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz GG. Vitamin D and intervention trials in prostate cancer: from theory to therapy. Ann Epidemiol. 2009;19:96–102. doi: 10.1016/j.annepidem.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 7.John EM, Schwartz GG, Dreon DM, Koo J. Vitamin D and breast cancer risk: the NHANES I epidemiologic follow-up study, 1971–1975 to 1992. National Health and Nutrition Examination Survey. Cancer Epidemiol Biomarkers Prev. 1999;8:399–406. [PubMed] [Google Scholar]
- 8.Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–9. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Shoshan M, Amir S, Dang DT, Dang LH, Weisman Y, Mabjeesh NJ. 1alpha,25-dihydroxyvitamin D3 (calcitriol) inhibits hypoxia-inducible factor-1/vascular endothelial growth factor pathway in human cancer cells. Mol Cancer Ther. 2007;6:1433–9. doi: 10.1158/1535-7163.MCT-06-0677. [DOI] [PubMed] [Google Scholar]
- 10.Gruber HE, Hoelscher G, Ingram JA, Chow Y, Loeffler B, Hanley EN., Jr 1,25(OH)2-vitamin D3 inhibits proliferation and decreases production of monocyte chemoattractant protein-1, thrombopoietin, VEGF, and angiogenin by human annulus cells in vitro. Spine. 2008;33:755–65. doi: 10.1097/BRS.0b013e3181695d59. [DOI] [PubMed] [Google Scholar]
- 11.Chung I, Han G, Seshadri M, Gillard BM, Yu WD, Foster BA, et al. Role of vitamin D receptor in the antiproliferative effects of calcitriol in tumor-derived endothelial cells and tumor angiogenesis in vivo. Cancer Res. 2009;69:967–75. doi: 10.1158/0008-5472.CAN-08-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardus A, Panizo S, Encinas M, Dolcet X, Gallego C, Aldea M, et al. 1,25-dihydroxyvitamin D3 regulates VEGF production through a vitamin D response element in the VEGF promoter. Atherosclerosis. 2009;204:85–9. doi: 10.1016/j.atherosclerosis.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 13.Grant WB. Role of vitamin D in up-regulating VEGF and reducing the risk of pre-eclampsia. Clin Sci (Lond) 2009;116:871. doi: 10.1042/CS20080562. [DOI] [PubMed] [Google Scholar]
- 14.Masood R, Nagpal S, Zheng T, Cai J, Tulpule A, Smith DL, et al. Kaposi sarcoma is a therapeutic target for vitamin D(3) receptor agonist. Blood. 2000;96:3188–94. [PubMed] [Google Scholar]
- 15.Gonzalez-Pardo V, Verstuyf A, Boland R, Russo de Boland A. Vitamin D analogue TX 527 down-regulates the NF-kappaB pathway and controls the proliferation of endothelial cells transformed by Kaposi sarcoma herpesvirus. Br J Pharmacol. 2013;169:1635–45. doi: 10.1111/bph.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez-Pardo V, Suares A, Verstuyf A, De Clercq P, Boland R, Boland AR. Cell cycle arrest and apoptosis induced by 1alpha,25(OH)D and TX 527 in Kaposi sarcoma is VDR dependent. J Steroid Biochem Mol Biol. 2013 Dec 5; doi: 10.1016/j.jsbmb.2013.11.014. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Pardo V, Martin D, Gutkind JS, Verstuyf A, Bouillon R, de Boland AR, et al. 1 Alpha,25-dihydroxyvitamin D3 and its TX527 analog inhibit the growth of endothelial cells transformed by Kaposi sarcoma-associated herpes virus G protein-coupled receptor in vitro and in vivo. Endocrinology. 2010;151:23–31. doi: 10.1210/en.2009-0650. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Pardo V, D’Elia N, Verstuyf A, Boland R, Russo de Boland A. NFkappaB pathway is down-regulated by 1alpha,25(OH)(2)-vitamin D(3) in endothelial cells transformed by Kaposi sarcoma-associated herpes virus G protein coupled receptor. Steroids. 2012;77:1025–32. doi: 10.1016/j.steroids.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Kim JH, Gandhi V, Psevdos G, Espinoza F, Park J, Sharp V. Evaluation of vitamin D levels among HIV-infected patients in New York City. AIDS Res Hum Retroviruses. 2012;28:235–41. doi: 10.1089/AID.2011.0040. [DOI] [PubMed] [Google Scholar]
- 20.Dao CN, Patel P, Overton ET, Rhame F, Pals SL, Johnson C, et al. Low vitamin D among HIV-infected adults: prevalence of and risk factors for low vitamin D Levels in a cohort of HIV-infected adults and comparison to prevalence among adults in the US general population. Clin Infect Dis. 2011;52:396–405. doi: 10.1093/cid/ciq158. [DOI] [PubMed] [Google Scholar]
- 21.Overton ET, Yin MT. The rapidly evolving research on vitamin D among HIV-infected populations. Curr Infect Dis Rep. 2011;13:83–93. doi: 10.1007/s11908-010-0144-x. [DOI] [PubMed] [Google Scholar]
- 22.Viard JP, Souberbielle JC, Kirk O, Reekie J, Knysz B, Losso M, et al. Vitamin D and clinical disease progression in HIV infection: results from the EuroSIDA study. AIDS. 2011;25:1305–15. doi: 10.1097/QAD.0b013e328347f6f7. [DOI] [PubMed] [Google Scholar]
- 23.Gibney KB, Mihrshahi S, Torresi J, Marshall C, Leder K, Biggs BA. The profile of health problems in African immigrants attending an infectious disease unit in Melbourne, Australia. Am J Trop Med Hyg. 2009;80:805–11. [PubMed] [Google Scholar]
- 24.Woolley IJ, Giles ML, Howard JE, Korman TM. Unrecognised vitamin D deficiency: low concentrations in African migrants with HIV in Australia. Sex Health. 2008;5:375–6. doi: 10.1071/sh08048. [DOI] [PubMed] [Google Scholar]
- 25.van der Meer IM, Middelkoop BJ, Boeke AJ, Lips P. Prevalence of vitamin D deficiency among Turkish, Moroccan, Indian and Sub-Sahara African populations in Europe and their countries of origin: an overview. Osteoporos Int. 2011;22:1009–21. doi: 10.1007/s00198-010-1279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huntington MK, Shafer CW, Pudwill R, Boer L, Kendall J. Prevalence of vitamin D deficiency among immigrants to South Dakota. S D Med. 2010;63:51–5. [PubMed] [Google Scholar]
- 27.Vescini F, Cozzi-Lepri A, Borderi M, Re MC, Maggiolo F, De Luca A, et al. Prevalence of hypovitaminosis D and factors associated with vitamin D deficiency and morbidity among HIV-infected patients enrolled in a large Italian cohort. J Acquir Immune Defic Syndr. 2011;58:163–72. doi: 10.1097/QAI.0b013e31822e57e9. [DOI] [PubMed] [Google Scholar]
- 28.Njemini R, Meyers I, Demanet C, Smitz J, Sosso M, Mets T. The prevalence of autoantibodies in an elderly Sub-Saharan African population. Clin Exp Immunol. 2002;127:99–106. doi: 10.1046/j.1365-2249.2002.01713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friis H, Range N, Pedersen ML, Molgaard C, Changalucha J, Krarup H, et al. Hypovitaminosis D is common among pulmonary tuberculosis patients in Tanzania but is not explained by the acute phase response. J Nutr. 2008;138:2474–80. doi: 10.3945/jn.108.094979. [DOI] [PubMed] [Google Scholar]
- 30.Aspray TJ, Yan L, Prentice A. Parathyroid hormone and rates of bone formation are raised in perimenopausal rural Gambian women. Bone. 2005;36:710–20. doi: 10.1016/j.bone.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Glew RH, Crossey MJ, Polanams J, Okolie HI, VanderJagt DJ. Vitamin D status of seminomadic Fulani men and women. J Natl Med Assoc. 2010;102:485–90. doi: 10.1016/s0027-9684(15)30556-3. [DOI] [PubMed] [Google Scholar]
- 32.Wejse C, Olesen R, Rabna P, Kaestel P, Gustafson P, Aaby P, et al. Serum 25-hydroxyvitamin D in a West African population of tuberculosis patients and unmatched healthy controls. Am J Clin Nutr. 2007;86:1376–83. doi: 10.1093/ajcn/86.5.1376. [DOI] [PubMed] [Google Scholar]
- 33.Krigel RL, Laubenstein LJ, Muggia FM. Kaposi’s sarcoma: a new staging classification. Cancer Treat Rep. 1983;67:531–4. [PubMed] [Google Scholar]
- 34.Krown SE, Testa MA, Huang J. AIDS-related Kaposi’s sarcoma: prospective validation of the AIDS Clinical Trials Group staging classification. AIDS Clinical Trials Group Oncology Committee. J Clin Oncol. 1997;15:3085–92. doi: 10.1200/JCO.1997.15.9.3085. [DOI] [PubMed] [Google Scholar]
- 35.White IE, Campbell TB. Quantitation of cell-free and cell-associated Kaposi’s sarcoma-associated herpesvirus DNA by real-time PCR. J Clin Microbiol. 2000;38:1992–5. doi: 10.1128/jcm.38.5.1992-1995.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 37.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19:73–8. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vescini F, Cozzi-Lepri A, Borderi M, Re MC, Maggiolo F, De Luca A, et al. Prevalence of hypovitaminosis D and factors associated with vitamin D deficiency and morbidity among HIV-infected patients enrolled in a large Italian cohort. J Acquir Immune Defic Syndr. 2011;58:163–72. doi: 10.1097/QAI.0b013e31822e57e9. [DOI] [PubMed] [Google Scholar]
- 39.Campbell GR, Spector SA. Hormonally active vitamin D3 (1alpha,25-dihydroxycholecalciferol) triggers autophagy in human macrophages that inhibits HIV-1 infection. J Biol Chem. 2011;286:18890–902. doi: 10.1074/jbc.M110.206110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Locardi C, Petrini C, Boccoli G, Testa U, Dieffenbach C, Butto S, et al. Increased human immunodeficiency virus (HIV) expression in chronically infected U937 cells upon in vitro differentiation by hydroxyvitamin D3: roles of interferon and tumor necrosis factor in regulation of HIV production. J Virol. 1990;64:5874–82. doi: 10.1128/jvi.64.12.5874-5882.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pauza CD, Kornbluth R, Emau P, Richman DD, Deftos LJ. Vitamin D3 compounds regulate human immunodeficiency virus type 1 replication in U937 monoblastoid cells and in monocyte-derived macrophages. J Leukoc Biol. 1993;53:157–64. doi: 10.1002/jlb.53.2.157. [DOI] [PubMed] [Google Scholar]
- 42.Bearden A, Abad C, Gangnon R, Sosman JM, Binkley N, Safdar N. Cross-sectional study of vitamin D levels, immunologic and virologic outcomes in HIV-infected adults. J Clin Endocrinol Metab. 2013;98:1726–33. doi: 10.1210/jc.2012-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goletti D, Kinter AL, Biswas P, Bende SM, Poli G, Fauci AS. Effect of cellular differentiation on cytokine-induced expression of human immunodeficiency virus in chronically infected promonocytic cells: dissociation of cellular differentiation and viral expression. J Virol. 1995;69:2540–6. doi: 10.1128/jvi.69.4.2540-2546.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campbell GR, Spector SA. Vitamin D inhibits human immunodeficiency virus type 1 and Mycobacterium tuberculosis infection in macrophages through the induction of autophagy. PLoS Pathog. 2012;8:e1002689. doi: 10.1371/journal.ppat.1002689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haug CJ, Aukrust P, Haug E, Morkrid L, Muller F, Froland SS. Severe deficiency of 1,25-dihydroxyvitamin D3 in human immunodeficiency virus infection: association with immunological hyperactivity and only minor changes in calcium homeostasis. J Clin Endocrinol Metab. 1998;83:3832–8. doi: 10.1210/jcem.83.11.5270. [DOI] [PubMed] [Google Scholar]
- 46.Villamor E. A potential role for vitamin D on HIV infection? Nutr Rev. 2006;64:226–33. doi: 10.1301/nr.2006.may.226-233. [DOI] [PubMed] [Google Scholar]
- 47.Mehta S, Hunter DJ, Mugusi FM, Spiegelman D, Manji KP, Giovannucci EL, et al. Perinatal outcomes, including mother-to-child transmission of HIV, and child mortality and their association with maternal vitamin D status in Tanzania. J Infect Dis. 2009;200:1022–30. doi: 10.1086/605699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tostmann A, Wielders JP, Kibiki GS, Verhoef H, Boeree MJ, van der Ven AJ. Serum 25-hydroxy-vitamin D3 concentrations increase during tuberculosis treatment in Tanzania. Int J Tuberc Lung Dis. 2010;14:1147–52. [PubMed] [Google Scholar]
- 49.Mehta S, Giovannucci E, Mugusi FM, Spiegelman D, Aboud S, Hertzmark E, et al. Vitamin D status of HIV-infected women and its association with HIV disease progression, anemia, and mortality. PLoS One. 2010;5:e8770. doi: 10.1371/journal.pone.0008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ross AC, Judd S, Kumari M, Hileman C, Storer N, Labbato D, et al. Vitamin D is linked to carotid intima-media thickness and immune reconstitution in HIV-positive individuals. Antivir Ther. 2011;16:555–63. doi: 10.3851/IMP1784. [DOI] [PMC free article] [PubMed] [Google Scholar]