Fig. 7. Anti-aggregation and unfoldase activity of TF.
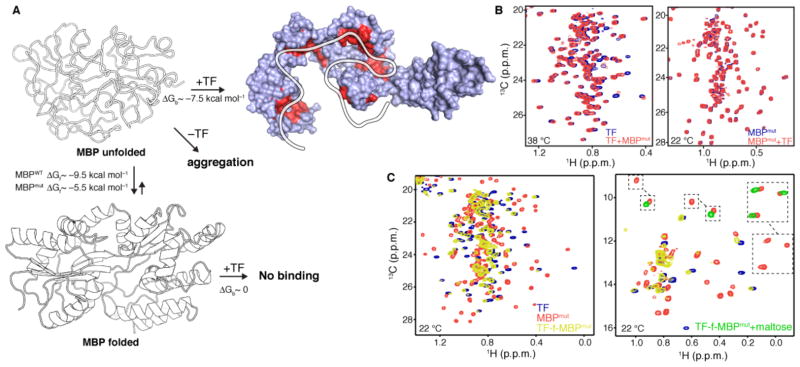
(A) Schematic of MBP unfolding and binding reactions between TF and MBPmut. ΔGf is the free energy of folding and ΔGb is the free energy of binding. The sites that TF uses to interact with the unfolded state of MBPmut, as determined by NMR, are colored red. (B) 1H-13C correlated methyl NMR spectra of the interaction between TF and MBPmut. The spectrum shown on the left was recorded at 38 °C using labeled TF and unlabeled MBPmut, whereas the spectrum shown on the right was recorded at 22 °C using the reverse labeling scheme. No interaction is observed at 22 °C, where the unfolded population of MBPmut is negligible, while binding was observed at 38 °C where the unfolded population of MBPmut is appreciable. For clarity, only the region of the Leu and Val methyl resonances is shown. The full-range spectra are shown in fig. S25. (C) 1H-13C correlated methyl NMR spectra of the fusion construct between TF and MBPmut (TF-f-MBPmut, yellow). The spectra of free TF (blue) and free MBPmut (orange) are also shown for comparison. The spectrum on the left depicts the region of the Leu and Val methyl resonances whereas the one on the right depicts the region of the Ile methyl resonances. The disappearance of the peaks belonging to MBPmut residues residing in folded regions and the appearance of peaks in the random-coil region indicates that MBPmut is unfolded when fused to TF. Addition of maltose (green resonances on the Ile spectrum) stabilizes MBPmut and prevents its unfolding by TF.
