Abstract
Objective
To determine if increased placental vascular impedance to flow is associated with changes in fetal cardiac function using spatiotemporal image correlation (STIC) and Virtual Organ Computer-aided AnaLysis (VOCAL).
Study Design
A cross-sectional study was performed in fetuses with an umbilical artery pulsatility index > 95th percentile (ABN). Ventricular volume (end-systole, end-diastole), stroke volume (SV), cardiac output (CO), adjusted CO, and ejection fraction (EF) were compared to those of 184 normal fetuses (NL).
Results
1) 34 fetuses were evaluated at a median gestational age of 28.3 (range 20.6 – 36.9) weeks; 2) mean ventricular volumes were lower for ABN than NL (end-systole, end-diastole) with a proportionally greater decrease for left ventricular volume (vs. right); 3) mean left and right SV, CO, and adjusted CO were lower for ABN (vs. NL); 4) right ventricular volume, SV, CO, and adjusted CO exceeded the left in ABN fetuses; 5) mean EF was greater for ABN than NL; and 6) median left EF was greater (vs. right) in ABN fetuses.
Conclusion
Increased placental vascular impedance to flow is associated with changes in fetal cardiac function.
Keywords: cardiac function, cardiac output, Contour Finder, ejection fraction, fetal echocardiography, fetus, 4D, IUGR, prenatal diagnosis, sonography, STIC, stroke volume, 3D, umbilical artery Doppler, ventricular volume, VOCAL
Introduction
Abnormal umbilical artery Doppler velocimetry reflects increased impedance to blood flow in the placenta.1–4 Mathematical modeling of the placental circulation shows that initially, placental resistance and pulsatility index (PI) increase very slowly with fractional terminal vessel obliteration.5 However, there is a steep increase of the PI after 60–90% of vessels are obliterated.5
In human pregnancies, structural heart disease,6–8 small for gestational age with normal umbilical artery Doppler velocimetry,9–10 intrauterine growth restriction (IUGR),11–15 twin-to-twin transfusion syndrome,16–18 and intra-amniotic infection19–20 (reported in animal models also21–22) can result in fetal cardiac dysfunction. The heart is a central organ in the fetal adaptive mechanisms to placental insufficiency and hypoxia.23 Therefore, it follows that placental insufficiency with increased placental vascular resistance may lead to fetal cardiovascular compromise,24 and even fetal metabolic acidosis and death.25 Indeed, severe IUGR due to placental insufficiency contributes to 30% of total perinatal loss and severe morbidity.26 Monitoring of fetal cardiac function has been proposed as an adjunct to current methods to predict adverse outcome and death in IUGR.27 Fetuses with abnormal umbilical artery Doppler velocimetry have been shown to have similar changes to those observed in adults with atherosclerosis. This may be important in relating placental vascular disease (detected by umbilical artery Doppler velocimetry) to the risk for adult cardiovascular disease.28 Studies report that fetuses with abnormal umbilical artery Doppler velocimetry have evidence of higher red blood cell count and hemoglobin concentration,29 endothelium activation,28 platelet activation (which promotes thrombosis),30 platelet consumption,31 an atherogenic lipoprotein profile,32 and evidence of intravascular inflammation.33 Epidemiologic studies and animal models have also established that low birthweight babies have an increased risk of cardiovascular disease later in life.34–35 Thus, the condition which is the focus of our study is the in-utero equivalent to fetal atherosclerosis, and this, along with fetal cardiac dysfunction may have important consequences in fetal programming of cardiac disease and the early-onset of disease.23 Examining fetal cardiovascular parameters is required to gain an understanding of the hemodynamic changes occurring in the setting of increased placental vascular impedance to flow.
However, the repeatability and reproducibility of most fetal echocardiographic measurements determined using two-dimensional (2D) sonography is poor, particularly for ventricular volume and volume flow estimations.36 This has been attributed to measurement variation in the atrioventricular and semilunar valves, which can lead to large differences in the estimated cardiac output.36–37 Errors in measuring velocity-time integral or valve area will greatly influence volume flow measurements, particularly because the valve area is related to the square of the radius, thus accentuating any errors.38 Moreover, the use of 2D measurements to estimate ventricular volume requires assumptions about the three-dimensional geometry of the heart which may be invalid, leading to inaccuracy in estimations of cardiac output.36–37
Three-39 and four-dimensional sonography have the potential to minimize the limitations inherent in 2D estimations of fetal cardiovascular parameters because: 1) geometric assumptions are not made when assessing ventricular volumes; 2) neither small outflow tract diameters nor angle-dependent Doppler measurements are required for calculation; and 3) from a single cardiac dataset obtained using spatiotemporal image correlation (STIC), all parameters required for calculation (left and right ventricular volumes) are present in the same volume, reducing the risk inherent in measuring two chambers at different times when using 2D ultrasound.40 Indeed, three-41–46 and four-dimensional echocardiography47–57 have been used to evaluate cardiovascular parameters in normal fetuses.
Yet, there is insufficient data regarding the fetal cardiovascular response to increased placental vascular impedance to flow determined using four-dimensional sonography. We have previously described a repeatable and reproducible technique to quantify ventricular volume calculations using STIC and Virtual Organ Computer-aided AnaLysis (VOCAL).58 Subsequently, we quantified fetal cardiovascular parameters (ventricular volume, stroke volume, cardiac output, and ejection fraction) in a group of 184 normal fetuses over a range of gestational ages.40 Therefore, the objective of this study was to use the same technique to determine if increased placental vascular impedance to flow is associated with changes in fetal cardiac function.
Materials and Methods
Study population
A cross-sectional study was conducted to include pregnancies with increased placental vascular impedance to flow (umbilical artery PI > 95th percentile59) by searching our database of women enrolled into research protocols that included examination of the fetal heart by three- and four-dimensional ultrasound. Women were eligible for inclusion if gestational age was determined by either a first or second trimester sonographic examination and there was a singleton fetus (> 19 weeks of gestation). Women were excluded in the presence of fetal hydrops, chromosomal, or congenital abnormalities. A control group, consisting of 184 normal fetuses whose cardiovascular parameters had been previously reported,40 was used for comparison.
IUGR was defined as an abdominal circumference < 5th percentile for gestational age60–61 with an umbilical artery PI > 95th percentile.59 Estimated fetal weight was not used to determine the presence of IUGR. Fetal Doppler recordings were obtained from the umbilical artery (free loop of cord), middle cerebral artery, and from the ductus venosus when possible. Preeclampsia was defined as the presence of systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg, and proteinuria of 300 mg/24 hours or ≥ +2 (dipstick) on two occasions six hours apart. All women provided written informed consent prior to undergoing sonographic examination. Participation was approved by the Institutional Review Board of the National Institute of Child Health and Human Development and by the Human Investigation Committee of Wayne State University.
Examination Technique
Ultrasound examinations were performed by eight experienced sonographers using systems with STIC capability (Voluson 730 Expert, Voluson E8 Expert; GE Medical Systems, Kretztechnik GmbH, Zipf, Austria) and utilizing a motorized curved array transabdominal transducer (2–5 or 4–8 MHz). Tissue harmonic imaging was used for each examination, and Compound Resolution Imaging (CRI) was used at the sonographer’s discretion. A transverse view of the fetal chest at the level of the four-chamber view was obtained, from which STIC datasets were acquired. The transducer was oriented such that the fetal spine was located posteriorly for each acquisition. Acquisition time was 10 seconds with a sweep angle that was sufficient to encompass the fetal cardiac structures (25 – 35 degrees). Color Doppler sonography was not utilized during the acquisition process. Adequate cardiac datasets were accepted for post-processing if acoustic shadowing (signal loss in the sound path secondary to echogenic structures), dropout (signal loss in the sound path without intervening structures), and motion artifact were absent. When multiple STIC datasets were available, the dataset obtained closest to the time of delivery was selected for analysis.
Cardiac datasets were acquired to investigate the following fetal cardiovascular parameters: 1) ventricular volume (mL); 2) stroke volume (mL) (end-diastolic volume – end-systolic volume); 3) cardiac output (mL/min) (stroke volume×fetal heart rate); and 4) ejection fraction (%) (stroke volume/end-diastolic volume×100%). Fetal biometry of the biparietal diameter (BPD), head circumference (HC), abdominal circumference (AC), and femoral diaphysis length (FL) were obtained using 2D sonography at the time of cardiac dataset acquisition. Cardiac output was expressed both as a function of estimated fetal weight,62 and as a function of biometric parameters (HC, AC, FL).
Analysis was performed offline (4D View versions 5.0 – 7.0; GE Healthcare, Milwaukee, WI) in a standardized manner. In the A plane of the multiplanar display, the fetal heart was re-oriented such that the left ventricle was located on the left side of the screen with the apex of the heart directed upwards. Next, the ventricular septum was rotated to 90 degrees in both the A and C planes. The atrioventricular (AV) valves were located by scrolling from front to back in the A plane. The image was optimized by selecting Chroma Color 1 (Sepia) with the addition of speckle reduction (SRI 5). After image brightness and contrast settings were optimized, end-systolic and end-diastolic phases were identified by scrolling through each frame, and locating the image preceding AV valve opening (systole) and following AV valve closure (diastole).
Ventricular volumes were calculated in a semi-automated fashion utilizing VOCAL. VOCAL II was selected and the Contour Finder: Trace option was utilized with 15 degrees of rotation and a sensitivity of 1 (default = 5). The image was enlarged and the reference dot repositioned into the ventricle of interest. Due to the complex geometry of the ventricles, the location of the reference dot within the ventricle was selected to meet the software requirement that the contour only cross the rotation line twice. With these selections, 12 rotational steps were made and a volume was computed. Datasets were accepted for analysis if the ventricular septum, ventricular walls, and AV valves were visible throughout each rotational step.
We previously reported the repeatability and reproducibility of ventricular volume measurements utilizing this technique.58 Volume measurements were repeatable with good agreement [coefficient of variation (CV) < 10%] and excellent reliability [intraclass correlation (ICC) > 0.95] for both intraobserver and interobserver measurements. Additionally, ventricular volumes were reproducible with negligible differences in agreement (CV < 1%), good reliability (ICC > 0.9), and minimal bias (mean percent difference −0.4%; 95% limits of agreement, −5.4% to 5.9%) when different STIC datasets for the same patient were compared.58
Statistical Analysis
Data were first assessed using numerical and graphical techniques, including scatter plots of each response vs. gestational age, to determine whether they met assumptions of the statistical tests being used for analysis. All but two scatter plots revealed the presence of curvilinear relationships and heteroscedasticity; hence, natural logarithmic transformations (from the Box-Cox family of transformations) of each response and gestational age were performed to linearize the data and correct for heteroscedasticity.
Analyses of covariance (ANCOVA) based on weighted regression were performed on the transformed data (Table 2), with the weights computed according to the procedure described by Altman.63–66 These weights are the best linear unbiased estimates (BLUE). Ejection fraction for the right and left ventricles were both linear and homoscedastic; therefore, analysis of covariance of two-factor interaction and main effects multiple regression models were used iteratively to analyze this untransformed data. Residual analysis was performed on all models as a diagnostic measure to assess the aptness of the models fit.
Table 2.
Comparison of cardiovascular parameters between fetuses with an umbilical artery pulsatility index > 95th percentile (ABN) and normal controls (NL)
| Cardiovascular parametera | NL (95% CI)b | ABN (95% CI)c | Proportion Changed |
|---|---|---|---|
| Ventricular volume in end-systole (mL) | |||
| Left | 0.43 (0.4 – 0.5) | 0.12 (0.02 – 0.2) | −72% |
| Right | 0.65 (0.6 – 0.7) | 0.39 (0.3 – 0.5) | −40% |
| Ventricular volume in end-diastole (mL) | |||
| Left | 1.28 (1.2 – 1.4) | 0.64 (0.4 – 0.9) | −50% |
| Right | 1.57 (1.5 – 1.7) | 1.09 (0.8 – 1.3) | −30% |
| Stroke volume (mL) | |||
| Left | 0.86 (0.79 – 0.93) | 0.53 (0.37 – 0.68)e | −38% |
| Right | 0.92 (0.85 – 0.99) | 0.71 (0.55 – 0.86)e | −23% |
| Cardiac output (mL/min) | |||
| Left | 119.6 (110 – 129) | 71.9 (50 – 94)e | −40% |
| Right | 127.5 (118 – 137) | 96 (74 – 118)e | −25% |
| Cardiac output divided by HC (mL/min/cm) | |||
| Left | 4.4 (4.1 – 4.8) | 2.8 (2.0 – 3.6)e | −36% |
| Right | 4.7 (4.4 – 5.0) | 3.7 (2.9 – 4.4)e | −21% |
| Cardiac output divided by AC (mL/min/cm) | |||
| Left | 4.8 (4.5 – 5.2) | 3.2 (2.4 – 4.0)e | −33% |
| Right | 5.1 (4.8 – 5.5) | 4.1 (3.3 – 4.9)e | −20% |
| Cardiac output divided by FL (mL/min/cm) | |||
| Left | 21.4 (19.8 – 23.0) | 14.2 (10.5 – 18.0)e | −34% |
| Right | 22.6 (21.1 – 24.2) | 18.4 (14.7 – 22.1)e | −19% |
| Ejection fraction (%) | |||
| Left | 70.4 (69 – 72) | 82.4 (79 – 86)e | 17% |
| Right | 60.8 (59 – 63) | 66 (62 – 70)e | 9% |
ABN, abnormal; AC, abdominal circumference; CI, confidence interval; FL, femoral diaphysis length; HC, head circumference; NL, normal
Mean values adjusted for gestational age
Data from Hamill N et al.40
NL vs. ABN; p < 0.0001 (main effects ANCOVA)
Proportion change calculated as [(1 – ABN/NL) × 100%]
ABN right ventricle vs. ABN left ventricle for median stroke volume, cardiac output, cardiac output divided by HC, AC, FL, and ejection fraction; p < 0.05 for all, except p < 0.001 for ejection fraction (paired test)
For bivariate analysis, the Shapiro-Wilk and Kolmogorov-Smirnov tests were used to test for normal distribution. Student’s t-test was used to determine the differences of the mean among groups, and Pearson’s correlation coefficient was utilized to assess correlations. For nonparametric data, the Mann-Whitney U test and Wilcoxon signed-rank test were used to determine the difference between parameter medians, and Spearman’s rank correlation coefficient (rs) was utilized to assess correlations. A P-value <0.05 was considered statistically significant for all comparisons. Statistical analyses were performed using SPSS package version 14 (SPSS Inc., Chicago IL), as well as The SAS System for Windows version 9.2 (SAS Institute Inc., Cary, NC).
Results
Patient Population
Thirty-four women met the inclusion criteria; clinical and sonographic data are shown in Table 1. Ventricular volume (end-systole, end-diastole), stroke volume (SV), cardiac output (CO), adjusted CO, and ejection fraction (EF) were determined and compared to that of 184 normal fetuses (NL) previously reported by our group (each with normal umbilical artery PI and abdominal circumference measurements).40
Table 1.
Clinical and sonographic characteristics of the study population (n = 34)
| Parameter | Value |
|---|---|
| Clinical characteristics | |
| Gestational age at delivery (weeks) | 31.6 (23.0 – 41.1) |
| Interval between acquisition of STIC datasets and delivery (days) | 10 (1 – 133) |
| Birth weight (grams) | 1000 (282 – 3750) |
| Term deliveries (≥ 37 weeks of gestation) | 20% (7/34) |
| Preeclampsia | 53% (18/34) |
| Perinatal death | 18% (6/34) |
| Sonographic characteristics | |
| Gestational age at evaluation (weeks) | 28.3 (20.6 – 36.9) |
| IUGR present | 71% (24/34) |
| aSymmetrical IUGR | 54% (13/24) |
| bAsymmetrical IUGR | 46% (11/24) |
| HC < 10th percentile | 50% (17/34) |
| Amniotic fluid index (mm) | 67 (17 – 178) |
| UA end-diastolic velocity present | 74% (25/34) |
| UA AEDV | 20% (7/34) |
| UA REDV | 6% (2/34) |
| MCA PI | 1.7 (0.74 – 2.59) |
| cDV PIV | 0.65 (0.24 – 3.06) |
| cDV (Reversed or absent a-wave) | 7% (2/29) |
AEDV, absent end-diastolic velocity; DV, ductus venosus; HC, head circumference;67 IUGR, intrauterine growth restriction (defined as abdominal circumference < 5th percentile for gestational age); MCA, middle cerebral artery; PI, pulsatility index; PIV, pulsatility index for veins; REDV, reversed end-diastolic velocity; STIC, spatiotemporal image correlation; UA, umbilical artery.
HC/AC < 95th percentile for gestational age68
HC/AC > 95th percentile for gestational age68
DV Doppler velocimetry results available in 29 cases
Data given as median (range) or %.
Sonographic evaluation was performed at a median gestational age of 28.3 (range: 20.6 – 36.9) weeks. Umbilical artery (UA) waveform analysis demonstrated the presence of end-diastolic velocity in 74% (n=25), absence in 20% (n=7), and reversed in 6% (n=2). Since cardiovascular parameters did not statistically differ between these 3 groups, they were considered as a single group with an UA PI > 95th percentile (ABN). Doppler velocimetry of the middle cerebral artery and ductus venosus was available for 100% (n=34) and 85% (n=29) of women, respectively.
The median gestational age at delivery was 31.6 (range: 23.0 – 41.1) weeks, with 20% (n=7) of cases delivering at term (≥ 37 weeks of gestation). The median interval between acquisition of STIC datasets and delivery was 10 (range: 1 – 133) days. The median birth weight was 1000 (range: 282 – 3750) grams. Of the 34 ABN cases, IUGR occurred in 71% (n=24), preeclampsia in 53% (n=18), and perinatal death in 18% (n=6). Since no significant differences were found between cardiovascular parameters in those with and without IUGR, preeclampsia, or perinatal death, they were analyzed as a single group.
Ventricular volumes are lower in the presence of increased placental vascular impedance to flow
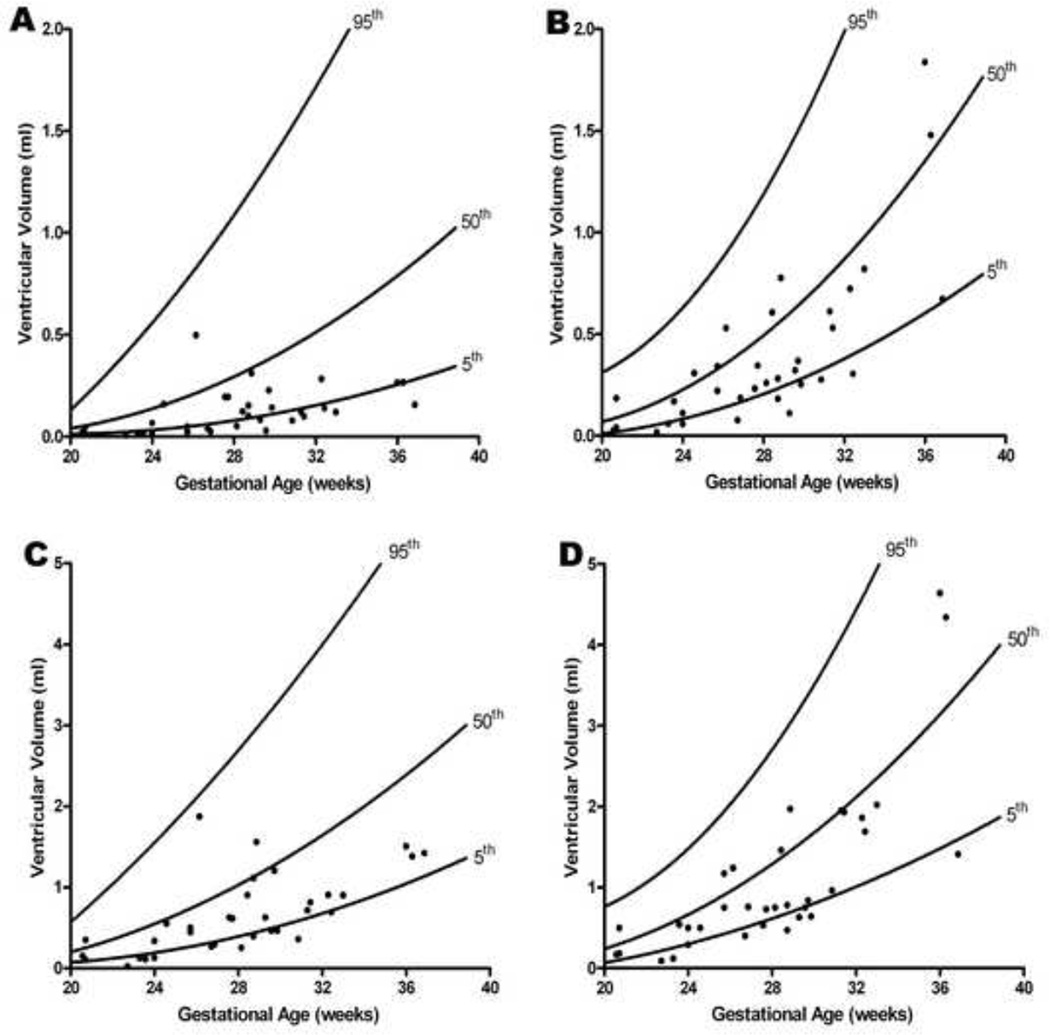
After adjusting for gestational age, mean volumes for the left ventricle (mL) were lower in end-systole (ABN: 0.12 vs. NL: 0.43; p < 0.0001) and end-diastole (ABN: 0.64 vs. NL: 1.28; p < 0.0001) (Figure 1 and Table 2). Similarly, after adjusting for gestational age, mean volumes for the right ventricle (mL) were also lower in end-systole (ABN: 0.39 vs. NL: 0.65; p < 0.0001) and end-diastole (ABN: 1.09 vs. NL: 1.57; p < 0.0001) (Figure 1 and Table 2). Moreover, there was a proportionately greater decrease [(1 – ABN/NL)×100%] in left ventricular volume as compared to the right, in both end-systole (left: −72% vs. right: −40%) and end-diastole (left: - 50% vs. right: −30%) (Table 2).
Figure 1. Ventricular volume in end-systole and end-diastole as a function of gestational age in the presence of increased placental vascular impedance to flow.
Ventricular volume calculations in fetuses with an umbilical artery pulsatility index > 95th percentile (ABN) were compared to 184 normal fetuses (NL)40 (A: left ventricle in end-systole; B: right ventricle in end-systole; C: left ventricle in end-diastole; D: right ventricle in end-diastole). For the left ventricle, mean volumes (adjusted for gestational age) (mL) were lower in both end-systole (ABN: 0.12 vs. NL: 0.43; p < 0.0001) and end-diastole (ABN: 0.64 vs. NL: 1.28; p < 0.0001). For the right ventricle, mean volumes (adjusted for gestational age) (mL) were also lower in both end-systole (ABN: 0.39 vs. NL: 0.65; p < 0.0001) and end-diastole (ABN: 1.09 vs. NL: 1.57; p < 0.0001)
Right ventricular volumes are greater than the left in the presence of increased placental vascular impedance to flow
Median right ventricular volumes (mL) were significantly greater than the left in end-systole (ABN Right: 0.28 vs. ABN Left: 0.10; p < 0.001) and end-diastole (ABN Right: 0.75 vs. ABN Left: 0.53; p < 0.001) (Table 3). When a ratio of right to left ventricular volume was calculated, right ventricular volumes were greater in end-systole (median Right/Left: 3.2, IQR: 1.8 – 5.4) and end-diastole (median Right/Left: 1.5, IQR: 1.1 – 2.6), an effect which was independent of gestational age (systole: rs = 0.15; p = NS; diastole: rs = 0.15; p = NS). For the same median ratio of right to left ventricular volume, this was significantly different between the ABN and NL groups in both end-diastole (ABN: 1.5, IQR: 1.1 – 2.6 vs. NL: 1.2, IQR: 0.9 – 1.7; p < 0.05) and end-systole (ABN: 3.2, IQR: 1.8 – 5.4 vs. NL: 1.6, IQR: 1.1 – 2.4; p < 0.05).
Table 3.
Comparison of fetal cardiovascular parameters between the left and right ventricles for fetuses with an umbilical artery pulsatility index > 95th percentile
| Cardiovascular parameter | Left Ventricle | Right Ventricle | P value |
|---|---|---|---|
| Volume in end-systole (mL) | 0.10 (0.03 – 0.17) | 0.28 (0.16 – 0.55) | < 0.001 |
| Volume in end-diastole (mL) | 0.53 (0.29 – 0.91) | 0.75 (0.5 – 1.52) | < 0.001 |
| Stroke volume (mL) | 0.43 (0.26 – 0.78) | 0.5 (0.31 – 0.92) | < 0.05 |
| Cardiac output (mL/min) | 61.1 (34.9 – 104.9) | 65.9 (45.3 – 127) | < 0.05 |
| Cardiac output adjusted by EFW (mL/min/kg) | 67.8 (44.7 – 84.1) | 77.4 (66.1 – 123.8) | < 0.05 |
| Cardiac Output adjusted by AC (mL/min/cm) | 3.06 (1.82 – 4.3) | 3.11 (2.36 – 5.39) | < 0.05 |
| Cardiac Output adjusted by HC (mL/min/cm) | 2.69 (1.48 – 3.91) | 2.63 (1.94 – 5.06) | < 0.05 |
| Cardiac Output adjusted by FL (mL/min/cm) | 13.3 (7.5 – 18.7) | 14.0 (10.1 – 24.0) | < 0.05 |
| Ejection fraction (%) | 83.7 (77.4 – 87.9) | 64.7 (58.1 – 76) | < 0.001 |
Data given as group-level median (interquartile range); non-parametric comparisons were performed; median right to left ratios presented in the text were determined using ratios calculated within each fetus to account for the paired nature of these measures, and thus, are not consistent with the right to left ratios of the group level measures presented in this table.
AC, abdominal circumference; EFW, estimated fetal weight; HC, head circumference; FL, femoral diaphysis length
Stroke volume and cardiac output are lower in the presence of increased placental vascular impedance to flow
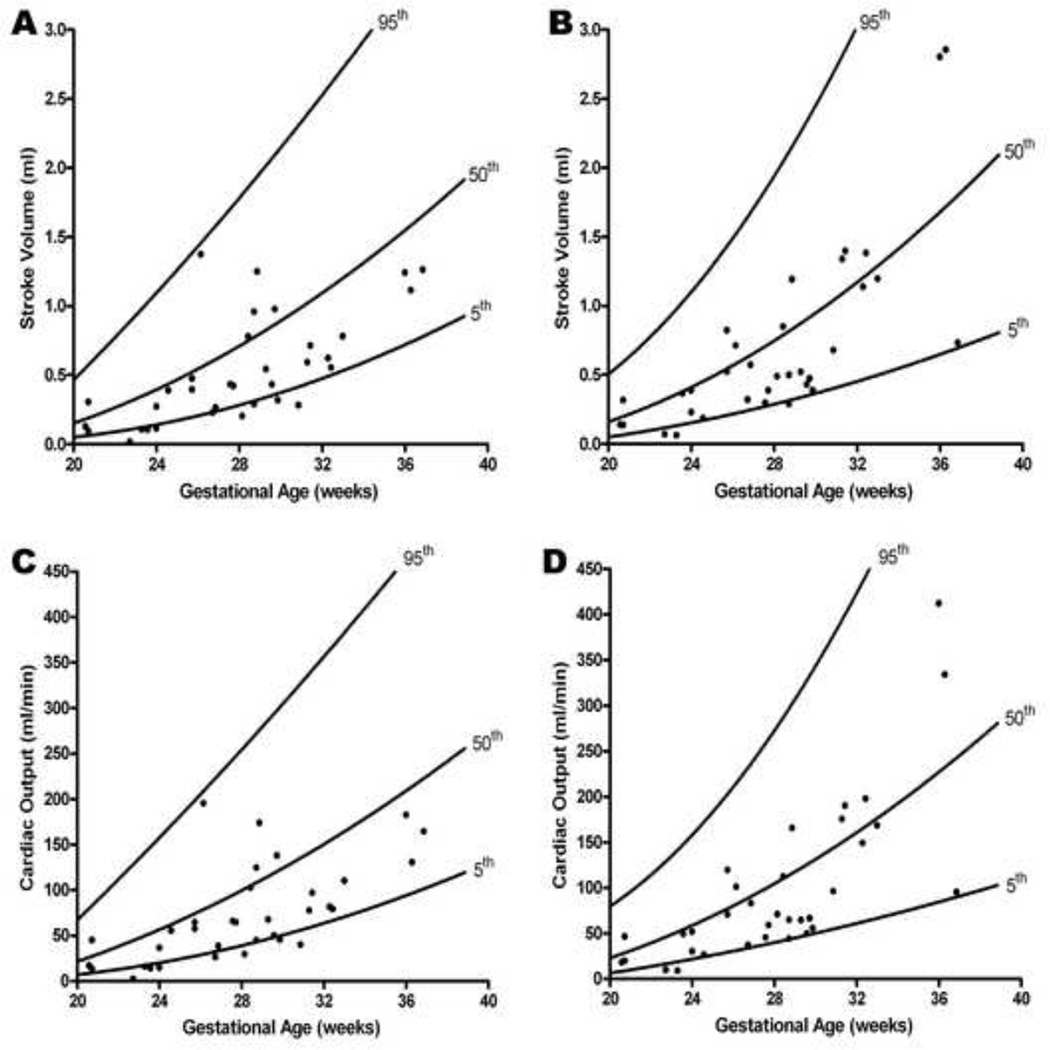
Mean stroke volume (adjusted for gestational age) (mL) was lower for the left (ABN: 0.53 vs. NL: 0.86; p < 0.0001) and right ventricle (ABN: 0.71 vs. NL: 0.92; p < 0.0001) (Figure 2 and Table 2). Similarly, mean cardiac output (adjusted for gestational age) (mL/min) was lower for the left (ABN: 71.9 vs. NL: 119.6; p < 0.0001) and right ventricle (ABN: 96 vs. NL: 127.5; p < 0.0001) (Figure 2 and Table 2). Median fetal heart rate (bpm) was not significantly different between the ABN and NL groups (ABN: 141, IQR: 131 – 145 vs. NL: 140, IQR: 134 – 147; p = 0.44).
Figure 2. Stroke volume and cardiac output as a function of gestational age in the presence of increased placental vascular impedance to flow.
Stroke volume (SV) and cardiac output (CO) calculations in fetuses with an umbilical artery pulsatility index > 95th percentile (ABN) were compared to 184 normal fetuses (NL)40 (A: left ventricular SV; B: right ventricular SV; C: left ventricular CO; D: right ventricular CO). Mean SV (adjusted for gestational age) (mL) was lower for both the left ventricle (ABN: 0.53 vs. NL: 0.86; p < 0.0001) and right ventricle (ABN: 0.71 vs. NL: 0.92 ; p < 0.0001). Similarly, mean cardiac output (adjusted for gestational age) (mL/min) was lower for both the left ventricle (ABN: 71.9 vs. NL: 119.6; p < 0.0001) and right ventricle (ABN: 96.0 vs. NL: 127.5; p < 0.0001)
Cardiac output adjusted for fetal size remains lower in the presence of increased placental vascular impedance to flow
Fetal cardiac output was adjusted for estimated fetal weight (EFW),62 which was calculated using biometric parameters (BPD, HC, AC, FL) obtained at the time of cardiac volume acquisition. Adjustment for EFW (CO/EFW) demonstrated that neither the left nor the right cardiac output (mL/min/kg) changed significantly as gestation advanced (Left CO/EFW ABN: rs = 0.13, p = NS; Right CO/EFW ABN: rs = 0.22, p = NS).
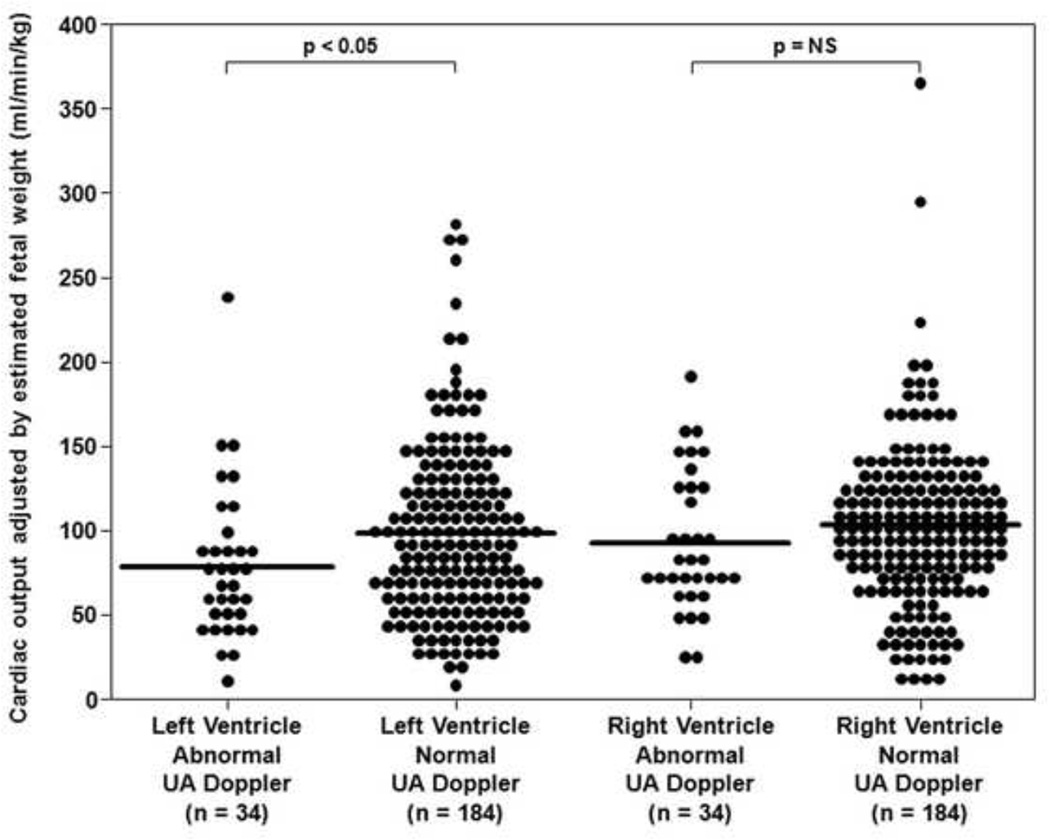
For the left ventricle, the median CO adjusted for EFW (mL/min/kg) was significantly lower in the presence of increased placental vascular impedance to flow (ABN: 67.8, IQR: 44.7 – 84.1 vs. NL: 90, IQR: 56 – 127.5; p < 0.05) (Figure 3). However, for the right ventricle, the median CO adjusted for EFW (mL/min/kg) was not significantly different between ABN and NL groups (ABN: 77.4, IQR: 66.1 – 123.8 vs. NL: 99.9, IQR: 72.2 – 126; p = NS) (Figure 3).
Figure 3. Cardiac output adjusted by estimated fetal weight of the left and right ventricles in the presence of increased placental vascular impedance to flow.
Cardiac output adjusted for estimated fetal weight (EFW) in fetuses with an umbilical artery (UA) pulsatility index > 95th percentile (ABN) were compared to 184 normal fetuses (NL).40 For the left ventricle, the median CO adjusted for EFW (mL/min/kg) was significantly lower in the presence of increased placental vascular impedance to flow (ABN: 67.8, IQR: 44.7 – 84.1 vs. NL: 90.0, IQR: 56.0 – 127.5; p < 0.05). However, for the right ventricle, the median CO adjusted for EFW (mL/min/kg) was not significantly different between ABN and NL groups (ABN: 77.4, IQR: 66.1 – 123.8 vs. NL: 99.9, IQR: 72.2 – 126.0; p = NS).
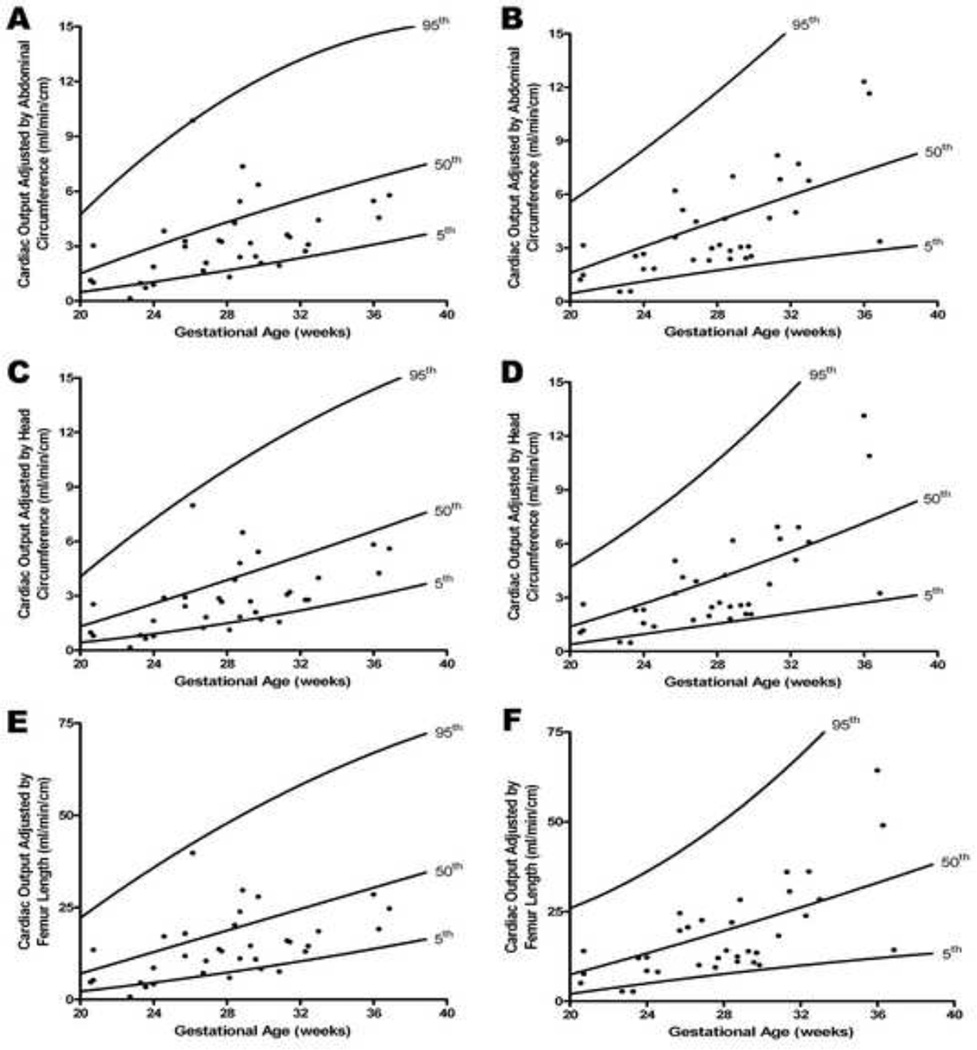
Fetal cardiac output was also adjusted for fetal size, by dividing the CO (mL/min) by the following biometric parameters: abdominal circumference [AC, cm; CO(AC)], head circumference [HC, cm; CO(HC)], and femoral diaphysis length [FL, cm; CO(FL)]. In the presence of increased placental vascular impedance to flow, values adjusted for gestational age were significantly lower for the mean left CO(AC) (mL/min/cm) (ABN: 3.2 vs. NL: 4.8; p = 0.0001), CO(HC) (mL/min/cm) (ABN: 2.8 vs. NL: 4.4; p < 0.0001), and CO(FL) (mL/min/cm) (ABN: 14.2 vs. NL: 21.4; p = 0.0001) (Figure 4 and Table 2). Similarly, values adjusted for gestational age were significantly lower for the mean right CO(AC) (mL/min/cm) (ABN: 4.1 vs. NL: 5.1; p < 0.0001), CO(HC) (mL/min/cm) (ABN: 3.7 vs. NL: 4.7; p < 0.0001), and CO(FL) (mL/min/cm) (ABN: 18.4 vs. NL: 22.6; p < 0.0001) (Figure 4 and Table 2).
Figure 4. Cardiac output adjusted by fetal biometric parameters as a function of gestational age in the presence of increased placental vascular impedance to flow.
Cardiac output (CO) obtained for left (A, C, E) and right (B, D, F) ventricles, divided by fetal biometric parameters: abdominal circumference [AC, cm; CO(AC)], head circumference [HC, cm; CO(HC)], and femoral diaphysis length [FL, cm; CO(FL)] in fetuses with an umbilical artery pulsatility index > 95th percentile (ABN) were compared to 184 normal fetuses (NL).40 In the presence of increased placental vascular impedance to flow, values were significantly lower for the mean left CO(AC) (adjusted for gestational age) (mL/min/cm) (ABN: 3.2 vs. NL: 4.8; p = 0.0001), CO(HC) (adjusted for gestational age) (mL/min/cm) (ABN: 2.8 vs. NL: 4.4; p< 0.0001), and CO(FL) (adjusted for gestational age) (mL/min/cm) (ABN: 14.2 vs. NL: 21.4; p = 0.0001). Similarly, values were significantly lower for the mean right CO(AC) (adjusted for gestational age) (mL/min/cm) (ABN: 4.1 vs. NL: 5.1; p < 0.0001), CO(HC) (adjusted for gestational age) (mL/min/cm) (ABN: 3.7 vs. NL: 4.7; p < 0.0001), and CO(FL) (adjusted for gestational age) (mL/min/cm) (ABN: 18.4 vs. NL: 22.6; p < 0.0001).
Right ventricular stroke volume, cardiac output, and adjusted cardiac output are greater than the left in the presence of increased placental vascular impedance to flow
Median right ventricular stroke volume (mL) was significantly greater than the left side (ABN Right: 0.5 vs. ABN Left: 0.43; p < 0.05). Median right cardiac output (mL/min) was also significantly greater than the left side (ABN Right: 65.9 vs. ABN Left: 61.1; p < 0.05) (Table 3).
After cardiac output was adjusted for EFW and each of the three biometric parameters, the median right ventricular cardiac output remained significantly greater than the left side: 1) EFW (mL/min/kg) (ABN Right: 77.4 vs. ABN Left: 67.8; p < 0.05); 2) abdominal circumference (mL/min/cm) (ABN Right: 3.11 vs. ABN Left: 3.06 ; p < 0.05); 3) head circumference (mL/min/cm) (ABN Right: 2.63 vs. ABN Left: 2.69; p < 0.05); and 4) femoral diaphysis length (mL/min/cm) (ABN Right: 14.0 vs. ABN Left: 13.3; p < 0.05) (Table 3).
Ejection fraction is higher in the presence of increased placental vascular impedance to flow, and is greater on the left side
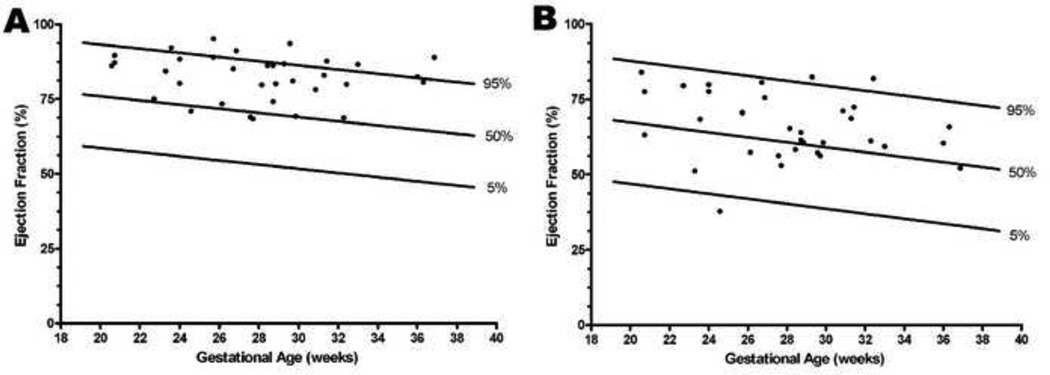
Mean ejection fraction (adjusted for gestational age) (%) was significantly higher for the left (ABN: 82.4 vs. NL: 70.4; p < 0.0001) and right ventricle (ABN: 66.0 vs. NL: 60.8; p < 0.0001) (Figure 5 and Table 2). Moreover, the median left ejection fraction (%) was significantly greater than the right side (ABN Left: 83.7 vs. ABN Right: 64.7; p < 0.001) (Table 3).
Figure 5. Ejection fraction as a function of gestational age in the presence of increased placental vascular impedance to flow.
Ejection fraction (EF) in fetuses with an umbilical artery pulsatility index > 95th percentile (ABN) were compared to 184 normal fetuses (NL)40 (A: left ventricular EF; B: right ventricular EF). Mean ejection fraction (adjusted for gestational age) (%) was significantly higher for both the left ventricle (ABN: 82.4 vs. NL: 70.4; p < 0.0001) and right ventricle (ABN: 66.0 vs. NL: 60.8; p < 0.0001).
Comment
Principal findings of this study
In the presence of increased placental vascular impedance to flow: 1) fetal ventricular volume (end-systole and end-diastole), stroke volume, and cardiac output are lower when compared to normal fetuses; 2) right ventricular volume, stroke volume, and cardiac output exceed those of the left side; 3) ejection fraction is higher when compared to normal fetuses; and 4) left ejection fraction is greater than that of the right side.
The fetal cardiovascular response to increased placental vascular impedance to flow determined using two-dimensional ultrasound
The heart is a central organ in the fetal adaptive mechanisms to placental insufficiency and hypoxia (crispi). Thus, placental insufficiency with increased placental vascular resistance may lead to fetal cardiovascular compromise,24 and even fetal metabolic acidosis and death.25 Examining fetal cardiovascular parameters in this setting could provide insight into the physiologic response to this condition. Fetuses with abnormal umbilical artery Doppler velocimetry have been shown to have similar changes to those observed in adults with atherosclerosis.28–33 Therefore, placental vascular disease28 (detected by umbilical artery Doppler velocimetry) along with fetal cardiac dysfunction may have important consequences in fetal programming of cardiac disease and the early-onset of disease.23
Several investigators have used 2D sonography to examine cardiovascular parameters (such as stroke volume and cardiac output) in fetuses with abnormal UA Doppler velocimetry.69–74 The results suggests that left ventricular stroke volume and cardiac output are decreased.72–73 Yet, for the right ventricle, some studies report an increased cardiac output,7,27 while others report a decreased cardiac output.71,73 One potential confounder is fetal size, which increases with gestational age.75 Yet, even when cardiac output is adjusted by EFW, conflicting results have been reported. In the presence of abnormal UA Doppler velocimetry, fetal cardiac output adjusted by EFW has been reported to be increased,72 decreased,69 and no different,73 when compared to that of control fetuses.
These discrepancies could be attributed to the limitations associated with using 2D methods to calculate fetal cardiovascular parameters. Errors in the calculation of stroke volume and cardiac output may arise from inaccurate measurement of vessel diameters and Doppler recordings.36 Simpson and Cook determined the repeatability of fetal Doppler echocardiographic measurements, and reported that intra- and inter-observer errors were high for vessel dimension, stroke volume, and cardiac output.36 Indeed, for Doppler measurements of the aorta (vessel diameter and velocity time integral), the coefficient of variation was > 10% for the calculated stroke volume (16%) and cardiac output (16%).36 These limitations also apply to adults, in which 2D echocardiography lacks accuracy when compared to the gold standards of magnetic resonance imaging or radionuclide ventriculography for quantification of ejection fraction and volumes.76
However, assessing fetal cardiac function using four-dimensional sonography (STIC) appears to overcome many of these pitfalls, since geometric assumptions are not made, and angle-dependent Doppler measurements are not required. Moreover, the performance of STIC is feasible, and examination times are reduced since acquisitions generally take no more than 12.5 seconds to complete, making a change in the fetal status unlikely.77–83
We have previously described a repeatable and reproducible approach to quantify ventricular volume calculations utilizing STIC,58 and then described cardiovascular parameters in a normal fetal population.40 There is insufficient data about the fetal cardiovascular response to increased placental vascular impedance to flow determined using four-dimensional sonography. Therefore, we employed this technique to study a cohort of fetuses with abnormal UA Doppler velocimetry.
The right side of the fetal heart is dominant in the setting of increased placental vascular impedance to flow
Right ventricular volume, stroke volume, and cardiac output were significantly greater than the left side in the presence of increased placental vascular impedance to flow. When a median ratio of right to left ventricular volume was calculated, the ratio was greater in end-diastole for ABN fetuses (1.5) when compared to the ratio (1.2) in normal fetuses, and was also greater in end-systole for ABN fetuses (3.2) when compared to the ratio (1.6) in normal fetuses.
Cardiac output was also adjusted by the EFW,62 and the right ventricular cardiac output remained significantly greater than the left side. However, estimates of fetal weight can carry substantial variation,84 which could introduce errors in the calculations. A systematic review of the literature evaluating sonographic estimation of fetal weight concluded that the size of random errors remains a major obstacle, with 95% CIs exceeding 14% of birth weight in all studies.85 Moreover, since a large proportion of our fetuses were affected with IUGR (71%), this may have confounded the calculation of cardiac output. Therefore, in order to minimize variability introduced into these calculations, cardiac output was also expressed as a function of 3 fetal biometric parameters, each of which has a reliability coefficient that approaches 1,86 and is measured directly without mathematical treatment or modeling of the observed results. The right ventricular cardiac output remained significantly greater than the left side after adjusting for HC, AC, and FL. In contrast, we previously reported in a population of normal fetuses no significant differences in stroke volume, cardiac output, or adjusted cardiac output (per estimated fetal weight and each of the 3 biometric parameters) between the right and left ventricles.40 It is noteworthy that the changes in cardiac function were independent of the fetal heart rate, which did not differ between the ABN and NL groups.
In contrast to the other cardiac parameters, the left ejection fraction was significantly greater than the right side in the presence of increased placental vascular impedance to flow. Taken together, these findings provide evidence that increased placental vascular impedance to flow is associated with changes in fetal cardiac function.
Fetuses respond with increased cardiac inotropy in the setting of increased placental vascular impedance to flow
In ABN fetuses, left and right ventricular volumes in end-systole and end-diastole were lower when compared to a normal population. This effect was most pronounced for the left ventricle, especially in end-systole. Moreover, ejection fraction was significantly higher in both ventricles when compared to normal fetuses. These observations suggest that fetal hearts pumping against increased placental vascular impedance to flow may compensate by increasing ventricular inotropy, the hallmark of which is reduced end-systolic volume and an increase in ejection fraction.87 In adults, the expected consequence of increased inotropy is an increase in cardiac output;87 however, the findings reported herein demonstrate lower stroke volume, cardiac output, and adjusted cardiac output.
Due to the discrepancy in the expected findings, evidence was sought in support of these observations. This may be found when examining the neurohumoral pathways, including the adrenergic nervous system, which is activated ex-utero in those with increased inotropy.88 There is empiric evidence in support of sympathetic activation in fetuses with growth restriction; specifically, norepinephrine concentrations are increased in amniotic fluid,89–90 as well as blood samples obtained from cordocentesis.91–92 Moreover, the pulsatility index of the UA has a significant correlation with norepinephrine concentrations in fetal blood.93
In growth restricted fetuses, M-mode echocardiography demonstrates significantly hypertrophied right and left ventricular free walls with greater cardiac size (adjusted for EFW) when compared to normal fetuses.94 The larger heart may result from an increase in afterload, which subsequently affects wall thickness.94 Indeed, Gruenwald reported that on pathologic examination, heart weights were consistently and moderately elevated in growth restricted fetuses, when compared to normally grown fetuses.95
Taken together, it is plausible that fetuses increase cardiac inotropy when there is increased placental vascular impedance to flow. Moreover, the inotropic response appears to be disproportionate between the left and right ventricles. Specifically, there is a proportionately greater decrease in end-systolic volume for the left ventricle (−72%) compared to the right (−40%), along with a greater increase in the left ejection fraction (17%) compared to the right (10%). It is possible that the left ventricle is more receptive to inotropic stimulation as an adaptive and protective mechanism to preserve the cerebral circulation. Indeed, animal and human studies have demonstrated that in the presence of hypoxia, there is a redistribution of blood flow with preferential perfusion of the brain (“brain-sparing” effect).96–99
Diastolic dysfunction may be a component of the fetal cardiovascular response to increased placental vascular impedance to flow
In ABN fetuses, fetal stroke volume and cardiac output were lower for both ventricles when compared to normal fetuses. The left cardiac output adjusted for EFW was significantly lower in ABN fetuses when compared to normal fetuses. However, there was no significant difference in right cardiac output adjusted for EFW between these groups. Moreover, cardiac output adjusted by AC, HC, and FL for both ventricles was significantly lower in ABN fetuses when compared to normal fetuses. Collectively, the lower left ventricular volume in end-systole along with the higher ejection fraction indicates increased inotropy, while the lower cardiac output suggests that even with increased inotropy, for some fetuses the left ventricle’s ability to compensate has been surpassed, suggesting that subclinical cardiac failure may have occurred.
Crispi et al. studied cardiac function longitudinally in growth restricted fetuses with abnormal UA Doppler velocimetry (pulsatility index > 2 SD).23 Three stages were defined based upon the status of end-diastolic velocity (stage 1: present; stage 2: absent; stage 3: reversed). In growth restricted fetuses, cardiac dysfunction (determined by modified myocardial performance index100 and early-to-late diastolic filling ratios) was identified in early stages, and increased progressively across stages. Therefore, the evidence suggests that in growth restricted fetuses with abnormal UA Doppler velocimetry, subclinical cardiac dysfunction is an early and progressive event across clinical stages of severity.23
In the adult, diastolic dysfunction has been recognized as a major cause of congestive heart failure. The major determinants of ventricular filling are ventricular relaxation and effective chamber compliance.101–102 In the normal fetus, cardiac compliance increases with gestational age, and a reduction in ventricular stiffness has been reported.103 In contrast, cardiac dysfunction in the growth-restricted fetus is characterized by increased peripheral resistance and decreased diastolic compliance, in which the heart can be described as being “stiff”.19 Indeed, Veille et al. reported that in growth-restricted fetuses, ventricles are not dilated, most likely because the fetal myocardium is inherently stiff.94 Similarly, in our group of ABN fetuses, cardiac failure may result partially from diastolic dysfunction, since there were lower end-diastolic volumes in both ventricles. The suboptimal ability of the heart to dilate could be due to a pathologic remodeling of the fetal heart, similar to the process present in adults with chronic hypertension.104 Alternatively, when there is increased placental vascular impedance to flow, it is possible that diastolic dysfunction occurs not through a pathologic mechanism, but due to the innate immaturity of the fetus. Specifically, in the setting of increased inotropy, the fetus may not yet possess the ability to actively relax the ventricles. Therefore, the expected increase in cardiovascular parameters does not occur. Evidence for subclinical diastolic dysfunction in growth restricted fetuses with abnormal UA Doppler velocimetry was reported by Crispi et al.23 In this population, cord blood levels of B-type natriuretic peptide (BNP) increased in a stage-dependent manner (based upon the status of umbilical artery end-diastolic velocity) compared with appropriately grown fetuses. In adults, BNP is considered the “gold standard” biomarker for heart failure, in which serum levels are elevated in early stages of subclinical diastolic dysfunction, and increase in proportion to severity.105
Limitations
There are several limitations of the current study. First, there is no invasive data to serve as a means of comparison to the calculation of fetal cardiovascular parameters. Due to the cardiovascular changes that occur shortly after delivery, neonatal data is not suitable to serve as a reference.106 Second, it is noteworthy that STIC produces a single, computer-generated cardiac cycle, which is an assemblage of between 20 and 30 real cardiac cycles, and a smoothing or averaging of the ventricular borders could occur, introducing error into the calculations.77 We have previously demonstrated that using STIC and VOCAL is both repeatable and reproducible for calculating fetal ventricular volumes.58 Moreover, the validity of STIC has been addressed using balloon models. Bhat et al. investigated volumes ranging between 2.5 and 10 mL,107 and Uittenbogaard et al. examined volumes ranging between 0.30 and 4.95 mL.108 Both groups concluded that STIC was acceptably accurate over these volume ranges. However, in the current study, there were volume calculations of less than 0.30 mL. Therefore, it remains unclear whether STIC compilation affects volume calculation because the absolute error of STIC was not described in volumes less than 0.30 mL. Third, there were no differences in fetal cardiovascular parameters when UA Doppler was stratified by type of end-diastolic velocity (present, absent, reversed). However, the number of cases with absent (n=7) or reversed (n=2) end-diastolic velocity was small, introducing the possibility of a type II error. Fourth, although the acquisition time of a STIC volume is 12.5 seconds at most, there is a significant learning curve and time commitment required to orient and analyze the data. Finally, the cross-sectional design of our study did not permit an evaluation of longitudinal changes in fetal cardiac function in response to increased placental vascular impedance to flow; thus, the results reported herein should be interpreted within this context. Future longitudinal studies are required to describe the natural history of cardiac function in these fetuses, and its relationship to fetal and neonatal outcome.
Conclusions
The findings of this study suggest that increased placental vascular impedance to flow is associated with changes in fetal cardiac function. Ventricular volume (especially the left ventricle in end-systole), stroke volume, and cardiac output are lower when compared to those of normal fetuses, and the right ventricle is dominant. Moreover, ejection fraction is higher when compared to normal fetuses, and the left ejection fraction is greater than the right. Taken together, the findings of lower left ventricular volume in end-systole and greater ejection fraction indicate increased inotropy. Yet, the lower cardiac output suggests that even with increased inotropy, in some fetuses the left ventricle’s ability to compensate has been surpassed, suggesting that subclinical cardiac failure may have occurred. Diastolic dysfunction may be a component of the fetal cardiovascular response to increased placental vascular impedance to flow.
Acknowledgments
Financial support: This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors declare that there is no conflict of interest.
Presentation information: The findings of this submission were presented at the 19th World Congress on Ultrasound in Obstetrics and Gynecology (International Society of Ultrasound in Obstetrics and Gynecology, ISUOG) in Hamburg, Germany on September 13-17, 2009 (Abstract number OP04.01).
References
- 1.Trudinger BJ, Giles WB, Cook CM, Bombardieri J, Collins L. Fetal umbilical artery flow velocity waveforms and placental resistance: clinical significance. Br J Obstet Gynaecol. 1985;92:23–30. doi: 10.1111/j.1471-0528.1985.tb01044.x. [DOI] [PubMed] [Google Scholar]
- 2.Trudinger BJ, Stevens D, Connelly A, et al. Umbilical artery flow velocity waveform and placental resistance: the effect of embolization of the umbilical circulation. Am J Obstet Gynecol. 1987;157:1443–1448. doi: 10.1016/s0002-9378(87)80241-7. [DOI] [PubMed] [Google Scholar]
- 3.Morrow RJ, Adamson SL, Bull SB, Ritchie JWK. Effect of placental embolization on the umbilical arterial velocity waveform in fetal sheep. Am J Obstet Gynecol. 1989;161:1055–1060. doi: 10.1016/0002-9378(89)90783-7. [DOI] [PubMed] [Google Scholar]
- 4.Mitra SC, Seshan SV, Riachi LE. Placental vessel morphometry in growth retardation and increaed resistance of the umbilical artery Doppler flow. J Matern Fetal Med. 2000;9:282–286. doi: 10.1002/1520-6661(200009/10)9:5<282::AID-MFM5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 5.Thompson RS, Trudinger BJ. Doppler waveform pulsatility index and resistance, pressure and flow in the umbilical placental circulation: an investigation using a mathematic model. Ultrasound Med Biol. 1990;16:449–458. doi: 10.1016/0301-5629(90)90167-b. [DOI] [PubMed] [Google Scholar]
- 6.Trines J, Hornberger LK. Evolution of heart disease in utero. Pediatr Cardiol. 2004;25:287–298. doi: 10.1007/s00246-003-0592-2. [DOI] [PubMed] [Google Scholar]
- 7.Allan LD, Crawford DC, Sheridan R, Chapman MG. Aetiology of non-immune hydrops: the value of echocardiography. Br J Obstet Gynaecol. 1986;93:223–225. doi: 10.1111/j.1471-0528.1986.tb07897.x. [DOI] [PubMed] [Google Scholar]
- 8.Kleinman CS, Donnerstein RL, DeVore GR, et al. Fetal echocardiography for evaluation of in utero congestive heart failure. N Engl J Med. 1982;306:568–575. doi: 10.1056/NEJM198203113061003. [DOI] [PubMed] [Google Scholar]
- 9.Comas M, Crispi F, Cruz Martinez R, Figueras F, Gratacos E. Tissue Doppler echocardiographic markers of cardiac dysfunction in small-for-gestational age fetuses. Am J Obstet Gynecol. 2011;205:57.e1–57.e6. doi: 10.1016/j.ajog.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Cruz-Martinez R, Figueras F, Hernandez-Andrade E, Oros D, Gratacos E. Changes in myocardial performance index and aortic isthmus and ductus venosus Doppler in term, small-for-gestational age fetuses with normal umbilical artery pulsatility index. Ultrasound Obstet Gynecol. 2011;38:400–405. doi: 10.1002/uog.8976. [DOI] [PubMed] [Google Scholar]
- 11.Bahtiyar MO, Copel JA. Cardiac changes in the intrauterine growth-restricted fetus. Semin Perinatol. 2008;32:190–193. doi: 10.1053/j.semperi.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Rizzo G, Capponi A, Rinaldo D, Arduini D, Romanini C. Ventricular ejection force in growth-retarded fetuses. Ultrasound Obstet Gynecol. 1995;5:247–255. doi: 10.1046/j.1469-0705.1995.05040247.x. [DOI] [PubMed] [Google Scholar]
- 13.Figueras F, Puerto B, Martinez JM, Cararach V, Vanrell JA. Cardiac function monitoring of fetuses with growth restriction. Eur J Obstet Gynecol Reprod Biol. 2003;110:159–163. doi: 10.1016/s0301-2115(03)00123-4. [DOI] [PubMed] [Google Scholar]
- 14.Comas M, Crispi F, Cruz-Martinez R, Martinez JM, Figueras F, Gratacos E. Usefulness of myocardial tissue Doppler vs. conventional echocardiography in the evaluation of cardiac dysfucntion in early-onset intrauterine growth restriction. Am J Obstet Gynecol. 2010;203:45.e1–47.e1. doi: 10.1016/j.ajog.2010.02.044. [DOI] [PubMed] [Google Scholar]
- 15.Naujorks AA, Zielinsky P, Beltrame PA, et al. Myocardial tissue Doppler assessment of diastolic function in the growth-restricted fetus. Ultrasound Obstet Gynecol. 2009;34:68–73. doi: 10.1002/uog.6427. [DOI] [PubMed] [Google Scholar]
- 16.Stirnemann JJ, Mougeot M, Proulx F, et al. Profiling fetal cardiac function in twin-twin transfusion syndrome. Ultrasound Obstet Gynecol. 2010;35:19–27. doi: 10.1002/uog.7488. [DOI] [PubMed] [Google Scholar]
- 17.Van Mieghem T, Klaritsch P, Done E, et al. Assessment of fetal cardiac function before and after therapy for twin-to-twin transfusion syndrome. Am J Obstet Gynecol. 2009;200:400.e1–400.e7. doi: 10.1016/j.ajog.2009.01.051. [DOI] [PubMed] [Google Scholar]
- 18.Van Mieghem T, Done E, Gucciardo L, et al. Amniotic fluid markers of fetal cardiac dysfunction in twin-to-twin transfusion syndrome. Am J Obstet Gynecol. 2010;202:48.e1–48.e7. doi: 10.1016/j.ajog.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Romero R, Espinoza J, Goncalves LF, et al. Fetal cardiac dysfunction in preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2004;16:146–157. doi: 10.1080/14767050400009279. [DOI] [PubMed] [Google Scholar]
- 20.DiNaro E, Cromi A, Ghezzi F, Giocolano A, Caringella A, Loverro G. Myocardial dysfunction in fetuses exposed to intraamniotic infection: new insights from tissue Doppler and strain imaging. Am J Obstet Gynecol. 2010;203:459.e1–459.e7. doi: 10.1016/j.ajog.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 21.Rounioja S, Rasanen J, Glumoff V, Ojaniemei M, Makikallio K, Hallman M. Intraamniotic lipopolysaccharide leads to fetal cardiac dysfunction – a mouse model for fetal inflammatory response. Cardiovasc Res. 2003;60:156–164. doi: 10.1016/s0008-6363(03)00338-9. [DOI] [PubMed] [Google Scholar]
- 22.Makikallio K, Rounioja S, Vuolteenaho O, Paakkari J, Hallman M, Rasanen J. Fetal cardiac natriuretric peptide expression and cardiovascular hemodynamics in endotoxininduced acute cardiac dysfunction in mouse. Pediatr Res. 2006;59:180–184. doi: 10.1203/01.pdr.0000196719.95046.19. [DOI] [PubMed] [Google Scholar]
- 23.Crispi F, Hernandez-Andrade E, Pelsers MM et al. Cardiac dysfunction and cell damage across clinical stages of severity in growth-restricted fetuses. Am J Obstet Gynecol. 2008;199:254.e1–254.e8. doi: 10.1016/j.ajog.2008.06.056. [DOI] [PubMed] [Google Scholar]
- 24.Makikallio K, Vuolteenaho V, Jouppila P, Rasanen J. Ultrasonographic and biochemical markers of human fetal cardiac dysfunction in placental insufficiency. Circulation. 2002;105:2058–2063. doi: 10.1161/01.cir.0000015505.24187.fa. [DOI] [PubMed] [Google Scholar]
- 25.Acharya G, Rasanen J, Makikallio K, et al. Metabolic acidosis decreases fetal myocardial isovolumic velocities in a chronic sheep model of increased placental vascular resistance. Am J Physiol Heart Circ Physiol. 2008;294:H498–H504. doi: 10.1152/ajpheart.00492.2007. [DOI] [PubMed] [Google Scholar]
- 26.Alberry M, Soothill P. Management of fetal growth restriction. Arch Dis Child Fetal Neonatal Ed. 2007;92:62–67. doi: 10.1136/adc.2005.082297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baschat AA, Harman CR. Venous Doppler in the assessment of fetal cardiovascular status. Curr Opin Obstet Gynecol. 2006;18:156–163. doi: 10.1097/01.gco.0000192988.07471.f9. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Athayde N, Trudinger B. Microvascular endothelial cell activation is present in the umbilical placental microcirculation in fetal placental vascular disease. Am J Obstet Gynecol. 2004;190:596–601. doi: 10.1016/j.ajog.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 29.Wilcox GR, Trudinger BJ. Erythrocytes in fetuses with abnormal umbilical artery flow velocity waveforms. Am J Obstet Gynecol. 2993;169:379–383. doi: 10.1016/0002-9378(93)90090-6. [DOI] [PubMed] [Google Scholar]
- 30.Trudinger B, Song JZ, Wu ZH, Wang J. Placental insufficiency is characterized by platelet activation in the fetus. Obstet Gynecol. 2003;101:975–981. doi: 10.1016/s0029-7844(03)00173-x. [DOI] [PubMed] [Google Scholar]
- 31.Wilcox GR, Trudinger BJ, Cook CM, Wilcox WR, Connelly AJ. Reduced fetal platelet counts in pregnancies with abnormal Doppler umbilical flow waveforms. Obstet Gynecol. 1989;73:639–643. [PubMed] [Google Scholar]
- 32.Wang J, Trudinger B. Is an atherogenic lipoprotein profile in the fetus a prerequisite for placental vascular disease? BJOG. 2000;107:508–513. doi: 10.1111/j.1471-0528.2000.tb13270.x. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Athayde N, Trudinger B. A proinflammatory cytokine response is present in the fetal placental vasculature in placental insufficiency. Am J Obstet Gynecol. 2003;189:1445–1451. doi: 10.1067/s0002-9378(03)00652-5. [DOI] [PubMed] [Google Scholar]
- 34.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth M. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298:564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vuguin PM. Animal models for small for gestational age and fetal programming of adult disease. Horm Res. 2007;68:113–123. doi: 10.1159/000100545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simpson JM, Cook A. Repeatability of echocardiographic measurements in the human fetus. Ultrasound Obstet Gynecol. 2002;20:332–339. doi: 10.1046/j.1469-0705.2002.00799.x. [DOI] [PubMed] [Google Scholar]
- 37.Simpson J. Echocardiographic evaluation of cardiac function in the fetus. Prenat Diagn. 2004;24:1081–1091. doi: 10.1002/pd.1065. [DOI] [PubMed] [Google Scholar]
- 38.Eik-Nes S, Marsal K, Kristofferson K. Methodology and basic problems related to blood flow studies in the human fetus. Ultrasound Med Biol. 1984;10:329–337. doi: 10.1016/0301-5629(84)90167-4. [DOI] [PubMed] [Google Scholar]
- 39.Nelson TR, Pretorius DH, Sklansky M, Hagen-Ansert S. Three-dimensional echocardiographic evaluation of fetal heart anatomy and function: acquisition, analysis, and display. J Ultrasound Med. 1996;15:1–9. [PubMed] [Google Scholar]
- 40.Hamill N, Yeo L, Romero R, et al. Fetal cardiac ventricular volume, cardiac output, and ejection fraction determined with four-dimensional ultrasound using spatiotemporal image correlation and virtual organ computer-aided analysis. Am J Obstet Gynecol. 2011;205:76.e1–76.e10. doi: 10.1016/j.ajog.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esh-Broder E, Ushakov FB, Imbar T, Yagel S. Application of free-hand threedimensional echocardiography in the evaluation of fetal cardiac ejection fraction: a preliminary study. Ultrasound Obstet Gynecol. 2004;23:546–551. doi: 10.1002/uog.1059. [DOI] [PubMed] [Google Scholar]
- 42.Meyer-Wittkopf M, Cole A, Cooper SG, Schmidt S, Sholler GF. Three-dimensional quantitative echocardiographic assessment of ventricular volume in healthy human fetuses and in fetuses with congenital heart disease. J Ultrasound Med. 2001;20:317–327. doi: 10.7863/jum.2001.20.4.317. [DOI] [PubMed] [Google Scholar]
- 43.Chang FM, Hus KF, Ko HC, et al. Fetal heart volume assessment by three-dimensional ultrasound. Ultrasound Obstet Gynecol. 1997;9:42–48. doi: 10.1046/j.1469-0705.1997.09010042.x. [DOI] [PubMed] [Google Scholar]
- 44.Peralta CF, Cavoretto P, Csapo B, Falcon O, Nicolaides KH. Lung and heart volumes by three-dimensional ultrasound in normal fetuses at 12-32 weeks’ gestation. Ultrasound Obstet Gynecol. 2006;27:128–133. doi: 10.1002/uog.2670. [DOI] [PubMed] [Google Scholar]
- 45.Barreto EQ, Milani HJ, Haratz KK, Araujo Junior E, Nardozza LM, Moron AF. Reference intervals for fetal heart volume from 3-dimensional sonography using the extended imaging virtual organ computer-aided analysis method at gestational ages of 20 to 34 weeks. J Ultrasound Med. 2012;31:673–678. doi: 10.7863/jum.2012.31.5.673. [DOI] [PubMed] [Google Scholar]
- 46.Bhat AH, Corbett V, Carpenter N, et al. Fetal ventricular mass determination on threedimensional echocardiography: studies in normal fetuses and validation experiments. Circulation. 2004;110:1054–1060. doi: 10.1161/01.CIR.0000139848.33468.22. [DOI] [PubMed] [Google Scholar]
- 47.Messing B, Cohen SM, Valsky DV, et al. Fetal cardiac ventricle volumetry in the second half of gestation assessed by 4D ultrasound using STIC combined with inversion mode. Ultrasound Obstet Gynecol. 2007;30:142–151. doi: 10.1002/uog.4036. [DOI] [PubMed] [Google Scholar]
- 48.Molina FS, Faro C, Sotiriadis A, Dagklis T, Nicolaides KH. Heart stroke volume and cardiac output by four-dimensional ultrasound in normal fetuses. Ultrasound Obstet Gynecol. 2008;32:181–187. doi: 10.1002/uog.5374. [DOI] [PubMed] [Google Scholar]
- 49.Rizzo G, Capponi A, Cavicchioni O, Vendola M, Arduini D. Fetal cardiac stroke volume determination by four-dimensional ultrasound with spatio-temporal image correlation 30 compared with two-dimensional and Doppler ultrasonography. Prenat Diagn. 2007;27:1147–1150. doi: 10.1002/pd.1870. [DOI] [PubMed] [Google Scholar]
- 50.Uittenbogaard LB, Haak MC, Spreeuwenberg MD, VanVugt JMG. Fetal cardiac function assessed with four-dimensional ultrasound imaging using spatiotemporal image correlation. Ultrasound Obstet Gynecol. 2009;33:272–281. doi: 10.1002/uog.6287. [DOI] [PubMed] [Google Scholar]
- 51.Schoonderwaldt EM, Groenenberg IA, Hop WC, Wladimiroff JW, Steegers EA. Reproducibility of echocardiographic measurements of human fetal left ventricular volumes and ejection fractions using four-dimensional ultrasound with the spatiotemporal image correlation modality. Eur J Obstet Gynecol Reprod Biol. 2012;160:22–29. doi: 10.1016/j.ejogrb.2011.09.036. [DOI] [PubMed] [Google Scholar]
- 52.Messing B, Cohen SM, Valsky DV, et al. Fetal heart ventricular mass obtained by STIC acquisition combined with inversion mode and VOCAL. Ultrasound Obstet Gynecol. 2011;38:191–197. doi: 10.1002/uog.8980. [DOI] [PubMed] [Google Scholar]
- 53.Luewan S, Yanase Y, Tongprasert F, Srisupundit K, Tongsong T. Fetal cardiac dimensions at 14-40 weeks’ gestation obtained using cardio-STIC-M. Ultrasound Obstet Gynecol. 2011;37:416. doi: 10.1002/uog.8961. [DOI] [PubMed] [Google Scholar]
- 54.Simioni C, Nardozza LM, Araujo Junior E, et al. Heart stroke volume, cardiac output, and ejection fraction in 265 normal fetus in the second half of gestation assessed by 4D ultrasound using spatio-temporal image correlation. J Matern Fetal Neonatal Med. 2011;24:1159–1167. doi: 10.3109/14767058.2010.545921. [DOI] [PubMed] [Google Scholar]
- 55.Rizzo G, Capponi A, Pietrolucci ME, Arduini D. Role of sonographic automatic volume calculation in measuring fetal cardiac ventricular volumes using 4-dimensional sonography: comparison with virtual organ computer-aided analysis. J Ultrasound Med. 2010;29:261–270. doi: 10.7863/jum.2010.29.2.261. [DOI] [PubMed] [Google Scholar]
- 56.Uittenbogaard LB, Haak MC, Tromp CH, Terwee CB, Van Vugt JM. Reliability of fetal cardiac volumetry using spatiotemporal image correlation: assessment of in-vivo and invitro measurements. Ultrasound Obstet Gynecol. 2010;36:308–314. doi: 10.1002/uog.7582. [DOI] [PubMed] [Google Scholar]
- 57.Tutschek B, Sahn DJ. Semi-automatic segmentation of fetal cardiac cavities: progress towards an automated fetal echocardiogram. Ultrasound Obstet Gynecol. 2008;32:176–180. doi: 10.1002/uog.5403. [DOI] [PubMed] [Google Scholar]
- 58.Hamill N, Romero R, Hassan S, et al. Repeatability and reproducibility of fetal cardiac ventricular volume calculations using spatiotemporal image correlation and virtual organ computer aided analysis. J Ultrasound Med. 2009;28:1301–1311. doi: 10.7863/jum.2009.28.10.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parra-Cordero M, Lees C, Missfelder-Lobos H, Seed P, Harris C. Fetal arterial and venous Doppler pulsatility index and time averaged velocity ranges. Prenat Diagn. 2007;27:1251–1257. doi: 10.1002/pd.1868. [DOI] [PubMed] [Google Scholar]
- 60.Turan OM, Turan S, Gungor S, et al. Progression of Doppler abnormalities in intrauterine growth restriction. Ultrasound Obstet Gynecol. 2008;32:160–167. doi: 10.1002/uog.5386. [DOI] [PubMed] [Google Scholar]
- 61.Smulian JC, Ananth CV, Vintzileos AM, Guzman ER. Revisiting sonographic abdominal circumference measurements: a comparison of outer centiles with established nomograms. Ultrasound Obstet Gynecol. 2001;18:237–243. doi: 10.1046/j.0960-7692.2001.473.x. [DOI] [PubMed] [Google Scholar]
- 62.Hadlock FP, Harrist RB, Carpenter RJ, Deter RL, Park SK. Sonographic estimation of fetal weight. The value of femur length in addition to head and abdomen measurements. Radiology. 1984;150:535–540. doi: 10.1148/radiology.150.2.6691115. [DOI] [PubMed] [Google Scholar]
- 63.Neter J, Kutner MH, Nachtsheim CJ, Wasserman W. Applied linear regression models. New York: McGraw-Hill; 1996. [Google Scholar]
- 64.Altman DG, Chitty LS. Charts of fetal size: 1. Methodology. Br J Obstet Gynaecol. 1994;101:29–34. doi: 10.1111/j.1471-0528.1994.tb13006.x. [DOI] [PubMed] [Google Scholar]
- 65.Altman DG. Construction of age-related reference centiles using absolute residuals. Stat Med. 1993;12:917–924. doi: 10.1002/sim.4780121003. [DOI] [PubMed] [Google Scholar]
- 66.Altman DG, Chitty LS. Design and analysis of studies to derive charts of fetal size. Ultrasound Obstet Gynecol. 1993;3:378–384. doi: 10.1046/j.1469-0705.1993.03060378.x. [DOI] [PubMed] [Google Scholar]
- 67.Lessoway VA, Schulzer M, Wittmann BK, Gagnon FA, Wilson RD. Ultrasound fetal biometry charts for a North American Caucasian population. J Clin Ultrasound. 1998;26:433–453. doi: 10.1002/(sici)1097-0096(199811/12)26:9<433::aid-jcu3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 68.Dashe JS, McIntire DD, Lucas MJ, Leveno KJ. Effects of symmetric and asymmetric fetal growth on pregnancy outcomes. Obstet Gynecol. 2000;96:321–327. doi: 10.1016/s0029-7844(00)00943-1. [DOI] [PubMed] [Google Scholar]
- 69.Makikallio K, Jouppila P, Rasanen J. Retrograde aortic isthmus net blood flow and human fetal cardiac function in placental insufficiency. Ultrasound Obstet Gynecol. 2003;22:351–357. doi: 10.1002/uog.232. [DOI] [PubMed] [Google Scholar]
- 70.Al-Ghazali W, Chapman MG, Allan LD. Doppler assessment of the cardiac and uteroplacental circulations in normal and complicated pregnancies. Br J Obstet Gynaecol. 1988;95:575–580. doi: 10.1111/j.1471-0528.1988.tb09486.x. [DOI] [PubMed] [Google Scholar]
- 71.Rizzo G, Arduini D. Fetal cardiac function in intrauterine growth retardation. Am J Obstet Gynecol. 1991;165:876–882. doi: 10.1016/0002-9378(91)90431-p. [DOI] [PubMed] [Google Scholar]
- 72.Reed KL, Anderson CF, Shenker L. Changes in intracardiac Doppler blood flow velocities in fetuses with absent umbilical artery diastolic flow. Am J Obstet Gynecol. 1987;157:774–779. doi: 10.1016/s0002-9378(87)80048-0. [DOI] [PubMed] [Google Scholar]
- 73.Kiserud T, Ebbing C, Kessler J, Rasmussen S. Fetal cardiac output, distribution to the placenta and impact of placental compromise. Ultrasound Obstet Gynecol. 2006;28:126–136. doi: 10.1002/uog.2832. [DOI] [PubMed] [Google Scholar]
- 74.Al-Ghazali W, Chita SK, Chapman MG, Allan LD. Evidence of redistribution of cardiac output in asymmetrical growth retardation. Br J Obstet Gynaecol. 1989;96:697–704. doi: 10.1111/j.1471-0528.1989.tb03285.x. [DOI] [PubMed] [Google Scholar]
- 75.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 76.Bellenger NG, Burgess MI, Ray SG, et al. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography radionuclide ventriculography and cardiovascular magnetic resonance: are they interchangeable? Eur Heart J. 2000;21:1387–1396. doi: 10.1053/euhj.2000.2011. [DOI] [PubMed] [Google Scholar]
- 77.DeVore GR, Falkensammer P, Sklansky MS, Platt LD. Spatio-temporal image correlation (STIC): new technology for evaluation of the fetal heart. Ultrasound Obstet Gynecol. 2003;22:380–387. doi: 10.1002/uog.217. [DOI] [PubMed] [Google Scholar]
- 78.Uittenbogaard LB, Haak MC, Spreeuwenberg MD, Van Vugt JM. A systematic analysis of the feasibility of four-dimensional ultrasound imaging using spatiotemporal image correlation in routine fetal echocardiography. Ultrasound Obstet Gynecol. 2008;31:625–632. doi: 10.1002/uog.5351. [DOI] [PubMed] [Google Scholar]
- 79.Goncalves LF, Lee W, Chaiworapongsa T, et al. Four-dimensional ultrasonography of the fetal heart with spatiotemporal image correlation. Am J Obstet Gynecol. 2003;189:1792–1802. doi: 10.1016/s0002-9378(03)00913-x. [DOI] [PubMed] [Google Scholar]
- 80.Vinals F, Poblete P, Giuliano A. Spatio-temporal image correlation (STIC): a new tool for the prenatal screening of congenital heart defects. Ultrasound Obstet Gynecol. 2003;22:388–394. doi: 10.1002/uog.883. [DOI] [PubMed] [Google Scholar]
- 81.Chaoui R, Hoffmann J, Heling KS. Three-dimensional (3D) and 4D color Doppler fetal echocardiography using spatio-temporal image correlation (STIC) Ultrasound Obstet Gynecol. 2004;23:535–545. doi: 10.1002/uog.1075. [DOI] [PubMed] [Google Scholar]
- 82.Bennasar M, Martinez JM, Gomez O, et al. Intra- and interobserver repeatability of fetal cardiac examination using four-dimensional spatiotemporal image correlation in each trimester of pregnancy. Ultrasound Obstet Gynecol. 2010;35:318–323. doi: 10.1002/uog.7570. [DOI] [PubMed] [Google Scholar]
- 83.Rizzo G, Capponi A, Muscatello A, Cavicchioni O, Vendola M, Arduini D. Examination of the fetal heart by four-dimensional ultrasound with spatiotemporal image correlation during routine second-trimester examintion: the ‘three-steps technique’. Fetal Diagn Ther. 2008;24:126–131. doi: 10.1159/000142142. [DOI] [PubMed] [Google Scholar]
- 84.Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements—a prospective study. Am J Obstet Gynecol. 1985;151:333–337. doi: 10.1016/0002-9378(85)90298-4. [DOI] [PubMed] [Google Scholar]
- 85.Dudley NJ. A systematic review of the ultrasound estimation of fetal weight. Ultrasound Obstet Gynecol. 2005;25:80–89. doi: 10.1002/uog.1751. [DOI] [PubMed] [Google Scholar]
- 86.Perni SC, Chervenak FA, Kalish RB, et al. Intraobserver and interobserver reproducibility of fetal biometry. Ultrasound Obstet Gynecol. 2004;24:654–658. doi: 10.1002/uog.1717. [DOI] [PubMed] [Google Scholar]
- 87.Schlant RC, Sonnenblick EH. Normal physiology of the cardiovascular system. In: Schlant RC, Alexander RW, editors. Hurst’s the heart. McGraw-Hill; 1994. pp. 124–126. [Google Scholar]
- 88.Villars PS, Hamlin SK, Shaw AD, Kanusky JT. Role of diastole in left ventricular function, I: Biochemical and biomechanical events. Am J Crit Care. 2004;13:394–403. [PubMed] [Google Scholar]
- 89.Divers WA, Wilkes MM, Babaknia A, Hill LM, Quilligan EJ, Yen SS. Amniotic fluid catecholamines and metabolites in intrauterine growth retardation. Am J Obstet Gynecol. 1981;141:608–610. doi: 10.1016/s0002-9378(15)33298-1. [DOI] [PubMed] [Google Scholar]
- 90.Suarez A, Rico F, Clavero-Nunez JA, Garcia-Barreno P. Amniotic fluid catecholamine metabolites in maternal smoking. Gynecol Obstet Invest. 1990;30:143–146. doi: 10.1159/000293242. [DOI] [PubMed] [Google Scholar]
- 91.Greenough A, Nicolaides KH, Lagercrantz H. Human fetal sympathoadrenal responsiveness. Early Hum Dev. 1990;23:9–13. doi: 10.1016/0378-3782(90)90124-2. [DOI] [PubMed] [Google Scholar]
- 92.Weiner CP, Robillard JE. Atrial natriuretic factor, digoxin-like immunoreactive substance, norepinephrine, epinephrine, and plasma rennin activity in human fetuses and their alteration by fetal disease. Am J Obstet Gynecol. 1988;159:1353–1360. doi: 10.1016/0002-9378(88)90555-8. [DOI] [PubMed] [Google Scholar]
- 93.Okamura K, Watanabe T, Tanigawara S, et al. Catecholamine levels and their correlation to blood gases in umbilical venous blood obtained by cordocentesis. Fetal Diagn Ther. 1990;5:147–152. doi: 10.1159/000263584. [DOI] [PubMed] [Google Scholar]
- 94.Veille JC, Hanson R, Sivakoff M, Hoen H, Ben-Ami M. Fetal cardiac size in normal, intrauterine growth retarded, and diabetic pregnancies. Amer J Perinatol. 1993;10:275–279. doi: 10.1055/s-2007-994739. [DOI] [PubMed] [Google Scholar]
- 95.Gruenwald P. Chronic fetal distress and placental insufficiency. Biol Neonat. 1963;5:215–265. doi: 10.1159/000239870. [DOI] [PubMed] [Google Scholar]
- 96.Peeters LLH, Sheldon RE, Jones MD, Makowski EL, Meschis A. Blood flow to fetal organs as a function of arterial oxygen content. Am J Obstet Gynecol. 1979;135:637–642. doi: 10.1016/s0002-9378(16)32989-1. [DOI] [PubMed] [Google Scholar]
- 97.Kjellmer I, Karlsson K, Olsson T, Rosen KG. Cerebral reactions during intrauterine asphyxia in the sheep. I. Circulation and oxygen consumption in the fetal brain. Pediatr Res. 1974;8:50–57. doi: 10.1203/00006450-197401000-00009. [DOI] [PubMed] [Google Scholar]
- 98.Wladimiroff JW, Tonge HM, Stewart PA. Doppler ultrasound assessment of cerebral blood flow in the human fetus. Br J Obstet Gynaecol. 1986;93:471–475. [PubMed] [Google Scholar]
- 99.Cohn HE, Sacks EJ, Heymann MA, Rudolph AM. Cardiovascular responses to hypoxemia and academia in fetal lambs. Am J Obstet Gynecol. 1974;120:817–824. doi: 10.1016/0002-9378(74)90587-0. [DOI] [PubMed] [Google Scholar]
- 100.Hernandez-Andrade E, Lopez-Tenorio, Figueroa-Diesel H, et al. A modified myocardial performance (Tei) index based on the use of valve clicks improves reproducibility of fetal left cardiac function assessment. Ultrasound Obstet Gynecol. 2005;26:227–232. doi: 10.1002/uog.1959. [DOI] [PubMed] [Google Scholar]
- 101.Naqvi TZ. Diastolic function assessment incorporating new techniques in Doppler echocardiography. Rev Cardiovasc Med. 2003;4:81–99. [PubMed] [Google Scholar]
- 102.Nishimura RA, Tajik AJ. Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography is the clinician’s Rosetta stone. J Am Coll Cardiol. 1997;30:8–18. doi: 10.1016/s0735-1097(97)00144-7. [DOI] [PubMed] [Google Scholar]
- 103.Veille JC, Smith N, Zaccaro D. Ventricular filling patterns of the right and left ventricles in normally grown fetuses: a longitudinal follow-up study from early intrauterine life to age 1 year. Am J Obstet Gynecol. 1999;180:849–858. doi: 10.1016/s0002-9378(99)70655-1. [DOI] [PubMed] [Google Scholar]
- 104.Kirkpatrick JN, Vannan MA, Narula J, Lang RM. Echocardiography in heart failure: applications, utility, and new horizons. J Am Coll Cardiol. 2007;50:381–396. doi: 10.1016/j.jacc.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 105.Doust JA, Pietrzak E, Dobson AJ, Glasziou PP. How well does B-type natriuretic peptide predict death and cardiac events in patients with heart failure: systematic review. BMJ. 2005;330:625–639. doi: 10.1136/bmj.330.7492.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Agata Y, Hiraishi S, Oguchi K, et al. Changes in left ventricular output from fetal to early neonatal life. J Pediatr. 1991;119:441–445. doi: 10.1016/s0022-3476(05)82060-8. [DOI] [PubMed] [Google Scholar]
- 107.Bhat AH, Corbett VN, Liu R, et al. Validation of volume and mass assessments for human fetal heart imaging by 4-dimensional spatiotemporal image correlation echocardiography: in vivo balloon model experiments. J Ultrasound Med. 2004;23:1151–1159. doi: 10.7863/jum.2004.23.9.1151. [DOI] [PubMed] [Google Scholar]
- 108.Uittenbogaard LB, Haak MC, Peters RJ, van Couwelaar GM, van Vugt JM. Validation of volume measurements for fetal echocardiography using four-dimensional ultrasound imaging and spatiotemporal image correlation. Ultrasound Obstet Gynecol. 2010;35:324–331. doi: 10.1002/uog.7561. [DOI] [PubMed] [Google Scholar]