Abstract
We have previously reported that a bystander effect is seen in cancer cells treated with Ad-IFNα and which are resistant to high concentrations of the interferon α protein (Intron A) (Zhang et al. Cancer Gene Therapy,14(3):241–50, 2007). We now provide further evidence for this bystander effect using conditioned medium (CM) collected from Ad-IFNα infected cancer and normal urothelial cells. The CMs collected from UC-9 and KU7 bladder cancer cells as well as normal urothelial cells following transfection with Ad-IFN produce cell death when added to various cancer cell types in culture but not normal urothelial cells. The CM could be filtered, frozen and thawed, and diluted to at least 1 part AdIFN-CM to 5 parts control medium and still show a similar cytotoxicity as a 100% concentration of AdIFN-CM. The CM could be partially inactivated by exposure to 65°C for 30min and totally inactivated by placement at 92°C for 3min. This cytotoxicity was observed by both flow cytometry and MTT assays as well as by phase microscopy and was seen whether the CM was collected 48, 72 or 96 hours after initial Ad-IFN treatment. Using KU7 bladder cancer cells as target cells AdIFN-CM produced no activation of caspase 8 and caspase 9 was observed although significant caspase 8 and caspase 9 cleavage occurred in Ad-IFN treated cells as a direct effect of Ad-IFN transfection. This bystander effect may play an important role in the current Ad-IFN clinical trial for superficial bladder cancer now underway.
Keywords: Adenoviral-mediated interferon α transfection, conditioned medium, bystander effects
INTRODUCTION
We have previously reported that Ad-IFNα is cytotoxic to bladder cancer cells which are resistant to high concentrations of the IFNα protein (Intron A) and has a strong bystander effect mediated by soluble factor(s) (1–2). Although the bystander effect can be shown using a co-culture system involving membrane inserts (2) the nature of the bystander factor(s) remain unknown. To better characterize the bystander factor(s), we prepared conditioned medium from Ad-IFN treated bladder cancer cell lines UC9 and KU7, which are both resistant to the IFN protein or from Ad-IFN normal human urothelial cells, NHU-Tert (3). Fortunately CM obtained from both Ad-IFN transfected normal and cancer bladder was cytotoxic when added to various cancer cell types but not normal cells. Therefore CM should be useful in trying to elucidate the identity of the soluble factor(s) causing this Ad-IFN produced bystander effect. This is now a particularly important goal since a Phase 1 clinical trial is now underway using intravesical Ad-IFN in the presence of Syn3 to increase gene transfer (1) and the bystander factor(s) produced by Ad-IFN may be an important component in its potential efficacy.
MATERIALS AND METHODS
Cell lines
The bladder cancer cell lines RT4 and UM-UC9 were provided by Dr. Barton Grossman and the breast cancer cell lines, MCF7 and ZR75T, by Dr. Khandan Keyomari at the UT MD Anderson Cancer Center. The prostate cancer cell line, DU145, and bladder cancer cell line KU7 were available in our laboratory. The hTERT immortalized normal urothelial cells, NHU-Tert , were provided by Dr Margaret Knowles [3]. The cancer cells were grown in MEM or RPMI 1640 with 10% Fetal Bovine Serum and incubated at 37°C in 5% CO2 and 95% air. NHU-Tert cells were grown in K-SFM medium with cholera toxin [3].
Reagents and Adenovirus Infection
Ad-β-gal and Ad-IFN α2b (Ad-IFN) were obtained from the Schering –Plough Research Institute (Kenilworth, NJ). Cells were exposed to the adenoviral vector at a 50 or 100 multiplicity of infection (50–100 MOI) for 3 hr in medium without serum. The virus was then removed and complete control medium added. Transfection frequency was checked by immunostaining staining in order to ensure the transfection rates were comparable in different experiments(2). Recombinant α-2b-interferon (Intron A) obtained from the Schering-Plough Research Institute (Kenilworth, NJ) was reconstituted at 1×107 IU/ml, and diluted to 1×104 IU/ml with medium containing 10% FBS.
Generation of Conditioned Medium (CM)
The UC9 and KU7 bladder cancer cells and NHU-Tert normal urothelial cells were plated into 100-mm dishes at 1×106 cells/ dish. After Ad-IFN infection as outlined above. The medium was harvested from the Ad-IFN infected dishes at various times after treatment and filtered through a 0.22 µm filter and termed either UC9-AdIFN-CM, or KU7-AdIFN-CM or NHU-AdIFN-CM. The medium harvested from the non-infected control cells or from the Ad β-gal treated cells were termed UC9 or KU7-Control-CM or UC9 or KU7-Ad βgal-CM, respectively. Give Intron A treatment or how CM obtained.
For Intron A control CM, the RT4, UC9, KU7 and NHU-Tert cells were treated with and kept in 1×104 iu/ml Intron A for 48hr. After harvest, the medium were filtered and named as RT4-Intron A-CM, UC9-Intron A-CM, KU7-Intron A-CM and NHU-Tert-Intron A-CM, respectively.
All of the conditioned medium were then divided into aliquots and stored at −80°C.
Quantification of Sub-diploid Population by Flow cytometry
The cells were plated into 100-mm dishes at 1×106 cells/dish 24 hr before infection. After Ad-IFN infection or CM exposure, the control and treated cells were harvested at various time points with trypsin-EDTA and washed in 1 ml of cold PBS. The cells were suspended in 0.5 ml of propidium iodide solution (50µg/ml propidium iodide, 0.1% Triton X-100, and 0.1% sodium citrate in PBS). Cells were then incubated at 4°C for 2 h in the dark, and then the fluorescence was read on a Coulter Epics (R) XL (Beckman-Coulter, Brea, CA). The percentage of cells showing sub-G1 DNA fragmentation was taken as a measure of apoptotic rate using PI staining and measuring with fluorescence-activated cell sorter.
MTT Assay
The various cells were seeded into 24-well plates at 1 × 104 cells/well on the day before treatment. The cells were infected with virus for 3 h or treated with conditioned medium as described above. The medium was then removed at different time points, and add 200 µl of medium added containing 1 mg/ml of 3-(4, 5-dimethylthiazol-2-)-2,5-diphenyltetrazolium bromide (MTT). After 3 h incubation at 37°C, the reaction was stopped with 200 µl of N, N-dimethylformamide lysis buffer, and the resultant solution was read at A595 with a microreader.
Western Blotting
The normal NHU-Tert cells or bladder cancer cell lines, UC-9 or KU7, were treated with various CMs. After 24h, 48h and 72h, respectively, cells were lysed in SDS buffer [150 mmol/L NaCl, 1% NP40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mmol/L Tris (pH 8.0), and protease inhibitors (Roche Diagnostics)], subjected to 15% SDS-PAGE, and transferred to nitrocellulose membranes. Membranes were blotted with anti-caspase 8 or caspase 9 antibodies: (Cell Signaling Technology, Beverly, MA). Equal loading was confirmed by blotting with anti-β-Actin antibody (Sigma, St. Louis, MO). The blots were incubated with chemiluminescent substrate (ECL, Amersham, Little Chalfont, UK.) and then developed by exposure to x-ray film.
Immunostaining
Immunochemical analysis was done according to previously described methods (4).
Virus replication test for conditioned medium
Routinely the virus titer was checked on 293 cells for the CM and for the medium from CM treated response cells. The 293 cells, grown in 6 well plates to 80% confluency, were washed once with medium and medium from the CM treated cells added at 1ml/per well. They were incubated at 5% CO2 37°C for 4hr and add another 1ml fresh MEM with 10% FBS added. The cells were then examine daily for cytopathic effects (CPE) daily for 5 days.
Statistical Analysis
Statistical analysis for the MTT and flow analysis were done using the General Lineal Models of the Statistica software (StatSoft, Inc., Tulsa, OK).
RESULTS
Production by Ad-IFN of soluble bystander factors in condition medium
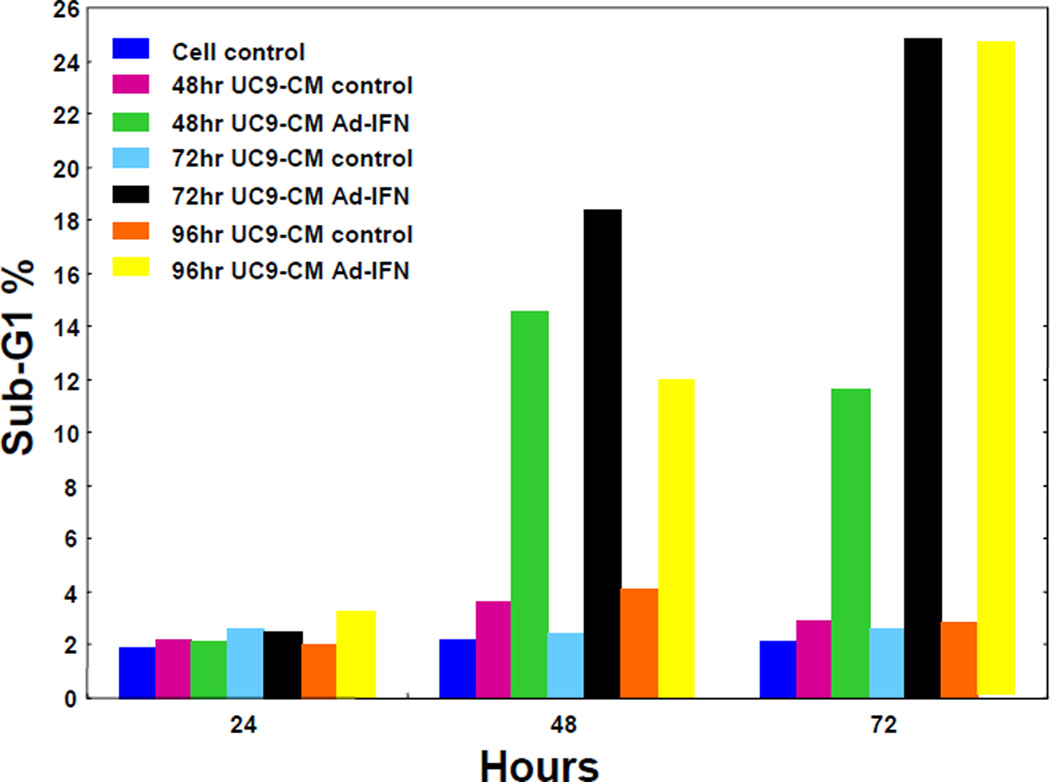
To determine if there was an optimal time for the production of bystander factors into the CM the UC9 cells were initially transfected with Ad-IFN and cultured in fresh medium without virus for different time periods (24, 48, 72, and 96 hours). The medium was then removed, filtered, aliquoted and frozen as AdIFN-CM for later use. Medium from cells which had received no Ad-IFN was obtained at each time point as control-CM. The undiluted CM from all time points were then added to UC9 cells for 24, 48 and 72 hours and then the sub-G1 population measured. No increase in the percentage of subG1 cells was found at any time exposure for AdIFN–CM removed 24h after transfection (data not shown). However, there was an increase in the percentage of subG1 cells in 48, 72 and 96 hr produced CM with longer periods of time in culture (Fig.1A). There was also a small increase the percentage of subG1 cells in Ad-IFN-CM obtained for 72 and 96 hours after Ad-IFN treatment compared to 48 hr.
Fig. 1.
Percentage of Sub-G1 cells produced UC9 cells following culture in CM. A. CM collected from UC9 cells with and without Ad-IFN infection was collected at 48, 72, and 96hr and added to UC9 cells for 24, 48 and 72 hr. No increase in the subG1 population was seen after 24hr of culture but a significant percentage of subG1 cells were observed after 48 and 72hr of culture for all three AdIFN-CM groups. B. Similar increase in the percentage of SubG1 KU7 cells following 48hr of culture with KU7 produced AdIFN-CM in undiluted CM compared to CM diluted 1:5 with fresh medium.
Whether or not the AdIFN-CM could be diluted with fresh medium was then tested with KU7 produced AdIFN-CM. Diluting the CM at a ratio of 1:5 and exposing the KU7 cells for 48 hr did not significantly decrease the percentage of apoptotic cells compared to undiluted CM as measured by the subG1 population (Fig.2B). Treatment with Ad-IFN and Intron A were used as controls. We repeated this result using UC9 produced Ad-IFN-CM (data not shown) and therefore used a 1:5 dilution in all subsequent experiments.
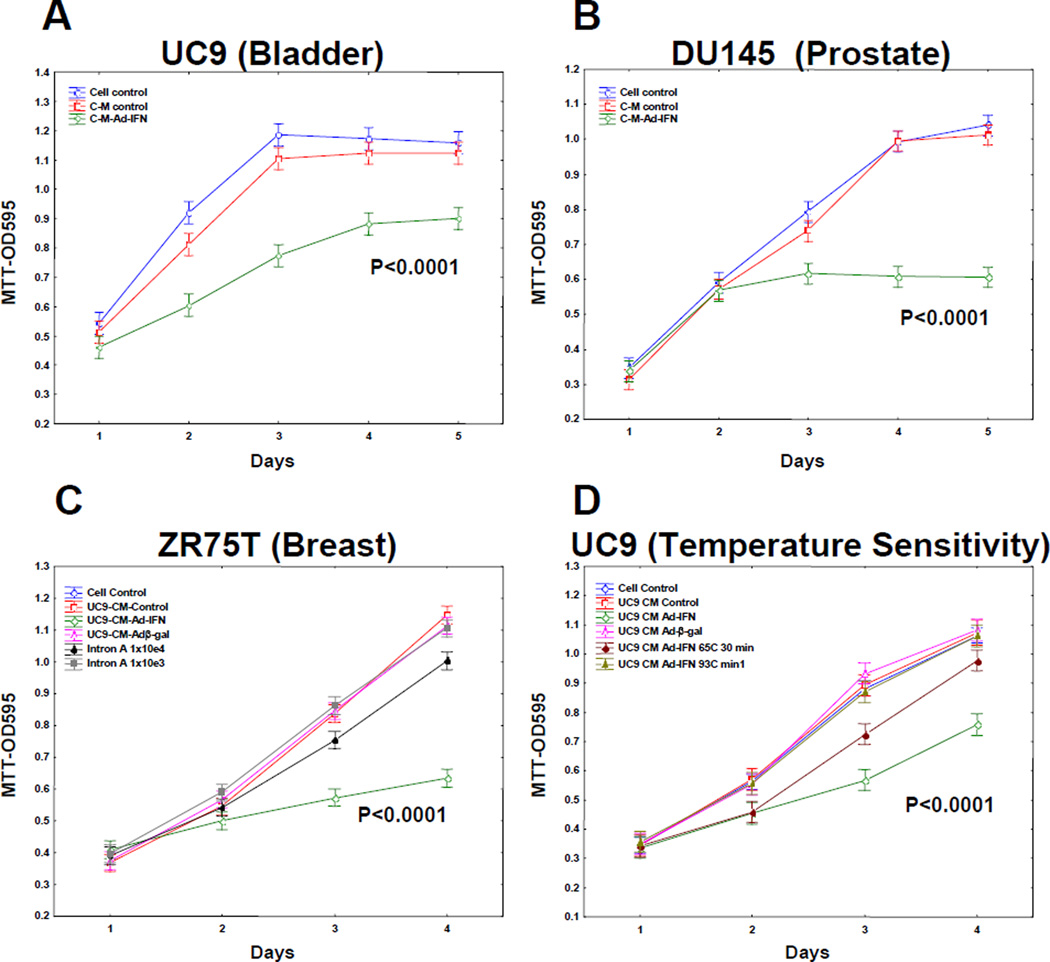
Fig. 2.
UC9 produced AdIFN–CM inhibits the growth of various cancer cells as measured by MTT and is temperature sensitive. A. UC9 bladder cancer cells B. DU145 prostate cancer cells C. ZR75T breast cancer cells D. Partial loss of UC Ad-IFN-CM activity after 30 min exposure to 65°C for 30min and was totally lost by placement at 92°C for 3min.
AdIFN-CM produced by UC9 bladder cancer cells is cytotoxic to other cancer types and is temperature sensitivity
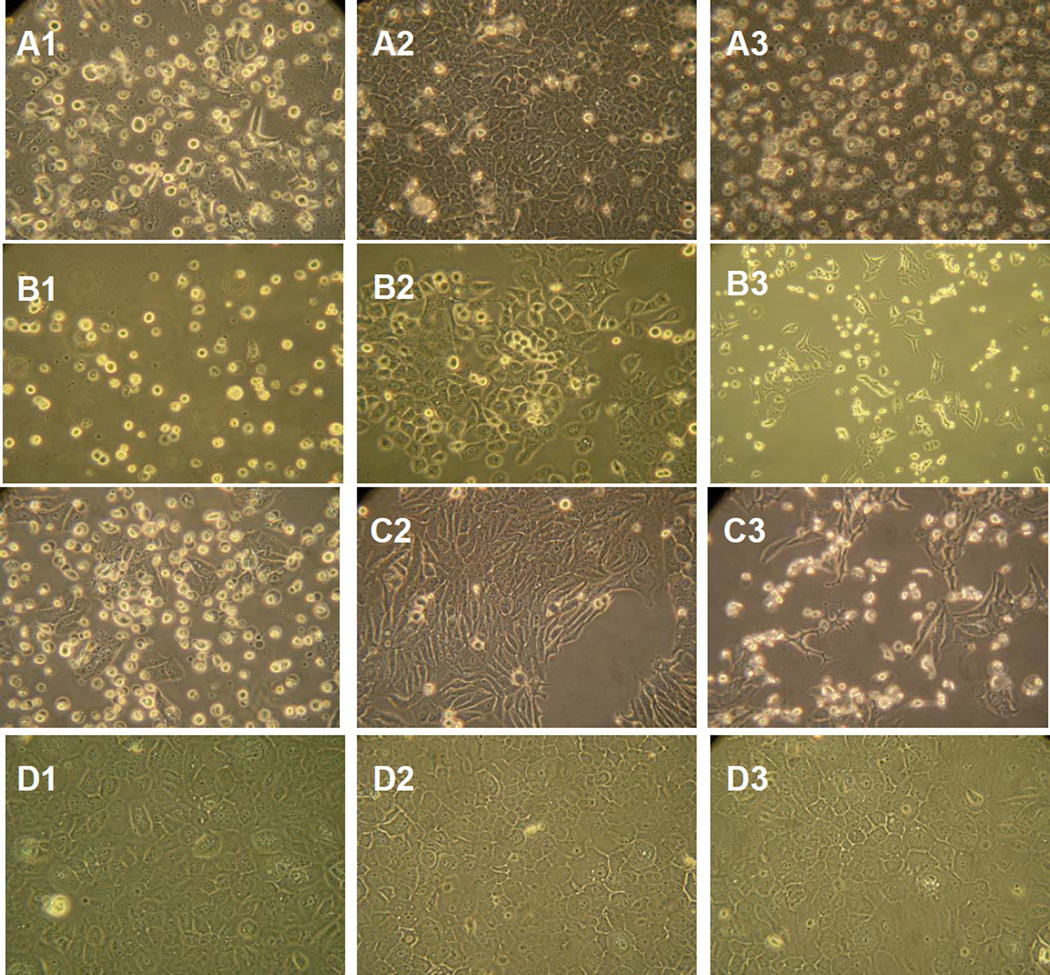
CM from Ad-IFN infected UC9 cells was diluted with fresh medium to ratio of 1:5 and cultured on DU145 prostate and ZRZ5T breast cancer cells as well as UC9 cells. All cell lines are resistance to high concentrations of Intron A (2 and Fig.2C). The UC9 produced Ad-IFN CM not only markedly decrease the growth of UC9 cells but also DU145 and ZR75T cells (Fig.2A,B and C, respectively). The cytotoxicity produced by the CM was also readily apparent by examination using phase microscopy (Fig.3). However, no cytotoxicity was observed when added to normal NHU-Tert cells (Fig.3) The ability of the CM to cause cytotoxicity was temperature sensitive since the activity was partially lost by exposure to 65°C for 30min and was totally inactivated by placement at 92°C for 3min. (Fig 2 D).
Fig. 3.
Cytotoxicity produced by AdIFN–CM as seen by phase microscopy in the same cells as examined in Fig.2. No cytotoxicity was found in normal human urothelial cells (NHU-tert cells).
Conditioned medium produced by Ad-IFN treatment in normal urothelial cells is cytotoxic to bladder cancer cells
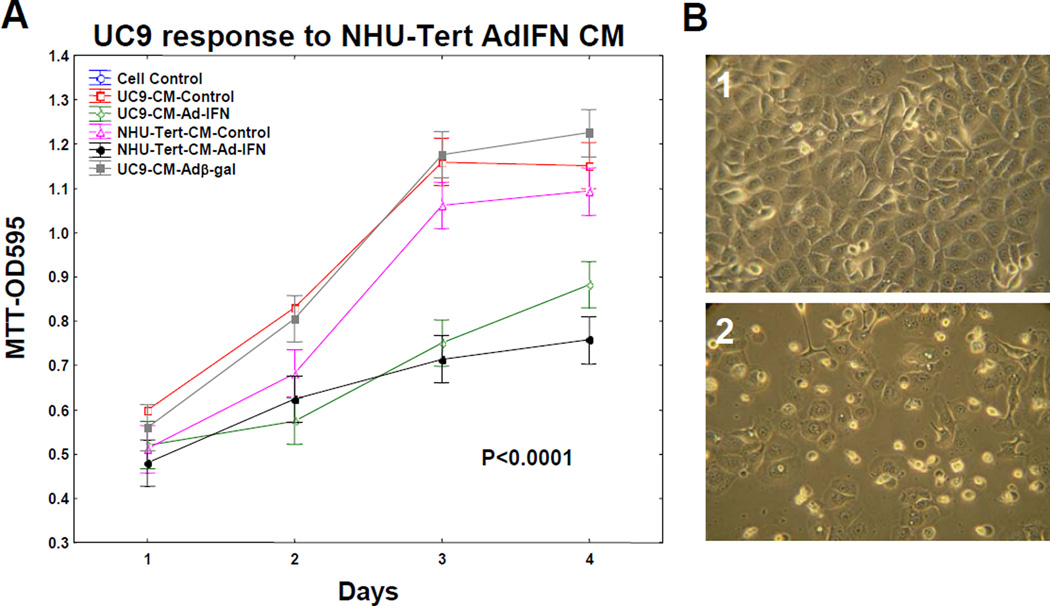
Although CM produced from cancer cells following Ad-IFN treatment was not cytotoxic to normal human urothelial cells (Fig. 3) nor did Ad-IFN treatment kill the NHU-tert cells (not shown) it was still possible that AdIFN-CM obtained by treatment of NHU-tert cells could be cytotoxic to human bladder cancer cells. In fact AdIFN-CM produced by NHU-tert cells and diluted 1:5 inhibited the growth of UC9 cells as well as AdIFN-CM obtained from UC9 cells (Fig.4A). The cytotoxicity produced by AdIFN-CM produced by NHU-tert cells to UC9 cells also was apparent by phase microscopy (Fig.4B2).
Fig. 4.
AdIFN–CM produced by normal human urothelial cells (NHU-tert) inhibits cell growth and is cytotoxic to UC9 bladder cancer cells. A. AdIFN–CM produced in NHU-tert cells inhibits UC9 cell grow similar to AdIFN–CM produced in UC9 cells B. 1.Absense of cytotoxicity of UC9 cells exposed to NHU-tert control CM for 48 hr. 2. Cytotoxicity of UC9 cells showing cells with apoptotic morphology 48 hr after addition of AdIFN–CM produced in NHU-tert cells.
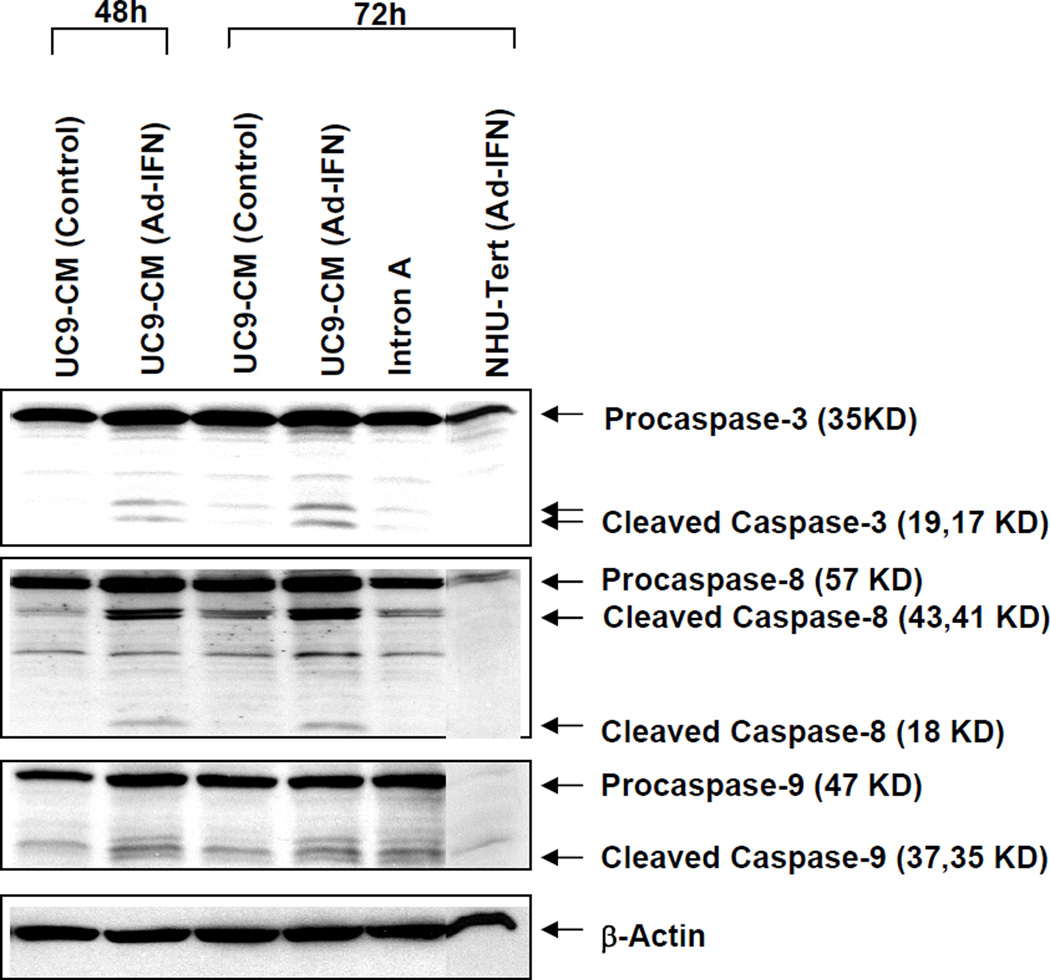
Ad-IFN produces activation of caspase 8 and 9 in cancer cells but not AdIFN-CM
We had previously shown that both caspase 8 and 9 were cleaved in UC9 and KU7 cells transfected with Ad-IFN as a measure of cell death (2). It was therefore of interest to determine if a similar caspase activation was produced during AdIFN-CM induced cytotoxicity. As shown in KU7 cells no caspase 8 or 9 cleavage was seen following AdIFN-CM treatment, indicating a different mechanism between Ad-IFN and AdIFN-CM produced cell death in Intron A resistant cancer cells.
DISCUSSION
We have previously reported that intravesical instillation of Ad-IFN in the presence of Syn3, which significantly increases adenoviral-mediated gene transfer, produced a large reduction in human bladder tumors obtained from KU7 cells growing superficially in the bladder of athymic mice (1,4). Unexpectedly the KU7 cell line was found to be highly resistant to Intron A (1,5) leading to the concept in Intron A resistant cells that Ad-IFN could kill cancer cells by mechanisms unrelated to a direct interferon α effect (1). In addition to the direct cytotoxicity produced by Ad-IFN in cancer cells transfected and expressing IFN a bystander effect was seen which was subsequently shown to be produced by a soluble factor(s) (2).
We now have found that the soluble bystander factors can be demonstrated in CM obtained after treatment of Ad-IFN in both human cancer and normal human urothelial. The CM can be frozen and thawed, diluted at least 1:5 with fresh medium and added to various cancer cell types and still produce its cytotoxic effects in cancer but not normal cells. Therefore AdIFN-CM should be an excellent source to try and isolate and identify these soluble bystander factors. This will now become a major focus of our laboratory. It should also be mentioned that a Phase 1 clinical trial is now underway using intravesical Ad-IFN in the presence of Syn3 for superficial bladder cancer and the bystander factors produced by Ad-IFN may be an important component in its potential efficacy.
Fig. 5.
Lack of caspase 8 and 9 cleavage in KU7 cells following addition of AdIFN-CM produced in KU7 cells. Western blot for caspase 8 and 9 following treatment of KU7 cells which was identical to that shown in Fig.1. Strong caspase 8 cleavage and marked decrease in procaspase 9 associated with caspase 9 cleavage was seen following Ad-IFN treatment whereas none was seen after addition of AdIFN-CM although a significant percentage of SubG1 occurred (See Fig.1B). β-actin was used as a control for equal loading.
Acknowledgments
Supported by a GU SPORE in Bladder Cancer (P50 CA91846) Project 5 to WFB
REFERENCES
- 1.Benedict WF, Tao Z, Kim CS, Zhang X, Zhou JH, Adam L, et al. Intravesical Ad-IFNα causes marked regression of human bladder cancer growing orthotopically in nude mice and overcomes resistance to IFNα protein. Mol Ther. 2004;10:525–532. doi: 10.1016/j.ymthe.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X-Q, Yang Z, Dong L, Papageorgiou A, McConkey D, Benedict WF. Adenoviral-mediated interferon a overcomes resistance to the interferon protein in various cancer types and has marked bystander effects. Cancer Gene Ther. 2007;14:241250. doi: 10.1038/sj.cgt.7701011. [DOI] [PubMed] [Google Scholar]
- 3.Chapman EJ, Hurst CD, Pitt E, Chambers P, Aveyard JS, Knowles MA. Expression of hTERT immortalises normal human urothelial cells without inactivation of the p16/Rb pathway. Oncogene. 2006;25:5037–5045. doi: 10.1038/sj.onc.1209513. [DOI] [PubMed] [Google Scholar]
- 4.Tao Z, Connor RJ, Ashoori F, Dinney CP, Munsell M, Philopena JA, et al. Intravesical adenoviral-mediated α-interferon in the presence of Syn3 causes marked regression of human bladder cancer growing orthotopically in nude mice: Correlations with dose and urine α-interferon concentrations. Cancer Gene Therapy. 2006;13:125–130. [Google Scholar]
- 5.Papageorgiou A, Lashinger L, Millikan R, Benedict WF, Dinney CP, McConkey DJ. Autocrine TRAIL production mediates interferon-induced apoptosis in human bladder cancer cells. Cancer Res. 2004;64:8973–8979. doi: 10.1158/0008-5472.CAN-04-1909. [DOI] [PubMed] [Google Scholar]