Abstract
Soil microbiota represent one of the ancient evolutionary origins of antibiotic resistance and have been proposed as a reservoir of resistance genes available for exchange with clinical pathogens. Using a high-throughput functional metagenomic approach in conjunction with a pipeline for the de-novo assembly of short-read sequence data from functional selections (termed PARFuMS), we provide evidence for recent exchange of antibiotic resistance genes between environmental bacteria and clinical pathogens. We describe multidrug-resistant soil bacteria containing resistance cassettes against five classes of antibiotics (β-lactams, aminoglycosides, amphenicols, sulfonamides, and tetracyclines) which have perfect nucleotide identity to genes from diverse human pathogens. This identity encompasses non-coding regions as well as multiple mobilization sequences, offering not only evidence of lateral exchange, but also a mechanism by which antibiotic resistance disseminates.
The continued evolution and widespread dissemination of antibiotic resistance genes in human pathogens is a preeminent clinical challenge (1). Environmental reservoirs have long been implicated as a source of resistance found in human pathogens (2). However, apart from certain opportunistic bacterial pathogens, where the same species can be found in the environment or infecting humans (3), examples of resistance genes from environmental bacteria with high identity to those of pathogens are rare (4, 5). The two documented examples are of Kluyvera and Shewanella isolates, which are found free-living in environmental settings (5, 6) yet have resistance genes (CTX-M β-lactamase and qnrA genes, respectively) with high identity (100% identity in clinical Kluyvera isolates) to those of pathogens (4, 5). The limited examples of resistance genes shared between environmental microbes and human pathogens raise questions regarding the clinical impact of environmental resistance. For instance, whether shared resistance is confined to genes of particular mechanisms (e.g. enzymatic β-lactam cleavage) or applies to many genes with diverse mechanisms of resistance is unknown. Additionally, whether a single horizontal gene transfer (HGT) event between environment and clinic can result in the de-novo acquisition of a multidrug-resistant phenotype is unclear. The two previous reports of high-identity resistance genes shared between environmental and pathogenic bacteria did not find evidence of co-localized resistance genes or of syntenic mobilization elements (4, 5), hallmarks of transferable multidrug resistance (7, 8). Determining the clinical impact of environmental resistance requires a deeper profiling of environmental reservoirs for the organisms and genotypes most likely to exchange resistance with human pathogens.
Soil, one of the largest and most diverse microbial habitats on earth, is increasingly recognized as a vast repository of antibiotic resistance genes (9–13). Not only does soil come into direct contact with antibiotics used extensively in rearing livestock (14) and plant agriculture (15), but it is also a natural habitat for the Actinomycete genus Streptomyces, whose species account for the majority of all naturally-produced antibiotics (16). Despite numerous studies demonstrating that soil contains resistance genes with biochemical mechanisms similar to those in common pathogens (3, 11–13), the sequence identities of these genes diverge from those of pathogens (17), providing little evidence that these resistomes have more than an evolutionary relationship. Therefore, whether soil has recently contributed to or acquired resistance genes from the pathogenic resistome remains an open question, and accordingly, the role of soil in the current global exchange of antibiotic resistance remains poorly defined.
To examine the capacity of non-pathogenic, soil-dwelling organisms to exchange antibiotic resistance with human pathogens, we sought to select for organisms prone to this exchange. As many major clinical pathogens are Proteobacterial (18), we cultured multidrug-resistant Proteobacteria from the soil (19), with the aim of enriching for resistance genes shared between soil and human pathogens. We interrogated the resistome of the resulting culture collection using functional metagenomic selections, which are ideally suited to characterize acquirable resistance as they identify any gene sufficient to confer resistance to a new host (e.g. pathogen) (20). To facilitate the rapid and efficient functional characterization of metagenomic libraries, we developed a massively-parallel, multiplexed functional selection platform that enables simultaneous sequencing, de-novo assembly, and functional annotation of hundreds of resistance fragments from many independent selections (termed PARFuMS: Parallel Annotation and Re-assembly of Functional Metagenomic Selections, fig. S1) (19).
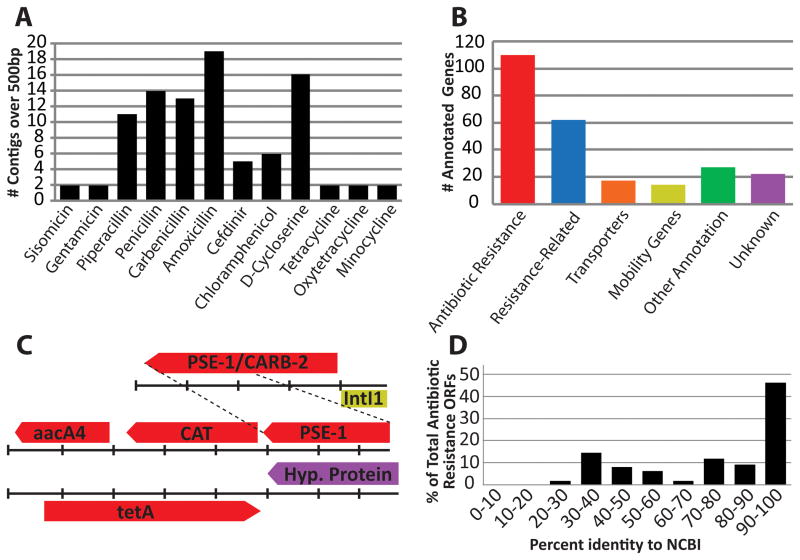
We applied PARFuMS to a collection of 95 soil-derived cultures (‘AB95’), representing bacteria with high-level resistance to various antibiotics. Cultures were obtained from 11 U.S. soils (table S1), passaged serially through minimal and rich media containing one of 18 antibiotics at 1000 mg/L (21) (tables S2, S3), and subjected to 16s rDNA profiling (19). We confirmed the culture collection was enriched for Proteobacteria and dominated by traditional soil-dwelling organisms (e.g. Pseudomonas, Pandoraea) (fig. S2). Equal proportions of the 95 cultures were pooled and bulk genomic DNA was extracted. One- to three-kilobase fragments of this metagenomic DNA were cloned into an expression vector and transformed into Escherichia coli. The resulting 2.57 gigabase library was selected on solid culture medium containing one of 12 antibiotics representing amino acid derivatives, aminoglycosides, amphenicols, β-lactams, and tetracyclines, at concentrations where the host-strain was susceptible (table S4). Resistance was detected against all 12 antibiotics and resistance-conferring fragments were sequenced, assembled, and annotated using PARFuMS, yielding 161 contigs (N50 >1.7Kb). Of the 252 open reading frames (ORFs) identified, 110 (44%) could confidently be annotated as antibiotic resistance genes (by similarity to a known resistance gene, consistent with functional selection) while another 62 (25%) were categorized as resistance-related (Fig. 1A–C, table S5).
Fig. 1.
Functional selection of the AB95 soil metagenomic library with 12 antibiotics (19). (A) Histogram depicting the number of distinct contigs over 500bp recovered from selection with each of the 12 antibiotics. (B) Functional classification of ORFs predicted by PARFuMS, across all selections. (C) Three representative metagenomic fragments; colors match catergorizations depicted in (B). Tick marks represent 300bp and dashed lines indicate common sequence on two distinct fragments. (D) Amino acid identity between antibiotic-resistance ORFs and the closest hit from GenBank, across all selections.
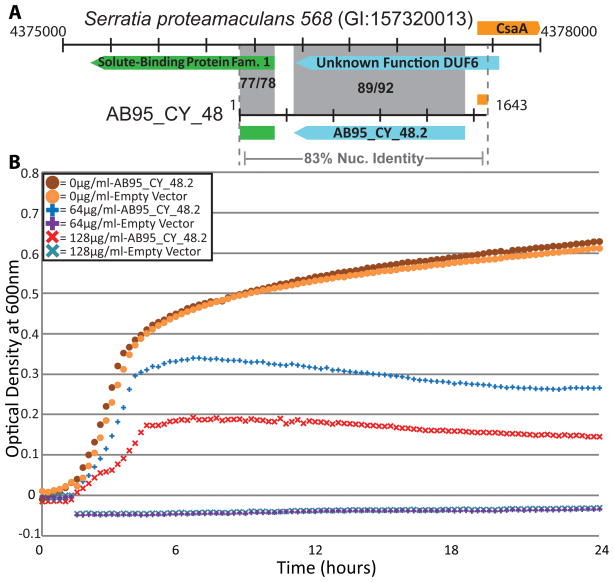
Of the 110 resistance genes, 18 had 100% amino acid identity to entries in GenBank, and another 32 were highly similar (≥90% identity). Thus, although we recovered several genes previously identified, most of the resistance genes discovered (54%) were formerly unknown (Fig. 1D). For instance, we identified a gene conferring D-cycloserine resistance from an AB95 isolate (most closely related to Serratia ficara) for which sequence alone could not predict resistance function (19). The ORF was 92% identical to a protein of unknown function from Serratia proteamaculans 568 (CP000826) (Fig. 2A), and enabled E. coli to tolerate high concentrations of D-cycloserine (128μg/mL) (Fig. 2B). The novel D-cycloserine resistance protein had low-level identity to a drug/metabolite transporter (46% identity over 91% of the sequence; YP_001583420), indicating the gene may have efflux-related function, consistent with known mechanisms of D-cycloserine tolerance (22).
Fig. 2.
A gene conferring resistance to D-cycloserine was captured for which sequence was unable to predict resistance function. (A) Resistance-conferring fragment AB95_CY_48 compared to its closest hit from the NCBI nucleotide collection. ORFs of the same color indicate homologous sequence; both nucleotide and amino acid identities are given in shaded regions (nuc/a.a.). Base-pair coordinates flank sequences and each tick mark represents 300bp. (B) Measurements of absorbance at 600nm, taken every 15 minutes, depict growth of E. coli, containing either AB95_CY_48.2 or an empty vector, at clinically-relevant concentrations of D-cycloserine. Measurements are corrected for background absorbance from media-only controls, and are averages of three trials (19).
Of the 110 AB95 resistance genes, 55 were β-lactamases. The majority of these sequences clustered with class C β-lactamases, and were dissimilar to entries currently in GenBank (fig. S3), a common result from metagenomic experiments (11, 20, 23). Notably, AB95 β-lactamases were highly divergent from those of the antibiotic-producing Streptomyces, indicating ancient evolutionary relationships (fig. S3, table S6). However, several β-lactamases with over 99% identity to sequences from both soil and enteric organisms were recovered (fig. S3).
We identified 16 sequences, from ten selections, with 100% nucleotide identity to antibiotic resistance genes previously sequenced from clinical isolates of many common human pathogens (Table 1). A bacterium was considered pathogenic only if it was isolated from an infection in a diseased human host. The 16 sequences represent seven unique genes, conferring resistance to five classes of antibiotics (β-lactams, aminoglycosides, amphenicols, sulfonamides, and tetracyclines) (Table 1). We discovered multiple examples of syntenic, soil-derived resistance genes shared with many common pathogens. For example, a chloramphenicol-acetyltransferase with 99.7% identity to K. pneumoniae clinical isolates was adjacent to both an aminoglycoside-acetyltransferase and a β-lactamase identical to genes found in many pathogens (JX009248). Additionally, an insert from two selections contained aadB (an aminoglycoside-adenyltransferase) adjacent to qacEΔ1 (an efflux pump conferring antiseptic resistance) and sul1 (a dihydropteroate synthase conferring sulfonamide resistance) in a class 1 integron-like structure (JX009286). All three genes and much of the surrounding integron (>2Kb), are 100% identical to numerous clinical pathogens. The seven soil-derived resistance genes (Table 1) are globally distributed amongst human pathogens: clinical isolates from many countries and all major continents contain genes with perfect nucleotide identity to genes from this set (fig. S4).
Table 1.
Non-redundant antibiotic resistance genes with 100% identity to known human pathogens.
| Gene Name | GenBankID | Number of Selections1 | Antibiotic Class | Annotation [Mechanism] | Pathogens Hit (GI#) |
|---|---|---|---|---|---|
| AB95_PI_68.1 | JX009363 | 4 | β-lactam | blaP1 [enzymatic degradation] | A. baumannii (94960156), K. pneumoniae (114147191), P. aeruginosa (117321883), S. typhimurium (12719011), P. mirabilis (157674381)2 |
| AB95_CH_13.1 | JX009364 | 1 | Amphenicol | Chloramphenicol Efflux [efflux] | A. baumannii (169147133), P. aeruginosa (260677483) |
| AB95_TE_2.2 | JX009366 | 3 | Tetracycline | tetA(G) [efflux] | A. baumannii (169147133), S. typhimurium (12719011) |
| AB95_TE_1.1 | JX009365 | 3 | Tetracycline | tetA [efflux] | A. baumannii (169147133), E. coli (312949035), K. pneumoniae (290792160), S. typhimurium (37962716)2 |
| AB95_GE_3.3 |
JX009367 JX009373 |
2 | Aminoglycoside | aadB [covalent modification] | E. cloacae (71361871), K. pneumoniae (206731403), P. aeruginosa (37955767), S. typhimurium (17383994)2 |
| AB95_GE_3.1 |
JX009368 JX009374 |
2 | Sulfonamide | sul1 [target modification] | C. diptheriae (323714042) E. cloacae (71361871), (206731403), K. pneumoniae P. aeruginosa (37955767), S. typhimurium (17383994), Yersinia pestis (165913934)2 |
| AB95_CH_21.1 | JX009369 | 1 | Aminoglycoside | aacA4 [covalent modification] | A. baumannii (164449567), (238865601), K. pneumoniae P. aeruginosa (219872982), S. typhi (34014739)2 |
Number of selections in which the entirety of a given gene was captured.
More pathogens exist for which 100% nucleotide identity was observed than listed
To identify soil isolates from the AB95 culture collection harboring the aforementioned resistance genes, we performed PCR using primers specific to the boundaries of the predicted ORFs (19). We identified two organisms isolated from farmland soil containing six of the resistance genes identical to pathogens, as well as two additional genes with over 99% identity to those in pathogens (tables S7, S8) (19). We confirmed that seven genes were present in an organism most closely related to Pseudomonas sp. K94.23 (a member of the P. fluorescens complex (24)), three originated from a strain most similar to Ochrobactrum anthropi, and two were in both genomes (19). P. fluorescens is not believed to cause human infection (25), and there are only limited examples of O. anthropi subgroups known to infect humans (26). Rather, these two organisms are predominantly found in environmental settings (25, 27). The substantial phylogenetic divergence between these traditionally non-pathogenic soil isolates and numerous human pathogens (table S9) contrasts with the 100% identity of numerous resistance genes found in both groups, confirming these genes moved between species via HGT.
Three ORFs from O. anthropi and P. fluorescens, conferring β-lactam, aminoglycoside, and amphenicol resistance and representing one gene shared by both organisms and one specific to each, were cloned from their genomic DNA, expressed in E. coli, and verified for resistance to seven antibiotics (19). In all cases, the ORFs conferred resistance at concentrations 16-fold greater than an empty-vector control and enabled growth in a minimum of 128μg/mL (and up to 2048μg/mL) of antibiotic (Table 2). These results mirror the minimum inhibitory concentrations of the source soil strains (Table 2), demonstrating the resistance genes retain functionality even when removed from all native genomic context, emphasizing their broad host-range compatibility.
Table 2.
Minimum inhibitory concentrations of various antibiotics towards both multidrug resistant soil isolates and E. coli clones expressing selected resistance genes (all concentrations are μg/mL).
| AX1 | CA | PE | PI | CF | CH | SI | GE | MN | OX | TE | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ochrobactrum soil isolate | >2048 | >2048 | >2048 | >2048 | <16 | 512 | 512 | 512 | <4 | 256 | 64 |
| Pseudomonas soil isolate | >2048 | >2048 | >2048 | >2048 | >1024 | 1024 | >1024 | >1024 | 8 | 128 | 32 |
| AB95_PI_68.1 | >2048 | >2048 | 2048 | 2048 | |||||||
| AB95_CH_33.1 | 256 | ||||||||||
| AB95_GE_3.3 | >1024 | >1024 | |||||||||
| E. coli + Empty Vector Control | <16 | <32 | 64 | 16 | <8 | <8 | <8 | <8 | <8 | 8 | 4 |
AX=amoxicillin, CA=carbenicillin, PE=penicillin, PI=piperacillin, CF=cefdinir, CH=chloramphenicol, SI=sisomicin, GE=gentamicin, MN=minocycline, OX=oxytetracycline, TE=tetracycline, blank cells indicate inhibitory concentrations were not determined.
Perfect nucleotide identity between full-length resistance genes from distinct species implies that recent HGT has occurred between these organisms (28), evidence that has not been previously reported between a non-pathogenic soil-dwelling organism and human pathogens. The seven resistance genes we discovered encompass all major mechanistic classes of antibiotic resistance (29) and are identical to genes found in diverse human pathogens, representing both Gram negative and positive bacteria. Moreover, for five of the soil-derived contigs that share resistance genes with pathogens, at least 80% of the contig is identical to sequence from a clinical isolate, encompassing coding and non-coding regions alike (the maximum span of identity is 2.28Kb, table S10). In support of recent mobilization, we found 11 unique sequences annotated as either an integrase or transposase from six antibiotic selections. Interestingly, two intl1 integrases were adjacent to resistance genes from both our organisms and pathogens, indicating a shared mechanism of HGT between soil and pathogenic bacteria. Four of the contigs assembled from our set are over 99% identical to a large span of sequence, found in numerous pathogens, that contains a high density of resistance genes and is flanked by multiple mobility elements (Fig. 3). This cluster of resistance genes exhibits extensive modularity; many combinations of the individual resistance elements are present in a multitude of clinical pathogens.
Fig. 3.
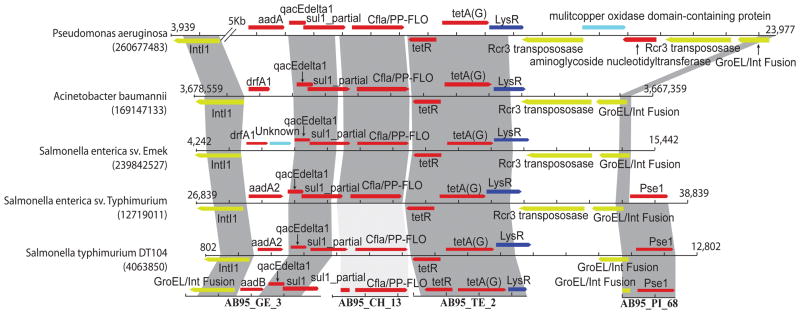
Comparison of four AB95-derived resistance fragments to five human pathogenic isolates. The four fragments are depicted along the bottom, and shading indicates high nucleotide identity between the fragments and pathogens (NCBI GenInfo numbers identify each pathogenic isolate). Dark gray shading indicates >99% identity; light grey shading indicates ~88% identity. Base-pair coordinates flank pathogenic sequences and each tick mark represents 800bp. Red ORFs represent resistance genes, yellow represents mobility elements, dark blue represents resistance-associated regulatory elements, and light blue represents other functions.
The closest homologs to each AB95 resistance gene include pathogenic resistance genes that are chromosomal as well as plasmid-borne, implying a diverse genetic organization of these genes. Four of the pathogen-identical genes from P. fluorescens, conferring resistance to the aminoglycosides, tetracyclines, amphenicols, and sulfonamides, were identified in a plasmid preparation, implicating conjugation or transformation as potential mechanisms of HGT (table S11). Additionally, we discovered nine integrases/transposases proximal to resistance genes not yet identified in pathogens, indicating additional resistance genes from these soil bacteria may be available for HGT with pathogens.
Given the extensive interspecific transfer of antibiotic resistance, and our data suggesting recent exchange between soil bacteria and clinical pathogens, it is important to identify routes of dissemination between these reservoirs. Possibilities include direct exchange between soil microbes and human pathogens or indirect transfer via the human intestinal microbiota. Many resistance genes from the intestinal microbiota are identical to those found in diverse human pathogens (20), and, accordingly, we compared the AB95 resistance genes against a set of resistance genes from cultured intestinal isolates (20), a collection of 128 representative gut organisms (table S12), and resistance genes from fecal metagenomes (19, 20). Most AB95 resistance genes were dissimilar to sequences from any intestinal dataset, with the average amino acid identity ranging from 30.2 to 45.5% (fig. S5). However, the two cultured datasets contained perfect matches to unique AB95 resistance genes (table S13). One such AB95 gene (JX009365) was not only identical to tetA from an intestinal isolate, but also to numerous pathogens, including A. baumannii, E. coli, K. pneumoniae, and S. typhimurium, indicating potential interconnections between the resistomes of the human gastrointestinal tract, soil, and clinical pathogens.
The exchange of resistance between soil and pathogens emphasizes the clinical importance of the soil resistome, regardless of whether resistance genes are moving from soil to the clinic, or vice versa. Transmission from soil to clinic establishes soil as a direct source of pathogenic resistance genes. Movement of resistance from pathogens into soil means pathogens can transfer resistance to soil organisms, of which many can cause nosocomial infection and may emerge as pathogens, akin to the rise of A. baumannii.
Powered by a novel method for characterizing functional selections at over 150-fold decreased cost (PARFuMS) (19), we describe antibiotic resistance genes found in traditional soil-dwelling bacteria and of all major mechanistic classes (29) with perfect identity to many diverse human pathogens. We also show, for the first time, these resistance genes are co-localized within long stretches of perfect nucleotide identity with pathogens, and are flanked by mobile DNA elements, providing not only evidence for recent HGT between soil and clinic, but also a mechanism through which this exchange may have occurred.
The Ochrobactrum and Pseudomonas isolates originated from farmland soils fertilized with manure from antibiotic-treated livestock. However, our current study design did not enable a statistically significant association of pathogen-identical resistance genes to specific soils. Rather, our results highlight the fact that soil and pathogenic resistomes are not distinct, emphasizing the clinical importance of environmental resistance. Our new method provides the increased throughput required to power future studies to identify soil (11), aquatic (5), and other (20) environments prone to resistance exchange with human pathogens, and to understand how specific anthropogenic practices influence the likelihood of this dissemination (3, 23).
Supplementary Material
Acknowledgments
We thank R. Mitra for initial discussions regarding simulations of metagenomic assembly, T. Druley for discussions surrounding the use of the six β-lactamase control fragments, J. Fay for discussions on dating horizontal gene transfer, J. Gordon for support, thoughtful discussion, and as the advisor to A.R., A. Moore for naming PARFuMS, and the Genome Technology Access Center at Washington University in St. Louis for generating Illumina sequence data. This work was supported by awards to G.D. through the Children’s Discovery Institute (award# MD-II-2011-117), the International Center for Advanced Renewable Energy and Sustainability at Washington University, and the National Academies Keck Futures Initiatives, Synthetic Biology - SB2. M.O.A.S. received funding from the Lundbeck foundation and the European Union FP7-HEALTH-2011-single-stage grant agreement no. 282004, EvoTAR. K.J.F. is a NSF graduate research fellow (award number DGE-1143954). A.R. is the recipient of an International Fulbright Science and Technology Award.
Footnotes
Materials and Methods
References (30–38)
Raw sequencing reads have been deposited to MG-RAST with accession numbers 4489630-39, 4489641-43, 4489645-46, 4489648-49, 4489650-51, 4489653-57, 4489659, 4489661-63, 4489665, and 4489667-68. Assembled sequences have been deposited to GenBank with accession numbers JX009202-JX009380.
The authors declare no competing financial interests.
The data reported in this paper are described in the Supporting Online Material.
References and Notes
- 1.Arias CA, Murray BE. Antibiotic-resistant bugs in the 21st century--a clinical super-challenge. N Engl J Med. 2009 Jan 29;360:439. doi: 10.1056/NEJMp0804651. [DOI] [PubMed] [Google Scholar]
- 2.Benveniste R, Davies J. Aminoglycoside antibiotic-inactivating enzymes in actinomycetes similar to those present in clinical isolates of antibiotic-resistant bacteria. Proc Natl Acad Sci U S A. 1973 Aug;70:2276. doi: 10.1073/pnas.70.8.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez JL. Antibiotics and antibiotic resistance genes in natural environments. Science. 2008 Jul 18;321:365. doi: 10.1126/science.1159483. [DOI] [PubMed] [Google Scholar]
- 4.Poirel L, Kampfer P, Nordmann P. Chromosome-encoded Ambler class A beta-lactamase of Kluyvera georgiana, a probable progenitor of a subgroup of CTX-M extended-spectrum beta-lactamases. Antimicrob Agents Chemother. 2002 Dec;46:4038. doi: 10.1128/AAC.46.12.4038-4040.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poirel L, Rodriguez-Martinez JM, Mammeri H, Liard A, Nordmann P. Origin of plasmid-mediated quinolone resistance determinant QnrA. Antimicrob Agents Chemother. 2005 Aug;49:3523. doi: 10.1128/AAC.49.8.3523-3525.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farmer JJ, 3rd, et al. Kluyvera, a new (redefined) genus in the family Enterobacteriaceae: identification of Kluyvera ascorbata sp. nov. and Kluyvera cryocrescens sp. nov. in clinical specimens. J Clin Microbiol. 1981 May;13:919. doi: 10.1128/jcm.13.5.919-933.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stalder T, Barraud O, Casellas M, Dagot C, Ploy MC. Integron involvement in environmental spread of antibiotic resistance. Frontiers in microbiology. 2012;3:119. doi: 10.3389/fmicb.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marshall BM, Levy SB. Food animals and antimicrobials: impacts on human health. Clin Microbiol Rev. 2011 Oct;24:718. doi: 10.1128/CMR.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Costa VM, et al. Antibiotic resistance is ancient. Nature. 2011 Sep 22;477:457. doi: 10.1038/nature10388. [DOI] [PubMed] [Google Scholar]
- 10.D’Costa VM, McGrann KM, Hughes DW, Wright GD. Sampling the antibiotic resistome. Science. 2006 Jan 20;311:374. doi: 10.1126/science.1120800. [DOI] [PubMed] [Google Scholar]
- 11.Allen HK, Moe LA, Rodbumrer J, Gaarder A, Handelsman J. Functional metagenomics reveals diverse beta-lactamases in a remote Alaskan soil. ISME J. 2009 Feb;3:243. doi: 10.1038/ismej.2008.86. [DOI] [PubMed] [Google Scholar]
- 12.Donato JJ, et al. Metagenomic analysis of apple orchard soil reveals antibiotic resistance genes encoding predicted bifunctional proteins. Appl Environ Microbiol. 2010 Jul;76:4396. doi: 10.1128/AEM.01763-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aminov RI, Mackie RI. Evolution and ecology of antibiotic resistance genes. FEMS Microbiol Lett. 2007 Jun;271:147. doi: 10.1111/j.1574-6968.2007.00757.x. [DOI] [PubMed] [Google Scholar]
- 14.Heuer H, Schmitt H, Smalla K. Antibiotic resistance gene spread due to manure application on agricultural fields. Curr Opin Microbiol. 2011 Jun;14:236. doi: 10.1016/j.mib.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 15.McManus PS, Stockwell VO, Sundin GW, Jones AL. Antibiotic use in plant agriculture. Annu Rev Phytopathol. 2002;40:443. doi: 10.1146/annurev.phyto.40.120301.093927. [DOI] [PubMed] [Google Scholar]
- 16.Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA. Practical Streptomyces Genetics. 1. John Innes Foundation; Norwich, UK: 2000. [Google Scholar]
- 17.Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010 Sep;74:417. doi: 10.1128/MMBR.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boucher HW, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009 Jan 1;48:1. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 19.Materials and methods are available as supplementary materials on Science online
- 20.Sommer MO, Dantas G, Church GM. Functional characterization of the antibiotic resistance reservoir in the human microflora. Science. 2009 Aug 28;325:1128. doi: 10.1126/science.1176950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dantas G, Sommer MO, Oluwasegun RD, Church GM. Bacteria subsisting on antibiotics. Science. 2008 Apr 4;320:100. doi: 10.1126/science.1155157. [DOI] [PubMed] [Google Scholar]
- 22.Clark VL, Young FE. Inducible resistance to D-cycloserine in Bacillus subtilis 168. Antimicrob Agents Chemother. 1977 May;11:871. doi: 10.1128/aac.11.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen HK, et al. Call of the wild: antibiotic resistance genes in natural environments. Nat Rev Microbiol. 2010 Apr;8:251. doi: 10.1038/nrmicro2312. [DOI] [PubMed] [Google Scholar]
- 24.Rezzonico F, Defago G, Moenne-Loccoz Y. Comparison of ATPase-encoding type III secretion system hrcN genes in biocontrol fluorescent Pseudomonads and in phytopathogenic proteobacteria. Appl Environ Microbiol. 2004 Sep;70:5119. doi: 10.1128/AEM.70.9.5119-5131.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silby MW, Winstanley C, Godfrey SA, Levy SB, Jackson RW. Pseudomonas genomes: diverse and adaptable. FEMS Microbiol Rev. 2011 Jul;35:652. doi: 10.1111/j.1574-6976.2011.00269.x. [DOI] [PubMed] [Google Scholar]
- 26.Romano S, et al. Multilocus sequence typing supports the hypothesis that Ochrobactrum anthropi displays a human-associated subpopulation. BMC Microbiology. 2009 Dec 18;9 doi: 10.1186/1471-2180-9-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chain PSG, et al. Genome of Ochrobactrum anthropi ATCC 49188(T), a Versatile Opportunistic Pathogen and Symbiont of Several Eukaryotic Hosts. Journal of Bacteriology. 2011 Aug;193:4274. doi: 10.1128/JB.05335-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smillie CS, et al. Ecology drives a global network of gene exchange connecting the human microbiome. Nature. 2011 Oct 30;480:241. doi: 10.1038/nature10571. [DOI] [PubMed] [Google Scholar]
- 29.Walsh C. Molecular mechanisms that confer antibacterial drug resistance. Nature. 2000 Aug 17;406:775. doi: 10.1038/35021219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.